Abstract
Abutilon indicum L. (Malvaceae) and Abutilon muticum DC. (Malvaceae) are traditional medicinal herbs used for analgesic, anthelmintic, hepatoprotective, and hypoglycemic properties. These effects may be correlated with the presence of antioxidant compounds. Extracts in organic solvents from the aerial parts and roots of both species were prepared and evaluated for their total antioxidant capacity (TAC), total phenolic content, and total flavonoid content. The Trolox equivalent antioxidant capacity (TEAC) of all the extracts of both plants was found, employing ABTS and FRAP assays. TEAC values ranged from 3.019 to 10.5 μM for n-hexane and butanol fractions of Abutilon indicum and from 2.247 to 14.208 μM for n-hexane and butanol fractions of Abutilon muticum, respectively, using the ABTS assay. The FRAP assay showed reducing powers of the fractions in the order of butanol > ethyl acetate > chloroform > n-hexane and butanol > chloroform > hexane > ethyl acetate for Abutilon indicum and Abutilon muticum, respectively. EC50 and TEC50 values for the extracts of both plants were determined using the DPPH free radical assay. The reaction kinetics with this free radical indicated the presence of both slow reacting and fast reacting antioxidant components in the extracts of both plants. The antioxidant/radical scavenging capacity of the extracts was found to be a dose-dependent activity. The results obtained in the present study indicate that both Abutilon species are potential sources of natural antioxidants.
Introduction
Oxidative stress (OS) is a general term used to describe the steady state level of oxidative damage in a cell, tissue, or organ, due to an imbalance between the production of reactive oxygen/nitrogen species and a biological system’s ability to readily detoxify the reactive intermediates or easily repair the resulting damage. All forms of life maintain a reducing environment through endogenously produced enzymes, body fluid, and diet. Disturbances in the normal redox state can cause toxic effects through the production of peroxides and free radicals that damage all components of the cell, including proteins, lipids, and DNA. The damage may cause cell injury and various degenerative disorders such as cardiovascular disease, aging, diabetes, Alzheimer’s disease mutations, and cancer (CitationCardozo-Pelaez et al., 2000; CitationRahimi et al., 2005). In recent years, considerable efforts have been directed toward identifying naturally occurring substances and food components that can protect against oxidative stress. Natural antioxidants have a wide range of biochemical activities, including inhibition of reactive oxygen species (ROS) generation, scavenging of free radicals, and alteration of intracellular redox potential. Increasing antioxidant levels in the blood may prevent the development of many chronic diseases such as cancer, heart failure, and hepatotoxicity (CitationHockenbery et al., 1993).
The genus Abutilon belongs to the family Malvaceae, and has about 150 species distributed throughout the Mediterranean to Central Asia, particularly in India and Pakistan. The chemical constituents and biological activities of this genus have been studied by many groups (CitationGaind & Chopra, 1976; CitationDennis & Kumar, 1987; CitationSharma & Ahmad, 1989; CitationSingh et al., 1997; CitationMatlawska & Sikorska, 2002). Several species of this genus have been used in folk medicine as an analgesic and for the treatment of digestive disorders, toothache, arthritis, and so forth (CitationBagi et al., 1985; CitationAhmad et al., 2000; Prochezian & Ansari, 2005). An infusion of the roots of Abutilon indicum L. (Malvaceae) has been described in indigenous medicine (CitationNadkarni, 1954; CitationChopra et al., 1958) as a diuretic and a demulcent in fever, chest infection, gonorrhea, arthritis, and leprosy, and has also been used in relieving strangury and hematuria. Recent experimental studies on immunological aspects revealed that A. indicum (whole plant) has some direct immunological effect (CitationMahmood et al., 2003). Abutilon muticum DC. (Malvaceae), another species of the genus Abutilon, occurs in plains throughout Pakistan, and is especially more common in Sindh and abundant in the deserts of Cholistan, Bahawalpur. The oil seeds of A. muticum have importance in the treatment of cold, cough, bronchial infection, inflammation of the urinary tract, diarrhea, and ulcers (CitationLander & Morrison, 1962). A literature survey showed that no comprehensive study has yet been carried out on the antioxidant and radical scavenging activity of A. indicum and A. muticum. Therefore, the present study focused on evaluation of the antioxidant properties of various organic extracts (n-hexane, chloroform, ethyl acetate, and n-butanol) of the aerial parts and roots of both plant species by different in vitro tests. The correlation between total phenolic and flavonoid contents and the antioxidant activity of various extracts was also examined.
