Abstract
In the present study, the hepatoprotective effects of petroleum ether (FRPE) and methanol (FRME) extract of Ficus racemosa Linn. (Moraceae) stem bark were studied using the model of hepatotoxicity induced by carbon tetrachloride (CCl4) in rats. CCl4 administration induced a significant decrease in serum total protein, albumin, urea and a significant increase (P ≤ 0.01) in total bilirubin associated with a marked elevation in the activities of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) as compared to control rats. Further, CCl4 intoxication caused significant increase in the TBARS and decrease in glutathione (GSH) levels in serum, liver and kidney. Pretreatment with FRPE and FRME restored total protein and albumin to near normal levels. Both the extracts resulted in significant decreases in the activities of AST, ALT and ALP, compared to CCl4-treated rats. However, a greater degree of reduction was observed in FRME pretreated group (FRPE 43%, 38%, and 33%; FRME 55%, 73%, and 38%). Total bilirubin content decreased from 2.1 mg/dL in CCl4-treated rats to 0.8 and 0.3 mg/dL in FRPE and FRME pretreated rats, respectively. The extracts improved the antioxidant status considerably as reflected by low TBARS and high GSH values. FRME exhibited higher hepatoprotective activity than a standard liver tonic (Liv52), while the protective effect of FRPE was similar to that of Liv52. The protective effect of F. racemosa was confirmed by histopathological profiles of the liver. The results indicate that F. racemosa possesses potent hepatoprotective effects against CCl4-induced hepatic damage in rats.
Introduction
Ficus racemosa Linn. (Moraceae), commonly known as “gular”, is widely distributed all over India and other parts of Asia (CitationCSIR, 1952; CitationKulkarni & Ansari, 2004). All parts of this plant are medicinally important in the traditional system of medicine in India and have been used extensively in biliary disorders, jaundice, dysentery, diabetes, diarrhea, and as an anti-inflammatory agent (CitationKirtikar & Basu, 1975; CitationNadkarni et al., 1976; CitationChopra et al., 1958). The stem bark of F. racemosa is used in folklore medicine for treatment of coughs and colds (CitationChopra et al., 1958). An infusion of bark is employed as a mouth wash for spongy gum condition and the decoction is used in treating various skin diseases and ulcers. It is used as a poultice in inflammatory swelling and boils. It is reported to be effective in the treatment of hemorrhoids, dysentery, asthma, gonorrhea, gleet, menorrhagia, leucorrhea, hemoptysis, and urinary diseases (CitationCSIR, 1952; CitationKirtikar & Basu, 1975; CitationNadkarni et al., 1976). F. racemosa is reported to possess various biological effects such as hepatoprotective, chemopreventive, antidiabetic, anti-inflammatory, antipyretic, antitussive, antidiarrheal and antidiuretic (CitationMandal et al., 2003; CitationKhan & Sultana, 2005; Rao et al., Citation2002a, Citation2002b, Citation2003; CitationMandal et al., 2000; CitationMukherjee et al., 1998; CitationRatnasooriya et al., 2003).
Carbon tetrachloride (CCl4), an industrial solvent, is a well-established hepatotoxin. Various studies have demonstrated that the liver is not the only target organ of CCl4 and it causes free radical generation in other tissues also such as kidneys, heart, lung, testis, brain and blood (CitationAhmad et al., 1987; CitationOhta et al., 1997; CitationOzturk et al., 2003). It has also been reported that exposure to CCl4 induces acute and chronic renal injuries (CitationPerez et al., 1987; CitationChurchill et al., 1983). In view of this, the present study evaluated the protective effects of F. racemosa bark on liver damage caused by CCl4 in Wistar rats and observed the changes in the antioxidant defenses.
Materials and methods
Chemicals and reagents
Total protein, albumin assay kits (Span Diagnostics, Surat, India), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin, urea, creatinine (Agappe Diagnostics, Ernakulam, India) assay kits and Liv52 (Himalaya Healthcare, Bangalore, India), a standard liver tonic, were used. 5,5-dithio (bis) nitro benzoic acid (DTNB) was purchased from Sigma Aldrich (Bangalore, India). All the chemicals and reagents used in the study were of analytical grade.
