Abstract
The current study is an effort to identify the hepatoprotective activity of the 50% ethanol extract of the whole plant of Amaranthus spinosus Linn. (Amaranthaceae) against d-galactosamine/lipopolysaccharide (d-GalN/LPS)-induced liver injury in rats. d-GalN/LPS (300 mg/kg body weight/30 µg/kg body weight)-induced hepatic damage was manifested by a significant (p <0.05) increase in the activities of marker enzymes (aspartate transaminase, alanine transaminase, alkaline phosphatase, lactate dehydrogenase and gamma glutamyl transferase) and bilirubin level in serum while phospholipids significantly decreased. All other parameters, i.e. cholesterol, triglycerides and free fatty acids were increased significantly in both serum and liver compared to the control group. Pretreatment of rats with A. spinosus extract (400 mg/kg) significantly (p <0.05) reversed these altered parameters to normal compared to the intoxicated group. The biochemical observations were supplemented by histopathological examination of liver sections. There were no significant changes in the activities of marker enzymes, bilirubin level and lipids in the rats treated with A. spinosus extract alone. Results of this study revealed that A. spinosus extract could afford a significant protection against d-GalN/LPS-induced hepatocellular injury.
Introduction
Hepatitis is a common disease in the world especially in the developing countries. It has been estimated that over 350 million people worldwide are infected with hepatitis B virus (HBV), and globally around 1 million die due to consequences of this infection annually (CitationAl-Mahtab et al., 2008), while hepatitis C virus infection is around 2%, with 170 million people chronically infected with the virus and 3 to 4 million newly infected each year (CitationMukhopadhya, 2008). Africa was reported to have the highest hepatitis C virus (HCV) prevalence rate (CitationElsheikh et al., 2007). The high prevalence rate of HCV in developing countries may be due to the lack of proper health facilities or poor economical status and less public awareness. Steroids, vaccines and antiviral drugs which have been employed to treat liver disease, have potential adverse effects especially when administered long term. Presently, the use of herbal medicines for prevention and control of chronic liver diseases is the focus of attention for the physicians, pharmaceutical manufacturers and patients; the reasons for such a shift toward the use of herbals include the expensive cost of conventional drugs, adverse drug reactions, and their inefficacy. Hence, hepatoprotective drugs from plant sources seem to be attractive alternative (CitationAghel et al. 2007; CitationSehrawat et al. 2006).
Amaranthus spinosus Linn. (Amaranthaceae) which is commonly known as “Kate Wali Chaulai” in Hindi, is used as a vegetable in India. In Indian traditional medicine (Ayurveda) the whole plant is used as an aid in upset stomachs, laxative, diuretic, stomachic, antipyretic, to improve the appetite, biliousness, blood diseases, burning sensation, leprosy, bronchitis, piles and leucorrhea (CitationKirtikar & Basu, 2001; CitationMishra, 1986). Juice of Amaranthus spinosus is used by tribes of Kerala, India to prevent swelling around the stomach, while the leaves are boiled without salt and consumed for 2-3 days to cure jaundice and are also employed to cure some types of rheumatic pain and stomachache (CitationHema et al., 2006). The leaves and roots are applied as a poultice to relieve bruises, abscesses, burns, inflammatory wounds, menorrhagia, gonorrhea, eczema and inflammatory swelling (CitationParrotta, 2001).
The aqueous extract of A. spinosus leaves possesses significant immunostimulating activity (CitationLin et al., 2005) and the stem extract has shown antimalarial properties (CitationHilou et al., 2006). The plant has high nutritive values due to the presence of high concentration of antioxidant components, fibers, proteins and essential amino acids, especially lysine (CitationOdhav et al., 2007; CitationTeutonico & Knorr, 1985). A. spinosus has been reported to possess a very good scavenging system to combat the effects of air pollution (CitationMandal & Mukherji, 2001).The betalains (180 mg/100 g) in the stem of A. spinosus were identified as amaranthine and isoamaranthine along with hydroxycinnamates, quercetin and kaempferol glycoside concentrations (305 mg/100 g) (CitationStintzing et al., 2004). Betalains are well known for hepatic disorders, jaundice, scanty urine, wounds, and for having antioxidant, anticancer, antiviral and antiparasitosis properties (Kapadia et al., 1995; CitationPatkai et al., 1997; CitationBerghofer & Schoenlechner, 2002). It also contains amaranthoside and amaricin, along with stigmasterol glycoside, spinoside (7-p-coumaroyl-apigenin-4-O-β-d-glucopyranoside), α-xylofuranosyl uracil, β-d-ribofuranosyl adenine, β-sitosterol glucoside, and betaines such as glycinebetaine and trigonelline (CitationAzhar-ul-Haq et al., 2004, Citation2006; CitationBlunden et al., 1999).
