Abstract
Pergularia daemia (Forsk.) Chiov. (Asclepiadaceae) is used traditionally as an anthelmintic, laxative, antipyretic, and expectorant, and also used to treat malarial intermittent fever. But the scientific parameters are not yet available to identify the true plant material. In the present investigation, various pharmacognostic standards for P. daemia have been established. Microscopically, thick root and thick taproot of P. daemia showed the presence of periderm, secondary phloem and secondary xylem. Abundant starch grains and calcium oxalate crystals are present in the cortical parenchyma masses included within the xylem. Powdered roots of the plant showed vessel elements, tracheids, fibers and xylem parenchyma. Total ash of the root of P. daemia was not more than 5% and water-soluble extractive value was two times higher than alcohol soluble extractive value. Phytochemically, the ethanol and aqueous extracts of the root of P. daemia showed maximum phytochemicals such as alkaloids, glycosides, steroids, flavonoids, saponin, tannin and phenolic compounds, terpenoids, carbohydrates, gums and mucilage. The results of this study should provide a standard for identification and preparation of monograph of this drug.
Introduction
The World Health Organization states that approx 85 to 90% of the world’s population consumes traditional herbal medicines, while the herbal drug industry has been in high growth since the late 1990s due to the growing demand in developing and developed countries (CitationDinesh Kumar, 2007). The plant biodiversity in India has served as the foundation for the development of many traditional system of medicine, including Ayurveda, Unani, Siddha, and Tibetan (CitationJadhav et al., 2003). In recent years there has been a rapid increase in the standardization of selected medicinal plants of potential therapeutic significance (CitationReddy et al., 1999; CitationVenkatesh et al., 2004). Standardization is an essential requirement for the whole plant, plant parts or extracts in order to assess the quality of drugs.
The whole plant of Pergularia daemia (Forsk.) Chiov. (Asclepiadaceae), known as “Veliparuthi” in Tamil, “Uttaravaruni” in Sanskrit and “Utranajutuka” in Hindi (CitationKhare, 2007), has traditionally been used as an anthelmintic, laxative, antipyretic, expectorant and also used to treat infantile diarrhea and malarial intermittent fever (CitationVarier, 1995; CitationKirtikar & Basu, 1999; CitationNadkarni, 2002). The latex is used as a remedy for toothache (CitationHebbar et al., 2004), while the stem bark is used as a remedy for cold (CitationDokosi, 1998). The aerial parts of this plant have been reported to exert various pharmacological activities, including hepatoprotection (CitationSureshkumar & Mishra, 2006, Citation2008), antifertility (CitationGolam Sadik et al., 2001), antidiabetic (CitationWahi et al., 2002), analgesic, antipyretic, and anti-inflammatory (CitationSathish et al., 1998). The roots of this plant have been used as an emetic (CitationMittal et al., 1962), to treat gonorrhea (CitationSamuelsson et al., 1991), asthma, and constipation (CitationReddy et al., 1988). Phytochemically the plant has been investigated for the presence of cardenolides, alkaloid, saponins (CitationSathish et al., 1998) and steroidal compounds (CitationAnjaneyulu et al., 1998). A review of literature revealed that no pharmacognostic standards have been recorded for this crude drug. Thus the present investigation has been undertaken with an objective to establish pharmacognostic standards for roots of P. daemia so that authentic plant material could be explored for its therapeutic claim.
Materials and method
Collection of plant material
The plant materials for the proposed study were collected in the month of November 2006 from Maruthamalai Hills, Coimbatore District, Tamil Nadu, India. The plant material was taxonomically identified by P. Jayaraman, Plant Anatomy Research Centre, Chennai, Tamil Nadu, India. The voucher herbarium specimens (PARC/2007/52) have been preserved in our laboratory for further reference.
Instruments used
Photographs of different magnifications were taken with a Nikon Labphot2 Microscopic Unit. For normal observations bright field was used. For the study of crystals, starch grains and lignified cells, polarized light was employed. Since these structures have birefringent property, under polarized light they appear bright against a dark background (CitationEsau, 1977).
Pharmacognostic studies
Macroscopic and microscopic studies
The gross morphological character of the root was described based on the shape, size, color, surface, fracture and appearance of the cut surface (CitationMukherjee, 2007). For microscopic examination the paraffin-embedded specimens were sectioned using a rotary microtome at a thickness of 10-12 μm. De-waxing of sections was made using a routine procedure (CitationJohansen, 1940) and the sections were stained with the Toluidine blue method (CitationO’Brien et al., 1964). Powdered root material was cleared with sodium hydroxide and mounted in glycerin medium after staining with phloroglucinol and hydrochloric acid.