Collection of plant material
Ficus racemosa stem bark was collected from Mukkadahally, Chamarajanagar district of Karnataka, India, during September 2007, subsequently identified by Shivprasad Hudeda, and the voucher specimen (BOT-001/2008) was deposited at the herbarium of the Department of Studies in Botany, University of Mysore. The bark was cut into small pieces, dried (50°C) and powdered, passed through 60 mesh sieve (BS) and stored in an airtight container at 4°C until further use.
Preparation of the extracts
The bark powder was extracted sequentially with petroleum ether and methanol. Evaporation of respective solvents in a rotary vacuum evaporator yielded petroleum ether extract - a sticky yellow mass (2.5% w/w), and methanol extract - a reddish brown solid mass (5.3% w/w). The extracts were homogenized with olive oil (1:5 w/v) and the rats were force fed using a feeding tube.
Animals
Healthy male Wistar rats between 8 and 9 weeks of age and weighing 140-160 g were obtained from the central animal house, Dept of Zoology, University of Mysore, and divided into 5 groups (n = 6): Control group, normal rats; FRPE group, petroleum ether extract-treated rats (500 mg/kg bw); FRME group, methanol extract-treated rats (500 mg/kg bw); LIV group, Liv52-treated rats (2.5 mL/kg bw); CCl4 group, untreated rats. All the groups other, than FRPE and FRME, received olive oil (1 mL/kg bw) in order to maintain uniformity. The rats were housed in polyacrylic cages and maintained at 27° ± 2°C, 45-60% relative humidity and 12 h photo period. The rats were provided with a standard pellet diet (Amrut Feeds, Pune, India) and water ad libitum. All animal procedures have been approved by the Animal Ethical Committee of the University of Mysore in accordance with animal experimentation and care.
Experimental design
Rats were treated with FRPE and FRME 500 mg/kg orally once a day for 7 days, and then a mixture 0.5 mL/kg (i.p.) of CCl4 in olive oil (1:1) was injected twice at 12 and 36 h after the final administration of FRPE and FRME. The animals were starved overnight before sacrifice in order to minimize variations in hepatic metabolism. The rats were anesthetized and decapitated 12 h after the final administration of CCl4 (CitationLim et al., 2000). Blood was collected by direct cardiac puncture, and liver and kidneys were immediately excised. Portions of the liver and kidneys were homogenized (1:5 w/v) in phosphate-buffered saline (pH 7.4) for estimation of TBARS and GSH, while the other portion of the greater hepatic lobes were excised for histo-pathological studies.
Assays for hepatotoxicity
The activities of alanine aminotransferase, aspartate aminotransferase and alkaline phosphatase activities were determined in serum according to the procedures given in the respective assay kits. The contents of glutathione (GSH) and TBARS in serum, liver and kidneys were determined by the methods of CitationEllman (1959) and CitationOhkawa et al. (1979) respectively. Total bilirubin, total protein, albumin, urea and creatinine contents in serum were also determined using assay kits.
Histo-pathological procedures
Liver fragments were fixed in a 10% solution of formaldehyde and then dehydrated in graduated ethanol (50-100%), cleared in xylene and embedded in paraffin. The hepatic sections (4–5 μm) were examined with a photomicroscope after staining with hematoxylin and eosin (H-E) dye. Liver morphological changes included cell necrosis, fatty and hydropic degenerative changes. The histopathological studies of liver sections were carried out at Ravi Diagnostic Laboratory, Mysore, India.
Statistical analysis
The values are expressed as mean ± SD. The data was analyzed by ANOVA followed by Tukey’s multiple comparisons test for significant differences using SPSS 11.0 software. The values were considered significant at p ≤ 0.05.
Results
Effect of FRPE and FRME on serum proteins, urea, creatinine and total bilirubin
The data on serum proteins, urea, creatinine and total bilirubin levels of various groups is given in . A significant decrease (p ≤ 0.05) in serum total protein (hypoproteinemia) was observed in CCl4 injected rats. However, the total protein concentration was restored nearer to control levels in FRME-treated rats. A similar trend was observed with respect to serum albumin, where a greater decrease in albumin was observed in CCl4 injected rats and FRME pretreatment restored albumin levels nearer to control levels, while FRPE and Liv52 restored albumin levels only to a marginal extent.