Due to the increasing awareness regarding health-beneficial effects of natural antioxidants, many research groups have examined plants for exploiting novel potential sources of natural antioxidants. Of the hundreds of previous studies on the antioxidant capacity of the higher plants, few have examined the herbs/shrubs that can be found in Asia, especially India and Pakistan. In doing so, we have identified plant species with antioxidant capacity that warrants further investigation, especially for a series of plants of the family Malvaceae. The bioactivity of A. indicum and A. muticum identified in this study has great potential for human and animal health because of its broad-ranging antioxidant capacity. Both plant species under study are medicinal herbs and used as animal fodder. We are reporting here for the first time their antioxidant capacity, as the purpose of this study was to determine new potential sources of natural antioxidants.
Materials and methods
Chemicals
Sodium acetate, 2,4,6-triphenylpyridyl-S-triazine (TPTZ), Folin–Ciocalteu reagent, 1,1-diphenyl-2-picrylhydrazyl (DPPH), ABTS [2,2′-azinobis (3-ethyl benzothiazoline-6-sulfonic acid)], 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid, 3,5,7,3′,4′-pentahydroxyflavone, FeCl3·6H2O, potassium dihydrogen phosphate (KH2PO4), dipotassium hydrogen phasphate (K2HPO4), gallic acid, linoleic acid, Tween-20, and ammonium thiocyanate were purchased from Aldrich Chemical Co. (Gillingham, Dorset, UK). All other reagents and solvents were of analytical grade and purchased from either Sigma or Merck representatives.
Plant material
The aerial parts (stem, leaves, and flowers) and roots of A. indicum and A. muticum, growing wild in Pakistan, were collected in April 2007, from the Cholistan Desert of Bahawalpur, Pakistan. Taxonomic identification with respect to morphology was carried out by Dr. Zaheer-ud-Din Khan, Head of the Botany Department, GC University, Lahore, Pakistan. Voucher specimens of both plants were deposited at the herbarium of the Department of Botany, GC University, Lahore, Pakistan (A. indicum: GC. Herb. Bot. 68; A. muticum: GC. Herb. Bot. 138). Freshly collected plant materials were cleaned to remove adherent dust and then dried under shade. The dried samples were pulverized in a Willy mill to 60 mesh size and used for solvent extraction.
Solvent extraction
The air-dried powdered plant samples were extracted individually with methanol. The extraction was repeated three times and the solvent was evaporated under vacuum conditions. These methanol extracts of different parts were suspended separately in 10 volumes of water and then partitioned successively with equal volumes of n-hexane, chloroform, ethyl acetate, and n-butanol, leaving a residual water-soluble fraction. Each fraction was concentrated by rotary vacuum evaporator and then dried. The dry extract obtained with each solvent was weighed and the percentage yield in terms of air-dried weight of plant material was calculated. The extracts thus obtained were stored at 4°C until they were used for the estimation of total phenolics, total flavonoid content, and antioxidant potential. For each plant sample, three replicates of the extracts were prepared.
Determination of total phenolic content
The concentration of total phenolic compounds in A. indicum and A. muticum (aerial parts and roots) was estimated following the procedure reported by CitationSingleton and Rossi (1965). Briefly, 40 μL aliquots from each of the replicates were mixed with 3.16 mL of distilled H2O and 200 μL of 0.2 N Folin–Ciocalteu reagent. After 8 min, 600 μL of saturated sodium carbonate (75 g/L) was added. The absorbance of the resulting blue solution was measured at 765 nm after incubation at 40°C for 30 min with intermittent shaking. Quantitative measurements were performed based on a six-point standard calibration curve of 20, 100, 200, 300, 400, 500 mg/L of gallic acid in 80% methanol. The total phenolic content was expressed as gallic acid equivalents (GAE) in milligrams per gram of dry plant material.