Physicochemical analysis
The dried powdered root material was subjected to physicochemical analysis including fluorescence analysis (CitationKokashi et al., 1958), moisture content, total ash, water soluble ash, acid insoluble ash, alcohol soluble extractive, and water soluble extractive (CitationMinistry of Health and Family Welfare, 1996; CitationKhandelwal, 2004) to determine the quality and purity of the plant material.
Preliminary phytochemical screening
The roots were shade-dried, powdered with a mechanical grinder and passed through a 40-mesh sieve. The sieved powder was stored in an airtight container and kept at room temperature until further study. The dried powdered material (250 g) was extracted with petroleum ether, chloroform, ethyl acetate, and ethanol by using Soxhlet apparatus and water by cold maceration. The solvents were completely removed under reduced pressure by using vacuum evaporator. The presence of various phytoconstituents, i.e. alkaloids (Dragendorff’s test), steroids and terpenoids (Liebermann Burchard test), tannin and phenolic compounds (Ferric chloride test), flavonoids (Shinoda test), amino acids (ninhydrin test), etc., was detected by the usual methods prescribed in standard texts (CitationKokate, 1994; CitationKhandelwal, 2004).
Results
Macroscopic characteristics of the root
The root is reddish brown in color, has no specific smell or taste, the surface is smooth or with minute fissures; the tap root is thick and soft, the lateral roots thin and wiry.
Anatomical characteristics of the root
Thick root
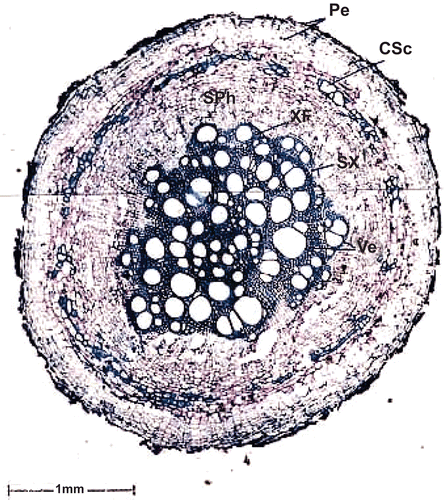
The thick root of the sample is 2.7 mm thick. It has the following tissue zones ().
Figure 1. Transverse section of thick root. Pe, periderm; CSc, cortical sclereids; SPh, secondary phloem; XF, xylem fibers; SX, secondary xylem; Ve, vessel.
Periderm.
The periderm is broad with dark crushed remnants of epidermis on the surface. It is 200 μm wide and consists of 8-10 layers of tabular phellem cells. Along the inner boundary of the periderm there is a thin, discontinuous cylinder of sclereids. The sclereids have thin lignified walls with wide lumen.
Secondary phloem.
The secondary phloem is wide and continuous around the xylem. It consists of radially running phloem rays which are slightly dilated at the periphery. Sieve elements occur in narrow radial blocks.
Secondary xylem.
The secondary xylem is uneven in outline; it has wide, mostly solitary vessels in the outer zone and narrow vessels in the center portion. The wide vessels are up to 180 μm in diameter. The central narrow vessels are 40 μm in diameter. The xylem fibers are thick walled with narrow lumen.
Thick taproot
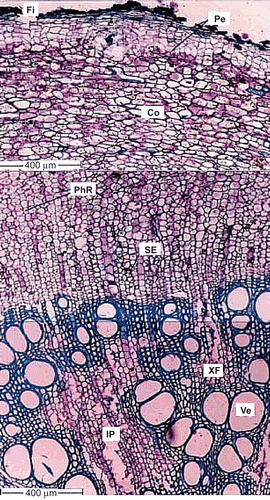
The sample taproot is 3.6 mm thick. The surface is minutely fissured; dark crushed cells are seen as crests on the surface ().
Figure 2. Transverse section of thick taproot. Fi, fissure; Pe, periderm; Co, cortex; PhR, phloem ray; SE, sieve element; XF, xylem fibers; Ve, Vessel; IP, included phloem.
Periderm.