Table 1. Effect of FRPE and FRME on serum proteins, urea, creatinine and total bilirubin.
A significant decrease (p ≤ 0.05) in serum urea concentration was observed in CCl4-treated rats, while no significant differences were observed with respect to the serum creatinine levels among various groups. FRPE and FRME treatment did not cause any significant change in serum urea and creatinine levels as compared to those in CCl4-treated rats. The total bilirubin content in FRME pretreated rats was comparable with that of control rats. The bilirubin content in FRPE pretreated rats was comparable with that of Liv52 pretreated rats and was significantly lower (p ≤ 0.05) then that of CCl4-treated rats.
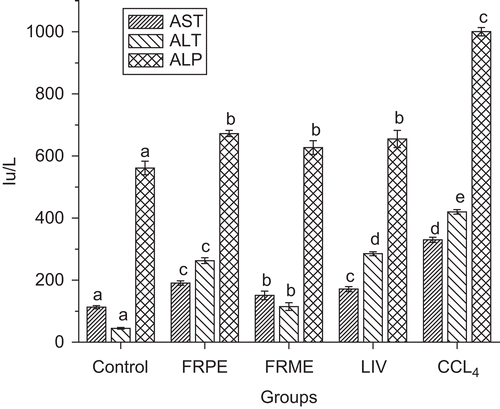
Effect of FRPE and FRME on serum transaminases, alkaline phosphatase (ALP)
Pretreatment with FRPE and FRME attenuated the increase in the activities of AST, ALT and ALP indicating their protective effect against CCl4-induced hepatic damage/injury (). FRME exhibited maximum inhibition of the activities of AST, ALT and ALP nearer to those of control levels. FRPE and Liv52 exhibited similar but significant hepatoprotective effect.
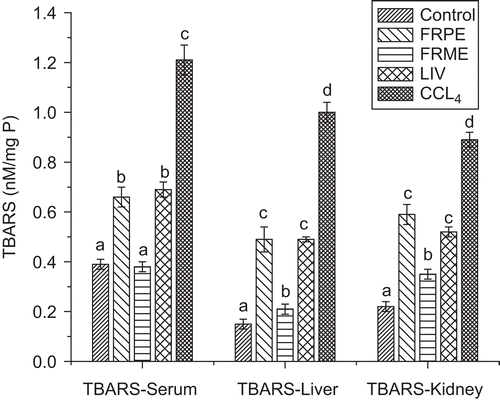
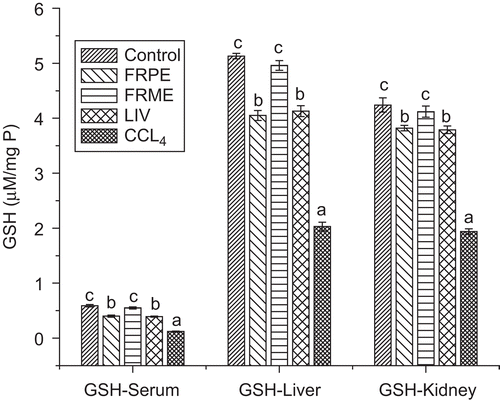
Effect of FRPE and FRME on serum, hepatic, renal TBARS and GSH
The effect of FRPE, FRME and Liv52 on antioxidant status is shown in . Pretreatment with FRPE and FRME significantly decreased (p ≤ 0.05) the lipid peroxidation induced by CCl4 as reflected by lower TBARS values in serum, liver and kidneys. Liv52 also decreased the oxidative stress significantly (p ≤ 0.05) and it was comparable with that of FRPE. However, none of the extracts resulted in complete reversal of the oxidative stress to normal levels. FRME significantly restored the depleted GSH levels similar to that of control rats. FRPE also restored the GSH but it did not reach the control levels. The Liv52 also exhibited GSH restoration effect which was similar to that of FRPE ().