Determination of total flavonoid content
Total flavonoid content was determined using a colorimetric method described by CitationDewanto et al. (2002). Briefly, 0.25 mL of the plant extract or quercetin standard solution was mixed with 1.25 mL of distilled water in a test tube followed by the addition of 75 μL of 5% NaNO2 solution. After 6 min, 150 μL of 10% AlCl3·6H2O solution was added and allowed to stand for another 5 min before 0.5 mL of 1 M NaOH was added. The volume of the mixture was raised to 2.5 mL with distilled H2O and it was mixed well. Absorbance was measured immediately against a blank at 510 nm. A calibration curve was prepared with quercetin, and the results are expressed as mg quercetin equivalents (QE)/100 g sample.
Total antioxidant activities
ABTS·+ decolorization assay
Total antioxidant activity in terms of Trolox equivalent antioxidant activity (TEAC) was measured using an improved ABTS method as described by CitationRe et al. (1999). The ABTS radical cation (ABTS·+) solution was prepared through the reaction of 7 mM ABTS and 2.45 mM potassium phosphate. The reaction mixture was incubated at room temperature in the dark for 12–16 h. The ABTS·+ solution was diluted with phosphate buffered saline (PBS, pH 7.4) to obtain an absorbance of 0.700 ± 0.02 at 734 nm and equilibrated at 30°C. After the addition of 10 μL of sample or standard solution in 3 mL of ABTS·+ solution (A734nm = 0.700 + 0.02) to 100 μL of diluted sample solution, the absorbance reading was taken at 30°C, exactly 1 min after initial mixing up to 6 min. Appropriate solvent blanks were run in each assay. The percentage inhibition of absorbance at 734 nm was calculated using the following formula:
where A0 and Af are the absorbance of ABTS·+ solution at 734 nm before and after the addition of plant extract or standard solution, respectively. The percentage inhibition was plotted as a function of the concentration of antioxidants and Trolox for standard reference data.
DPPH free radical scavenging activity
The DPPH radical scavenging effect was determined according to the method described by CitationSanchez-Moreno et al. (1998). DPPH solution (3.9 mL, 25 mg/L) in methanol was mixed with sample solution (0.1 mL). Upon reduction, the violet color of the solution fades proportionate to the amount of antioxidants present in the sample. The reaction progress was monitored by noting the absorbance at 515 nm after every minute, for 30 min or until the absorbance was stable. The percentage of DPPH remaining (% DPPHrem) was calculated using the following formula:
where [DPPH]T = t and [DPPH]T = 0 are the absorbances of DPPH solution at the start of reaction with a sample (i.e. T = 0) and at T = t ( i.e. until the absorbance becomes zero). The percentage of remaining DPPH against the sample/standard concentration was plotted to obtain the amount of antioxidant necessary to decrease the initial concentration of DPPH by 50% (EC50).
FRAP assay
The reducing capacity of plant extracts was measured according to the method of CitationBenzie and Strain (1996) with some modifications. Freshly prepared FRAP (ferric reducing antioxidant power) solution contained: 25 mL of 300 mM acetate buffer (pH 3.6) plus 2.5 mL of 10 mM TPTZ solution in 40 mM HCl solution and 2.5 mL of 20 mM ferric chloride solution. The sample was incubated at 37°C throughout the monitoring period. Three milliliters of FRAP reagent were warmed to 37°C and a reagent blank reading was taken at 593 nm. The reagent was then mixed with 100 μL of sample and 300 μL of distilled water. An absorbance reading was taken at 593 nm after every minute for 6 min. Absorbance of the reagent blank and sample (absorbance of the appropriately diluted sample without FRAP reagent) was subtracted from the final absorbance at 6 min, and the results were compared with the standard curve prepared using different concentrations of Trolox. The final FRAP value for each sample was the mean value of three replications.
Lipid peroxidation value in linoleic acid emulsion system
Lipid peroxidation value of the extracts was determined according to the ferric thiocyanate method in linoleic acid emulsion as described by Mitsuda et al. (1966). Plant extract (100 μL) was added to 2.4 mL of potassium phosphate buffer (0.04 M, pH 7.0) and 2.5 mL of linoleic acid emulsion. The linoleic acid emulsion (50 mL) was prepared by mixing 175 μg Tween-20, 155 μL linoleic acid, and 0.04 mL potassium phosphate buffer (0.04 M, pH 7.0). The solution was incubated at 37°C. During incubation, 100 μL of solution was regularly taken off at intervals of 24 h and the lipid peroxidation value was determined spectrophotometrically at 500 nm, after reacting with FeCl2 and thiocyanate. A 5.0 mL solution consisting of 2.5 mL linoleic acid emulsion and 2.5 mL of potassium phosphate buffer (0.04 mol/L, pH 7.0) was used as blank. Trolox was used as standard antioxidant for comparison.