The taproot periderm is well developed and has wider, rectangular phellem cells. It is 150 μm wide. There is no distinct phelloderm layer.
Cortex.
The cortex is about 500 μm wide; it consists of radially compressed, tangentially elongated, compact parenchyma cells. The cells have dense reserve ergastic substances.
Secondary phloem.
The taproot secondary phloem is broad and continuous. It consists of well differentiated phloem rays and phloem elements in radial blocks. The phloem rays are undilated and run in uniform parallel rows; the ray cells are rectangular in transactional view. The sieve elements and the axial parenchyma cells occur in thick radial parallel bands. The sieve elements are rectangular in sectional view with narrow lateral ends where the companion cells are situated.
Secondary xylem.
The secondary xylem has an undulate line. It is dense and solid. It consists of vessels, fibers and deeply intruded parenchyma masses. No growth rings are evident. The vessels are diffuse in distribution. The cross-sectional outline varies from circular, to elliptic, to angular. The secondary xylem vessels are thick-walled and lignified. The vessel is 150 μm in diameter and narrow vessels are 50 μm wide. The xylem fibers are thick-walled, lignified and are of lileriform type.
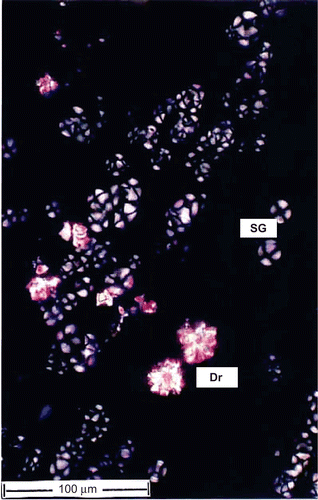
Cell inclusions.
Starch grains and calcium oxalate crystals are seen in the cortical parenchyma masses include within the xylem (). The starch grains are more abundant than the crystals. The starch grains are of concentric type with a central hilum and x-shaped dark marks; they are 15 μm in diameter. The crystals are druses or spherocrystal type; they are sparse and occur in only a few cells. The druses are 20-30 μm wide.
Microscopic characteristics of the powdered root
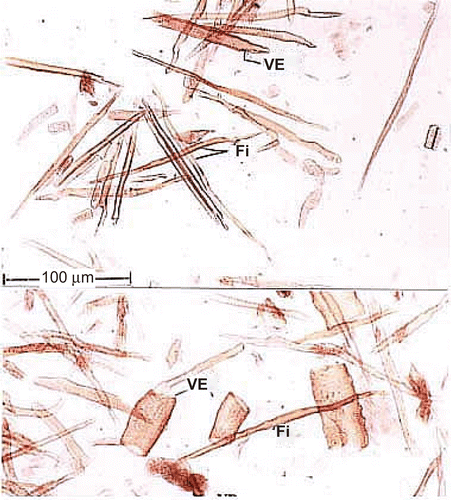
Powdered and macerated preparation of root shows the following elements ( and ).
Figure 5. Powder microscopy of the root. Tr, tracheid; PP, perforation plate; DVE, drum shaped vessel element; VE, vessel; FTr, fiber tracheid; Pa, parenchyma.
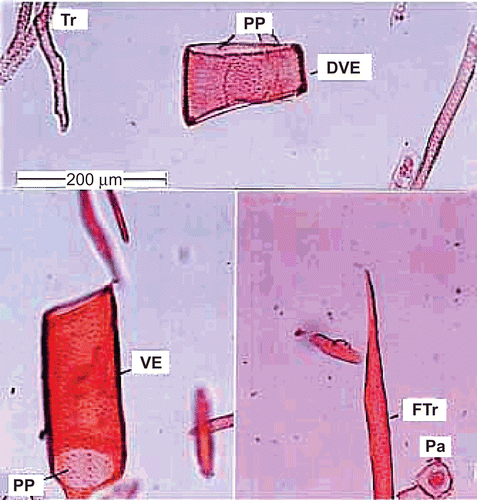
Vessel elements.
The vessel elements are wide, short and drum shaped or cylindrical and narrow. They have a simple perforation plate which is horizontal in orientation. The lateral walls have circular dense broad pits. The drum-shaped vessel element is 160 μm long and 180 μm wide. The cylindrical vessel elements are up to 350 μm long and 110 μm wide.
Tracheids.
These are long, narrow cylindrical cells. They have abundant bordered pits on lateral walls similar to vessels. They are longer (450 μm) than the vessel elements.