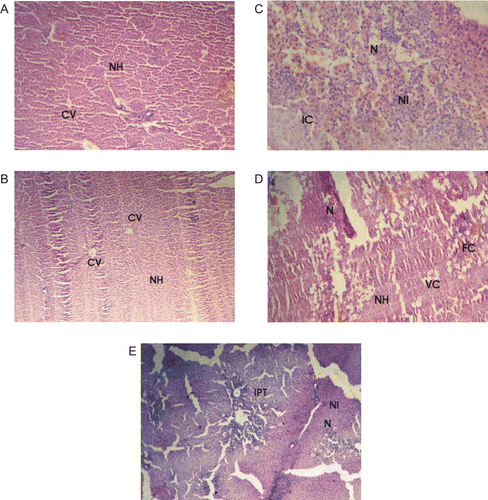
Effect of FRPE and FRME on the histopathology of liver
The protective effects of FRPE and FRME were confirmed by histopathological examination of the liver sections. In control animals, liver sections showed normal hepatic cells with well-preserved cytoplasm, prominent nucleus, nucleolus and central vein () similar observations were also found in FRME pretreated rats (). In CCl4-treated rats the sections showed intense necrosis, steatosis and inflammation with neutrophil infiltration of the hepatic cytoplasm (). Pretreatment with FRPE, and Liv52 showed considerable improvement in the hepatocellular architecture over CCl4-treated rats, as reflected from a significant reduction in hepatic necrosis ( & , respectively), however, fatty cellular changes were seen in both the groups. Vacuolated cells were seen in FRPE rats, and on the other hand, severe inflammation around the portal triad was seen in Liv52 pretreated rats.
Figure 4. (A) Section of the liver tissue of control rats showing normal hepatocytes and central vein. (C) Section of the liver tissue of rats treated with CCl4 showing severe necrosis, neutrophil infiltrate and inflammatory changes. (D) Section of the liver tissue of FRPE-pretreated rats showing normal hepatocytes necrosis and fatty changes. (B) Section of the liver tissue of FRME-pretreated rats showing normal hepatocytes and central vein. (E) Section of the liver tissue of Liv52-pretreated rats showing inflammatory changes and necrosis. NH, normal hepatocytes; CV, central vein; N, necrosis; NI, neutrophil infiltrate; IC, inflammatory cells; FC, fatty changes; VC, vacuolated cells; IPT, inflammatory infiltrate around the portal triad.
Discussion
Carbon tetrachloride is one of the most commonly used hepatotoxins in the experimental study of liver diseases. The hepatotoxic effects of CCl4 are largely due to its active metabolite, trichloromethyl radical (CitationJohnston & Kroening, 1998; CitationSrivastava et al., 1990). It is well established that CCl4 administration causes a significant decrease in serum urea, total protein and albumin levels (CitationOgeturk et al., 2004) and in the present investigation a significant decrease in the levels of total protein, albumin and albumin/globulin ratio was observed. However, pretreatment with the extracts of F. racemosa significantly restored the serum protein levels nearer to control levels. The decrease in serum protein content might be ascribed to liver damage caused by CCl4 as reports indicate that liver damage causes decreased amino acid uptake or hepatic protein synthesis (CitationEl-Shenawy & Abdel-Nabi, 2006). As reported in other studies (CitationOgeturk et al., 2004) CCl4 administration decreased serum urea levels considerably in comparison with control rats which marginally increased towards normalization in groups pretreated with F. racemosa extracts. However, some investigators have reported an increase in serum urea concentrations in CCl4-treated rats and attributed these changes in urea levels to the reduction in the glomerular filtration rate as a result of CCl4 intoxication, since the serum concentration of these two parameters depends largely on the glomerular filtration (CitationMoawad, 2007). Such an increase in urea levels may also depend on the CCl4 intoxication levels. The protective effect exhibited by Liv52 was similar to that of FRPE. FRME exhibited maximum protection and restored serum protein levels effectively. We observed a marginal increase in the serum creatinine levels of CCl4-treated rats compared to control rats but the difference did not reach statistical significance. The effect of CCl4 on serum creatinine levels is controversial. Some investigators have reported a decrease in serum creatinine in CCl4 toxicity, while others have found no significant changes (CitationOzdogan et al., 2002). In our study, creatinine values in CCl4 administered rats did not differ from control values.