Whole plants of A. spinosus are used for the treatment of jaundice in the traditional system of medicine (CitationStintzing et al., 2004; CitationHema et al., 2006) but there are no scientific studies on the protective potential of 50% ethanol extract of A. spinosus against experimentally induced hepatitis in rats. Thus, the aim of the present study is to further evaluate the hepatoprotective activity of A. spinosus extract correlating biochemical and histological changes against d-GalN/LPS-induced hepatitis in rats.
Materials and methods
Plant material and preparation of extracts
Whole plants of Amaranthus spinosus Linn. (Amaranthaceae) were freshly collected from the botanical garden of the National Botanical Research Institute, Lucknow, India in October 2006. The plant material was identified and authenticated taxonomically by R.L.S. Shikarwar at the National Botanical Research Institute. A voucher specimen (NAB 75006) of the collected sample was deposited in the institutional herbarium for future reference. The plants were washed with distilled water to remove dirt, and then shade dried. The dried materials were powdered and passed through a 10-mesh sieve. The coarsely powdered material (1 kg) was extracted with petroleum ether thrice to remove the fatty material and further marc was extracted thrice with ethanol (50%, v/v). The extracts were filtered, pooled and concentrated at reduced temperature (5°C) on a rotary evaporator (Buchi, USA) and then freeze dried (Freezone® 4.5, Labconco, Kansas City MO) at high vacuum (133 × 10−3 mBar) and at temperature -40° ± 2°C (A. spinosus extract, yield 6.12%, w/w). For the pharmacological tests the A. spinosus extract was suspended in double distilled water containing carboxymethyl cellulose (1%, w/v, CMC).
Chemicals
d-GalN and LPS (Sero type 011.B4 extracted by phenol water method from Escherichia coli) were obtained from Sigma (St. Louis, MO). Diagnostic kits used for the estimation of biochemical assays were purchased from Qualigen (Mumbai). Anesthetic ether i.p. was procured from Sherad Laboratories (Andhra Pradesh). All other chemicals used were of analytical grade.
Animals
Sprague-Dawley rats weighing (140 ± 20 g) of either sex were procured from the National Laboratory Animal Centre (NLAC), Central Drug Research Institute, Lucknow. They were kept in a departmental animal house in a well cross ventilated room at 27° ± 2°C, and relative humidity 44-56%, light and dark cycles of 10 and 14 h, respectively, for one week before and during the experiments. Animals were provided with standard rodent pellet diet (Amrut, India) and the food was withdrawn 18-24 h before the experiment, though water was allowed ad libitum. All studies were performed in accordance with the guidelines for the care and use of laboratory animals, as adopted and promulgated by the Institutional Animal Care Committee, CPCSEA, India (Reg. No. 222/2000/CPCSEA). All the chemicals used were of analytical grade from standard companies and the water represents double distilled water. A standard orogastric cannula was used for oral drug administration.
Experimental design
The animals were divided into four groups. Each group had six animal. Group 1 served as solvent control which received CMC (1 ml/kg body weight orally) for 10 days. Group 2 rats were given A. spinosus extract alone (400 mg/kg body weight orally) for 10 days. Hepatitis was induced in group 3 rats through intraperitoneal injections of D-GalN and LPS (300 mg/kg body weight and 30 µg/kg body weight, respectively) 18 h before the experiment (CitationOmar et al., 1996). Group 4 rats were pretreated with A. spinosus extract for 10 days prior to the induction of D-GalN/LPS as in group 3.