Fiber-tracheids.
These elements resemble the fibers in length and width, and tracheids in the presence of well-developed dense lateral wall pits. Their walls are thicker than fibers and vessels.
Fibers.
Xylem fibers are more abundant in the powder. They are long, thick-walled cells with narrow lumen. They have simple pits in one vertical row or the pits are absent in most of the fibers. The fibers are up to 600 μm long.
Xylem parenchyma.
The parenchyma cells are square-shaped, thin-walled and contain protoplast. They have no pits.
Phytochemical screening
Preliminary phytochemical screening of the various extracts of Pergularia daemia root showed positive results for the presence of steroids, flavonoids, tannins, alkaloids and glycosides (), and percentage yield of extracts was calculated ().
Table 1. Phytochemical screening of Pergularia daemia root extracts.
Table 2. Percentage yield of successive extracts of Pergularia daemia root.
Physicochemical analysis
The ash values revealed that the root of Pergularia daemia was not more than 5.06% w/w of total ash, not more than 0.99% w/w of acid insoluble ash and not more than 3.56% w/w of water soluble ash. The water soluble extractive value was not less than 9.86 w/w and alcohol soluble extractive value was not less than 7.73% w/w. The moisture content of the root material was 4.66% w/w. (). The fluorescence analysis of powder is presented in .
Table 3. Physicochemical analysis of Pergularia daemia root.
Table 4. Fluorescence analysis of Pergularia daemia root powder.
Discussion and conclusions
Traditional medicaments play an important role in our day to day life but only a few poly herbal formulations are accepted in modern medicine due to lack of accurate method for their standardization and evaluation. The main aim of pharmacognostic study is to assess the true identity of the raw material, which would reduce drastically many errors in wrong identification and handling of the final product for the required standard. Thus diagnostic features have been evolved to identify and to differentiate the P. daemia roots from other crude drugs and adulterants. Microscopic evaluation is an indispensable tool for the identification of medicinal herbs and is one of the essential parameters in preparation of modern monograph. The transverse section of thick root of P. daemia shows periderm and it consists of 8-10 layers of tabular phellem cells. Lignified sclereids are present in periderm. Sieve elements occur in narrow radial blocks present in secondary phloem. Xylem fibers are thick walled with narrow lumen present in secondary xylem. The transverse section of thick taproot shows well developed periderm and cortex about 150 and 500 µm wide respectively. The cortex parenchyma cells have dense reserve ergastic substance. Secondary phloem consists of well differentiated phloem rays and phloem elements in radial blocks. Xylem vessels and thick-walled lignified xylem fibers are present in secondary xylem. Starch grains and calcium oxalate crystals are seen in the cortical parenchyma masses included within the xylem. The starch grains are more abundant than the calcium oxalate crystals. Powdered roots of P. daemia show wide, short and drum or cylindrical narrow vessels elements. Long, narrow and cylindrical tracheids are present in the root powder. Xylem fibers are more abundant in the powder and xylem parenchyma cells are square, thin walled and contain protoplast. The results of preliminary phytochemical screening, corroborating well with the previous reports (CitationSathish et al., 1998; CitationAnjaneyulu et al., 1998), showed presence of alkaloids, saponins and steroidal compounds. Various physicochemical parameters are established to validate standardization data of P. daemia.
The diagnostic microscopic characters, powder characters and the numerical standards reported in this work could be useful for selecting authentic plant material for exploring its therapeutic potential.
Acknowledgements
The authors are grateful to Professor Dr. B. Jayakar, Principal, Vinayaka Mission College of Pharmacy, Vinayaka Mission University, Salem, Tamilnadu, India and Professor Dr. A. Balasubaramaniam, Director, Technocrats Institute of Technology – Pharmacy, Bhopal, for providing necessary facilities to conduct this study.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Anjaneyulu ASN, Raju DVSN, Srinivasa Rao S (1998): Chemical evaluation of Pergularia extensa. Indian J Chem 37B: 318–320.
- Dinesh Kumar C (2007): Pharmacognosy can help minimize accidental misuse of herbal medicine. Current Sci 93: 1356–1358.
- Dokosi OB (1998): Herbs of Ghana. Accra, Ghana University Press, pp. 313–314.
- Esau K (1977): Anatomy of Seed Plants, second edition. New York, John Wiley, pp. 550.