Hepatotoxic compounds such as CCl4 are known to cause elevation in serum transaminases. As expected, in the present study CCl4 induced severe hepatic damage as represented by markedly elevated levels of ALT, AST, ALP and total bilirubin. The significant reductions in the activities of serum transaminases (AST and ALT), alkaline phosphatase and total bilirubin content brought about by the pretreatment with FRME could be due to the presence of bergenin, an isocoumarin (CitationVeerapur et al., 2007) reported to exhibit significant hepatoprotective activity (CitationLim et al., 2000). Liv 52 is reported to exhibit significant hepatoprotective effect and improve liver functionality in CCl4 intoxicated rats (CitationDhawan & Goel, 1994).
CCl4 induces hepatotoxicity by metabolic activation; therefore it selectively causes toxicity in liver cells maintaining semi-normal metabolic function. CCl4 is metabolically activated by the cytochrome P450-dependent mixed oxidase in the endoplasmic reticulum to form a trichloromethyl free radical (·CCl3) which combines with cellular lipids and proteins in the presence of oxygen to induce lipid peroxidation (CitationRecknagel et al., 1977; CitationDe Groot & Noll, 1986). Both FRPE and FRME decreased lipid peroxidation to near normal levels. Similar observations are reported with respect to Liv52 which significantly decreased malonaldialdehyde content in CCl4 intoxicated rats (CitationPandey et al., 1994). Reports indicate that some natural extracts containing antioxidants protect against the CCl4-induced lipid peroxidation and impairment in GSH status (CitationKo et al., 1995). The reduction in oxidative stress by the extracts of F. racemosa could be attributed to the presence of tannins, kaempferol, rutin, bergapten, psoralenes, flavonoids, ficusin, coumarin and phenolic glycosides (CitationBaruah & Gohain, 1992), bergenin and racemosic acid (CitationLi et al., 2004) that are reported to act as strong antioxidant and anti-inflammatory agents (CitationKhan & Sultana, 2005).
In states of oxidative stress, GSH is converted to glutathione disulfide and depleted, leading to lipid peroxidation. Therefore, the role of GSH as a reasonable marker for the evaluation of oxidative stress is important (CitationRecknagel et al., 1977). FRPE, FRME and Liv52 inhibited lipid peroxidation and elevated depleted serum, hepatic and renal GSH levels towards normalization.
Histopathological studies showed that CCl4 caused severe necrosis, neutrophil infiltration and hydropic degeneration of the liver tissue. Pretreatment with extracts exhibited considerable hepatic protection, which confirmed the results of biochemical studies. All the effects of the extracts were comparable with those of Liv52, a proven hepatoprotective tonic (CitationChauhan et al., 1994; CitationKothari et al., 1990; CitationKataria & Singh, 1997; CitationGopumadhavan et al., 1993; CitationDhawan & Goel, 1994). It is noteworthy to state that FRME exhibited maximum hepatoprotective effect as reflected by both biochemical parameters and histopathology of liver sections. The effect was far superior to Liv52 which can be attributed to the presence of bergenin, a known hepatoprotective agent and racemosic acid, a strong antioxidant (CitationLi et al., 2004; CitationLim et al., 2000). The hepatoprotective effect of FRPE may be attributed to the presence of lupeol acetate, a triterpenoid (CitationRahman et al., 1994). The results of our study indicate that simultaneous treatment with F. racemosa bark extracts protects the liver against CCl4-induced hepatotoxicity.
Conclusion
The present study is suggestive of the fact that CCl4 causes hepatic damage in rats through enhanced lipid peroxidation and alterations in antioxidant defenses. The results demonstrate that F. racemosa has potent hepatoprotective action against CCl4-induced hepatic damage in rats and this effect may be mediated through its antioxidative action. These observations justify the use of F. racemosa as traditional medicine in the treatment of jaundice. Further investigations are underway to isolate the phytoconstituents responsible for its hepatoprotective effect.
Acknowledgement
The authors acknowledge the University Grants Commission, New Delhi, India, for financial assistance (F.31-278/2005).
Declaration of interest: The financial support has been acknowledged in the Acknowledgements.
References
- Ahmad FF, Cowan DL, Sun AY (1987): Detection of free radical formation in various tissues after acute carbon tetrachloride administration in gerbil. Life Sci 41: 2469–2475.