Statistical analysis
Different statistical techniques such as analysis of variance (ANOVA), Duncan’s multiple range method, and regression analysis were carried out for analyzing the data obtained from different samples and to study the relationship between antioxidant activity (AA), total phenolic content, and total flavonoid content. Each parameter was measured three times. Differences at p < 0.05 were considered statistically significant.
Results and discussion
Recovery percent and phenolic and flavonoid contents of extracts
The yields, total phenolic content (TPC), and total flavonoid content (TFC) of the extracts obtained from the aerial parts and roots of two varieties of the genus Abutilon using different organic solvents are shown in . A wide range of polyphenol contents (0.448–26.910 mg/g dry weight) was found in the organic extracts of different parts of both plant species. Despite low values of extraction yields, the phenolic and flavonoid contents determined were very good, indicating that the extraction was efficient. Aerial parts were found to contain relatively higher amounts of antioxidant components as compared to the roots. The highest phenolic content was found in the case of the ethyl acetate fraction, and these findings are in agreement with earlier reports (CitationYen et al., 1996), demonstrating that ethyl acetate and methanol are effective solvents for the extraction of antioxidants. The values for flavonoid and phenolic contents are comparable to previously exploited natural sources of antioxidants (CitationYen & Duh, 1993).
Table 1. Yield, total phenolic content (TPC), and total flavonoid content (TFC) of different parts of A. indicum and A. muticum.
A major group of phytochemicals in foods are the phenolic compounds, which include simple phenols, phenyl propanoids, benzoic acid and derivatives, flavonoids, stilbenes, tannins, lignans, and lignins. Plant phenolics are multifunctional and can act as reducing agents (free radical terminators), metal chelators, and singlet oxygen quenchers (CitationShahidi & Naczk, 2003). The phenolic compounds are very important plant constituents because of their scavenging ability due to their hydroxyl groups. Flavonoid contents extracted ranged from 0.111 to 16.981 mg/g dry weight of herbs for n-hexane and ethyl acetate fractions, respectively. Flavonoids are phytochemicals that have been demonstrated to be potent antioxidants based on their phenolic hydroxyl groups. They are known as primary antioxidants, and can delay or inhibit the oxidation of lipids or their molecules by inhibiting the initiation or propagation of oxidative chain reactions (CitationAndlauer & Furst, 1998).
ABTS radical cation scavenging activity
ABTS is a method based on reduction of the 2,2′-azinobis (3-ethyl benzothiazoline-6-sulfonic acid) radical. The reaction of ABTS·+ with free radical scavengers present in the test sample occurs rapidly, and can be assessed by following the decrease in sample absorbance at 734 nm. The TEAC (Trolox equivalent antioxidant capacity) method measures the antioxidant activity by measuring the extent of reduction of the radical cation as the percentage inhibition of absorbance at 734 nm on reaction with the sample solution. The extent of inhibition of the absorbance of ABTS·+ was plotted as a function of the concentration and compared with the standard Trolox curve in order to determine the TEAC.
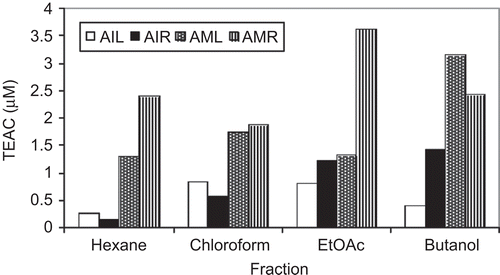
TEAC values of the different extracts of the aerial parts and roots of A. indicum and A. muticum are presented in . Although all the samples exhibited good ABTS radical scavenging activity, the butanol extract of A. muticum (roots) and ethyl acetate extract of A. muticum (aerial parts) showed the highest TEAC (14.208 and 13.21 μmol/g, respectively). The lowest TEAC values were obtained for n-hexane extracts of roots of A. indicum and aerial parts of A. muticum (1.796 and 2.247 μmol/g, respectively). Low and high molecular weight phenolics, including flavonoids, phenolic acids, and tannins, have been shown to be good quenchers of free radicals (CitationHagerman et al., 1998; CitationHeim et al., 2002). The free radical scavenging activity of different parts of A. indicum and A. muticum may also be attributed to the presence of phenolics and flavonoids.