- Golam Sadik, Gafur MA, Shah Alam Bhuiyan M, Khurshid Alam AHM, Helal U, Biswas M, Parvez Hassan, Abdul Mannan, Omar Faruk Khan M, Chowdhury AKA (2001): Antifertility activity of Pergularia daemia. The Sciences 1(1): 22–24.
- Hebbar SS, Harsha VH, Shripathi V, Hegde GR (2004): Ethnomedicine of Dharward District in Karnataka, India-plants used in oral health care. J Ethnopharmacol 94: 261–266.
- Jadhav RB, Patil CR, Ganbote AJ (2003): Plant biodiversity and its conservation. Indian J Pharm Edu 37: 162–165.
- Johansen DA (1940): Plant Micro Technique. New York, McGraw Hill Book Co., pp. 523.
- Khandelwal KR (2004): Practical Pharmacognosy. 11th Ed. Pune, India, Nirali Prakashan, pp. 149–153 and 157–159.
- Khare CP (2007): Indian Medicinal Plants: An Illustrated Dictionary. New York, Springer, pp. 472.
- Kirtikar KR, Basu BD (1999): Indian Medicinal Plants, Vol II. Dehradun, India, Bishen Singh & Mahendra Pal Singh, pp. 1546–1547.
- Kokashi CJ, Kokashi RJ, Sharma M (1958): Fluorescence of powdered vegetable drugs in ultra-violet radiation. J Am Pharm Assoc 47: 715–717.
- Kokate CK (1994): Practical Pharmacognosy, 4th Ed. Delhi, Vallaph Prakashan, pp. 107–111.
- Ministry of Health and Family Welfare (1996): Indian Pharmacopoeia, Vol. II. New Delhi, Government of India, Ministry of Health and Family Welfare, pp. A53–54.
- Mittal OP, Tamm C, Reichstein T (1962): The glycosides of Pergularia extensa. Helv Chim Acta 45: 907.
- Mukherjee PK. (2007): Quality Control of Herbal Drugs, second edition. New Delhi, Business Horizon, pp. 132–133.
- Nadkarni AK (2002): Indian Materia Medica, Vol. I, Mumbai, Popular Prakashan, pp. 266.
- O’Brien TP, Feder N, McCull ME (1964): Polychromatic staining of plant cell walls by Toluidine blue, Protoplasma 59: 364–373.
- Reddy MB, Reddy KR, Reddy MN (1988): A survey of medicinal plants of Chenchu tribes of Andhra Pradesh, India. Int J Crude Drug Res 26: 189–196.
- Reddy YSR, Venkatesh S, Ravichandran T,Suburaju T, Surseh B (1999): Pharmacognostical studies of Wrightia tinctoria bark. Pharm Biol 37: 291–295.
- Samuelsson G, Farah MH, Claeson P, Hagos M, Thulin M, Hedberg O, Warfa AM, Hassan AO, Elmi AH, Abdurahman AD, Elmi AS, Abdi YA, Alin MH (1991): Inventory of plants used in traditional medicine in Somalia-Plants of the families Acanthaceae-Chenopodiaceae. J Ethnopharmacol 35: 25–63.
- Sathish CJ, Sharma RA, Jain R, Mascolo N, Capasso F, Vijayvergia R, Mittal C (1998): Ethnopharmacological evaluation of Pergularia daemia (Forsk.) Chiov. Phytother Res 12: 378–380.
- Sureshkumar SV, Mishra SH (2006): Hepatoprotective effect of extracts of Pergularia daemia Forsk. J Ethnopharmacol 107: 164–168.
- Sureshkumar SV, Mishra SH (2008): Hepatoprotective effect of Pergularia daemia (Forsk.) ethanol extract and its fraction. Indian J Exp Biol 46: 447–452.
- Varier PS (1995): Indian Medicinal Plants (a compendium of 500 species), Vol. IV. Hyderabad, India, Orient Longman, pp. 236–238.
- Venkatesh S, Madhava RB, Surseh B, Swamy MM, Ramesh M (2004): Pharmacognostical identification of Rumex nepallensis Spreng (Polygonaceae) – an adulterant for Indian rhubarb. Nat Prod Sci 10: 43–47.
- Wahi AK, Ravi J, Hemalatha S, Singh PN (2002): Anti-diabetic activity of Daemia extensa, J Nat Remed 2(1): 80–83.