- Baruah KK, Gohain AK (1992): Chemical composition and nutritive value of dimaru (Ficus glomerata Roxb.) leaves. Indian J Anim Nutr 9: 107–108.
- Chauhan BL, Mohan AR, Kulkarni RD, Mitra SK (1994): Bioassay for evaluation of the hepatoprotective effect of Liv.52, a polyherbal formulation, on ethanol metabolism in chronic alcohol-exposed rats. Indian J Pharm 26: 117–120.
- Chopra RN, Chopra IC, Handa KL, Kapur LD (1958): Indigenous Drugs of India, second edition, Calcutta, Academic Publishers, pp. 508–674.
- Churchill DN, Finn A, Gault M (1983): Association between hydrocarbon exposure and glomerulonephritis. An araisal of the evidence. Nephron 33: 169–172.
- CSIR (1952): The Wealth of India, New Delhi, Council of Scientific and Industrial Research, pp. 35–36.
- De Groot H, Noll T (1986): The crucial role of low steady state oxygen partial pressures in haloalkane free-radical mediated lipid peroxidation. Biochem Pharmacol 35: 15–19.
- Dhawan D, Goel A (1994): Hepatoprotective effects of Liv.52 and its indirect influence on the regulation of thyroid hormones in rat liver toxicity induced by carbon tetrachloride. Res Exp Med 194: 203–215.
- Ellman GL (1959): Tissue sulfhydryl groups. Arch Biochem Biophys 82: 70–72.
- El-Shenawy NS, Abdel-Nabi IM (2006): Hypoglycemic effect of Cleome droserifolia ethanolic leaf extract in experimental diabetes, and on non-enzymatic antioxidant, glycogen, thyroid hormone and insulin levels. Diabetologia Croatica 35: 15–22.
- Gopumadhavan S, Jagadeesh S, Chauhan BL, Kulkarni RD (1993): Protective effect of Liv.52 on alcohol-induced fetotoxicity. Clin Exp Res 17: 1089–1092.
- Johnston DE, Kroening C (1998): Mechanism of early carbon tetrachloride toxicity in cultured rat hepatocytes. Pharmacol Toxicol 83: 231–239.
- Kataria M, Singh LN (1997): Hepatoprotective effect of Liv.52 and kumaryasava on carbon tetrachloride induced hepatic damage in rats. Indian J Exp Biol 35: 655–657.
- Khan N, Sultana S (2005): Chemomodulatory effect of Ficus racemosa extract against chemically induced renal carcinogenesis and oxidative damage response in Wistar rats. Life Sci 29: 1194–1210.
- Kirtikar KR, Basu BD (1975): Indian Medicinal Plants, second edition, Dehra Dun, India, pp. 2327–2328.
- Ko KM, Ip SP, Poon MK, Wu SS, Che CT, Ng KH, Kong YC (1995): Effect of a lignan-enriched Fructus schisandrae extract on hepatic glutathione status in rats: Protection against carbon tetrachloride toxicity. Planta Med 61: 134–137.
- Kothari S, Reddy BP, Rathore HS (1990): Protective role of Liv.52 against histological damage due to CdCl2 toxicity in the intestine of teleost fish. Probe 3: 220–228.
- Kulkarni PH, Ansari A (2004): The Ayurvedic Plants, Delhi, Satguru Publications, pp. 334.
- Li RW, Leach DN, Myers SP, Lin GD, Leach GJ, Waterman PG (2004): A new anti-inflammatory glucoside from Ficus racemosa L. Planta Med 70: 421–426.
- Lim HK, Kim HS, Choi HS, Seikwan Oh, Choi J (2000): Hepatoprotective effects of bergenin, a major constituent of Mallotus japonicus, on carbon tetrachloride-intoxicated rats. J Ethnopharmacol 72: 469–474.
- Mandal SC, Maity TK, Das J, Saba BP, Pal M (2000): Anti-inflammatory evaluation of Ficus racemosa Linn. leaf extract. J Ethnopharmacol 72: 87–92.
- Mandal SC, Maity, TK, Das J, Pal M, Saha, BP (2003): Hepatoprotective activity of Ficus racemosa leaf extract on liver damage caused by carbon tetrachloride in rats. Phytotherapy Res 13: 430–432.