Figure 1. Comparative analysis of TEAC (Trolox equivalent antioxidant capacity) values (μM) of different fractions of A. indicum and A. muticum using ABTS modified assay (pH 7.4). AIL, Abutilon indicum leaf extract; AIR, Abutilon indicum root extract; AML, Abutilon muticum leaf extract; AMR, Abutilon muticum root extract.
DPPH free radical scavenging activity
DPPH is a stable free radical that accepts an electron or hydrogen radical to become a stable diamagnetic molecule (CitationSoares et al., 1997). The reduction capability of DPPH radicals on reaction with sample extracts was determined as a function of time by the decrease in absorbance at 517 nm (CitationOyaizu, 1986). illustrates a significant (p < 0.05) decrease in the concentration of DPPH radical due to the scavenging ability of soluble solids in the extracts of the aerial parts and roots of A. indicum, A. muticum, and standard Trolox solution. The graphs show a sharp fall in the absorbance of DPPH in the first 5 min after the addition of extract/standard solution, which then became more moderate and gradual for the next 35 min. The ethyl acetate and n-hexane extracts of different parts of both plant species showed a significantly stronger DPPH scavenging activity than the n-butanol and chloroform extracts. Results indicate that both plants have a noticeable effect on the scavenging of free radicals. Different concentrations of each extract were applied to obtain EC50 and TEC50 values () as a function of time. A dose–response scavenging of DPPH free radical was observed. The kinetic studies showed that, as compared to the ethyl acetate extracts of roots and aerial parts of A. muticum, the chloroform extracts of the roots of A. indicum and aerial parts of A. muticum contained antioxidant components which were slow reacting and took a longer period of time to exhibit their complete antioxidant action. Based on the data obtained from the present study, both plant species may be considered as potent free radical scavengers, showing their ability to limit free radical damage occurring in the human body.
Figure 2. DPPH free radical scavenging activity of different fractions of different parts of A. indicum and A. muticum. Data are mean ± SD (n = 3).
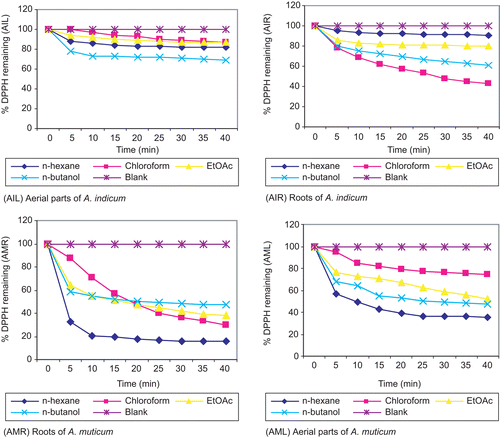
Table 2. EC50 and TEC50 values of different fractions of different parts of A. indicum and A. muticum.
FRAP assay
Like the TEAC assay, the FRAP method also involves a single electron transfer reaction between a recipient (here Fe3+-TPTZ) and a single electron donor (ArOH+). There is not much difference between the TEAC and FRAP assays except that TEAC is carried out at neutral pH while FRAP is performed at acidic pH.
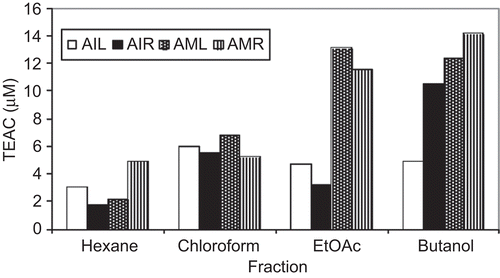
FRAP values for the investigated extracts varied over a wide range between 0.568 μg/mL for the chloroform extract of roots of A. indicum and 3.132 μg/mL for the butanol extract of aerial parts of A. muticum (). Hexane extracts of the aerial parts and roots of both species were found to be almost inactive with this method. The antioxidant activity increased proportionate to polarity of the solvent used for extraction, i.e., butanol > ethyl acetate > chloroform > hexane. In addition, the root extracts of A. muticum showed greater activity than the other extracts. The capability of different phenolic substances to scavenge various types of oxidation-initiating radicals has been reported in the polar phase (CitationBors et al., 1990; CitationRice-Evans et al., 1996). There is evidence that the antioxidant activity of many compounds of botanical origin is proportional to the TPC, suggesting a causative relationship between TPC and antioxidant activity (CitationVelioglu et al., 1998). Polarity-dependent correlation studies between TPC and FRAP values of different extracts of both plants confirmed this.
Antioxidant activity using linoleic acid peroxidation method
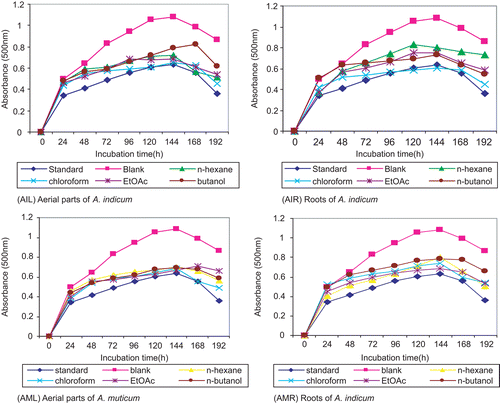
Lipid peroxidation values of the extracts were determined using the linoleic acid emulsion system. In principle, linoleic acid is allowed to oxidize in the presence of sample extract. Peroxyl radicals produced as a result of oxidation of linoleic acid are scavenged by the antioxidants present in the sample. A sample low in antioxidant components fails to capture peroxyl radicals that oxidize Fe2+ to Fe3+. These Fe3+ ions are determined spectrophotometrically through the formation of a colored complex with the SCN− ion (thiocyanate). Higher absorbance values show low concentrations of antioxidant components in the sample. Almost all of the extracts of both plant species exhibited inhibition of peroxidation of linoleic acid. The root extracts of both A. muticum and A. indicum gave comparable values to that of the standard antioxidant, Trolox (). Thus, A. indicum and A. muticum extracts may be taken as alternative and more cost effective sources of natural antioxidants.
Figure 4. Antioxidant activity (in terms of peroxidation value) of different fractions of different parts of A. indicum and A. muticum. Data are mean ± SD (n = 3).
The higher antioxidant potential of butanol and ethyl acetate extracts of both plants shown in the current study indicates that most of the active constituents of these plants are polar phenolic and flavonoid components. Further research is in progress for the isolation and identification of these active components. Taken collectively, these results lead to the conclusion that the aerial parts and root extracts of both A. indicum and A. muticum have powerful antiradical and antioxidant components which may be helpful in controlling complications during degenerative diseases.
Acknowledgements
The authors would like to thank Plant Taxonomist, Dr. Zaheer-ud-Din Khan (Department of Botany, GC University, Lahore, Pakistan) for the collection of plant material, and to Dr. Islam-Ullah Khan (Material Chemistry Laboratory, Department of Chemistry, GC University, Lahore, Pakistan) for research facilities.
Declaration of interest
The authors are grateful to the Higher Education Commission of Pakistan for research grant No. 042-121289-PS2-212. No additional support of funding has been received by the authors to carry out this study. This work is a part Ph.D (scholarship based).
References
- Ahmad M, Amin S, Islam M, Takahashi M, Okuyama E, Hossain CF (2000): Analgesic principle from Abutilon indicum. Pharmazie 55: 314–316.
- Andlauer W, Furst P (1998): Antioxidative power of phytochemicals. Cereal Food World 43: 356–360.
- Bagi MK, Kalyani GA, Denis TJ, Kumar KA, Kakrani HK (1985): A preliminary pharmacological screening of Abutilon indicum: II Analgesic activity. Fitoterapia 56: 169–171.
- Benzie IFF, Strain JJ (1996): The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”; the FRAP assay. Anal Biochem 239: 70–76.
- Bors W, Heller W, Michel C, Saran M (1990): Flavonoids as antioxidants: determination of radical scavenging efficiencies. Methods Enzymol 186: 343–355.