- Moawad KM (2007): Possible prophylactic effects of vitamin E or lycopene treatment on renal toxicity induced by CCl4 administration in albino rats. World J Zool 2: 19–28.
- Mukherjee PK, Saha K, Murugesan T, Mandal SC, Pal M, Saha BP (1998): Screening of anti-diarrhoeal profile of some plant extracts of a specific region of West Bengal. India. J Ethnopharmacol 60: 85–89.
- Nadkarni KM, Nadkarni AK, Chopra RN (1976): Indian Materia Medica, Bombay, Popular Prakashan, pp. 548–550.
- Ogeturk M, Kus I, Kavakli A, Zararsiz I, Ilhan N, Sarsilmaz M (2004): Effects of melatonin on carbon tetrachloride-induced changes in rat serum. J Physiol Biochem 60: 205–210.
- Ohkawa H, Ohishi N, Yagi K (1979): Assay for lipid peroxides in animal tissues by thiobarbituric reaction. Anal Biochem 95: 351–358.
- Ohta Y, Nishida K, Sasaki E, Kongo M, Ishiguro I (1997): Attenuation of disrupted hepatic active oxygen metabolism with the recovery of acute liver injury in rats intoxicated with carbon tetrachloride. Res Commun Mol Pathol Pharmacol 95: 191–207.
- Ozdogan O, Goren MZ, Ratip S, Giral A, Moini H, Enc F, Birsel S, Berkman K, Tozun N (2002): Role of endothelin-1 in a cirrhotic rat model with endotoxin induced acute renal failure. Hepatol Res 24: 114–124.
- Ozturk F, Ucar M, Ozturk IC, Vardi N, Batcioglu K (2003): Carbon tetrachloride-induced nephrotoxicity and protective effect of betaine in Sprague-Dawley rats. Urology 62: 353–356.
- Pandey S Gujrati VR, Shanker K, Singh N, Dhawan KN (1994): Hepato protective effect of Liv-52 against CCl4 induced lipid peroxidation in liver of rats. Indian J Exp Biol 32: 674–675.
- Perez AJ, Courel M, Sobrado J, Gonzalez L (1987): Acute renal failure after topical application of carbon tetrachloride. Lancet 1:515–516.
- Rahman NN, Khan M, Hasan R (1994): Bioactive components from Ficus glomerata. Pure Appl Chem 66: 2287–2290.
- Rao BR, Anipama K, Swaroop A, Murugesan T, Pal M, Mandal SC (2002a): Evaluation of anti-pyretic potential of Ficus racemosa bark. Phytomedicine 9: 731–733.
- Rao BR, Murugesan T, Pal M, Saha BP, Mandal SC (2003): Antitussive potential of methanol extract of stem bark of Ficus racemosa Linn. Phytother Res 17: 1117–1118.
- Rao BR, Murugesan T, Sinha S, Saha BP, Pal M, Mandal SC (2002b): Glucose lowering efficacy of Ficus racemosa bark extract in normal and alloxan diabetic rats. Phytother Res 16: 590–592.
- Ratnasooriya WD, Jayakody JRAC, Nadarajah T (2003): Antidiuretic activity of aqueous bark extract of Sri Lankan Ficus racemosa in rats. Acta Biol Hung 54: 357–364.
- Recknagel RO, Glende EA, Hruszkewycz AM (1977): Free Radicals in Biology, Vol. III, New York, Academic Press, pp. 97–132.
- Srivastava SP, Chen NO, Holtzman JL (1990): The in vitro NADPH dependent inhibition by CCl4 of the ATP-dependent calcium uptake of hepatic microsomes from male rats. Studies on the mechanism of inactivation of the hepatic microsomal calcium pump by the CCl3 radical. J Biol Chem 265: 8392–8399.
- Veerapur VP, Prabhakar KR, Parihar VK, Kandadi MR, Ramakrishana S, Mishra B, Satish Rao BS, Srinivasan KK, Priyadarsini KI, Unnikrishnan MK (2007): Ficus racemosa stem bark extract: A potent antioxidant and a probable natural radioprotector. Evid Based Complement Alternat Med 119: 1–8.