- Cardozo-Pelaez F, Brooks PJ, Stedeford T, Song S, Sanchez-Ramos J (2000): DNA damage, repair, and antioxidant systems in brain regions: a correlative study. Free Radic Biol Med 28: 779–785.
- Chopra RN, Chopra IC, Handa KL, Kapoor LD (1958): Indigenous Drugs of India. Calcutta, Dhur UN and Sons Pvt. Ltd., pp. 456, 561.
- Dennis TJ, Kumar KA (1987): Chemical examination of the roots of Abutilon indicum Linn. J Oil Technol Assoc India 15: 82–83.
- Dewanto V, Wu X, Adom KK, Liu RH (2002): Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem 50: 3010–3014.
- Gaind KN, Chopra KS (1976): Phytochemical investigation of Abutilon indicum. Planta Med 30: 174–185.
- Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW (1998): High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agric Food Chem 46: 1887–1892.
- Heim KE, Tagliaferro AR, Bobilya DJ (2002): Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem 13: 572–584.
- Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ (1993): Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 75: 241–251.
- Lander V, Morrison EA (1962): The Wealth of India. A Dictionary of Indian Raw Materials and Industrial Products, Vol. 6. New Delhi, CSIR, pp. 120.
- Mahmood K, Naseer R, Rehan A, Farooq HM, Qadir MA, Athar MA (2003): Biochemical analysis of oil from seeds of Abutilon muticum. Pak J Biol Sci 6: 1880–1882.
- Matlawska I, Sikorska M (2002): Flavonoid compounds in the flowers of Abutilon indicum (L.) Sweet (Malvaceae). Acta Pol Pharm 59: 227–229.
- Mitsuda H, Yasumoto K, Iwami K (1996): Antioxidative action of indole compounds during the autooxidation of linoleic acid. Euyo to Shokuryo 19: 210–214.
- Nadkarni AK (1954): Indian Materia Medica, 3rd ed., Vol. 1. Bombay, Popular Book Depot, p. 6.
- Oyaizu M (1986): Studies on product of Brownian reaction prepared from glucosamine. Jpn J Nutr 44: 307–315.
- Prochezian, E, Ansari SH (2005): Hepatoprotective activity of Abutilon indicum on experimental liver damage in rats. Phytomedicine 12: 62–64.
- Rahimi R, Nikfar S, Larijani B, Abdollahi M (2005): A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother 59: 365–373.
- Re R, Pellegrini N, Protegent A, Pannale A, Yang M, Rice-Evans C (1999): Antioxidant activity applying an improved ABTS radical cation decolourization assay. Free Radic Biol Med 26: 1231–1237.
- Rice-Evans CA, Miller NJ, Paganga G (1996): Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20: 933–956.
- Sanchez-Moreno C, Larrauri JA, Saura-Calixto F (1998): A procedure to measure the antiradical efficiency of polyphenols. J Sci Food Agric 76: 270–276.
- Shahidi F, Naczk M (2003): Phenolics in Food and Nutraceuticals. London, CRC Press, pp. 403–422.
- Sharma PV, Ahmad ZA (1989): Two sesquiterpene lactones from Abutilon indicum. Phytochemistry 28: 3525.
- Singh V, Mishra UC, Khare GC, Gupta PC (1997): A neutral seed gum from Abutilon indicum. Carbohydr Polym 33: 203–205.
- Singleton VL, Rossi JR Jr (1965): Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid. Am J Enol Vitic 16: 144–148.
- Soares JR, Dinis TCP, Cunha AP, Amedia LM (1997): Antioxidant activity of some extracts of Thymus zygis. Free Radic Res 26: 469–478.
- Velioglu YS, Mazza G, Gao L, Oomah BD (1998): Antioxidative activity and total phenolics in selected fruits, vegetables and grain products. J Agric Food Chem 46: 4113–4117.
- Yen GC, Duh PD (1993): Antioxidative properties of methanolic extracts from peanut hulls. J Am Oil Chem Soc 70: 383–386.
- Yen GC, Wu SC, Duh PD (1996) Extraction and identification of antioxidant components from the leaves of mulberry (Morus alba L). J Agric Food Chem 44: 1687–1690.