The rats were anesthetized with anesthetic ether i.p. (Sherad Laboratories, Andhra Pradesh) and sacrificed by cervical dislocation after the experiment. Blood was collected and the serum was isolated for various biochemical assays. The liver tissue excised was washed with ice cold saline. A portion of the liver was then homogenized in 0.1 M Tris buffer and the homogenate was used for biochemical estimations.
Biochemical assays
The activities of serum aspartate transaminase (AST), alanine transaminase (ALT) (CitationReitman & Frankel, 1957), alkaline phosphatase (ALP) (CitationKing & Armstrong, 1934), lactate dehydrogenase (LDH) (CitationKing, 1965), gamma glutamyl transferase (γ-GT) (CitationRosalki & Rau, 1972) and levels of bilirubin (CitationMalloy & Evelyn, 1937) were assayed. The extraction of serum and liver tissue lipids (CitationFolch et al., 1957) followed by the estimation of total cholesterol (CitationZlatkis et al., 1953), triglycerides (CitationFoster & Dunn, 1973), free fatty acids (CitationFalholt et al., 1973) and phospholipids (CitationZilversmit & Davis, 1950) were carried out.
Histopathology
The liver tissues were fixed with 10% phosphate buffered neutral formalin, dehydrated in graded (50-100%) alcohol and embedded in paraffin. Thin sections (5 μM) were cut and stained with routine hematoxylin and eosin (H & E) stain for photo microscopic assessment. The initial examination was qualitative, with the purpose of determining histopathological lesions in liver tissue (CitationAmresh et al., 2007a).
Statistical analysis
All the data were presented as mean ± SEM and analyzed by SPSS for Windows, version 9 (SPSS, Chicago, IL) for the possible significant interrelation between the various groups, the independent samples t-test. A value of p < 0.05 was considered statistically significant.
Results
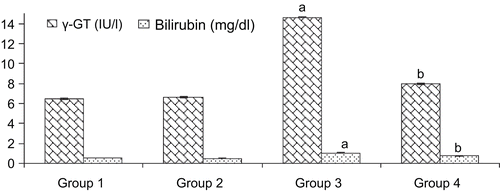
Group 3 rats intoxicated with d-GalN/LPS caused significant elevation (p < 0.05) in serum activities with higher levels of AST (3.77-fold), ALT (3.91-fold), ALP (2.60-fold), LDH (1.80-fold), γ-GT (2.62-fold) and bilirubin (1.85-fold) compared to control group 1. While the pretreatment of A. spinosus extract (400 mg/kg) significantly reduced the elevated serum parameters by 96.36, 75.07, 55.92, 13.34, 22.87, and 33.33% of the AST, ALT, ALP, LDH, γ-GT, and bilirubin, respectively compared to the intoxicated group 3 ( and ).
Figure 1. Levels of serum AST, ALT, ALP and LDH in control and experimental groups of rats. Values expressed as mean ± SD, n = 6. ap < 0.05 compared with group 1; bp < 0.05 compared with group 3. Group 1, control (only vehicle); group 2, ASE (400 mg/kg alone); group 3, D-GalN and LPS 300 mg/kg and 30 µg/kg intoxicated; group 4, pretreatment of ASE 400 mg/kg and D-GalN/LPS.
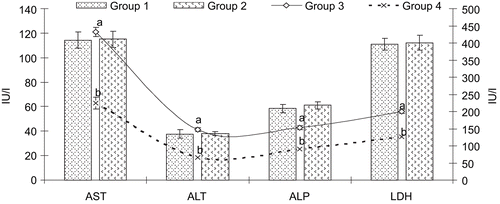
Figure 2. Levels of serum γ-GT and bilirubin in control and experimental groups of rats. Values expressed as mean ± SD, n = 6. ap < 0.05 compared with group 1; bp < 0.05 compared with group 3. Group1, control (only vehicle); group 2, ASE (400 mg/kg alone); group 3, d-GalN and LPS 300 mg/kg and 30 µg/kg intoxicated; group 4, pretreatment of ASE 400 mg/kg and d-GalN/LPS.
The rats treated with d-GalN/LPS in group 3 showed significant (p < 0.05) increase in the levels of cholesterol (1.34 and 1.77-fold), triglycerides (2.54 and 1.96-fold) and free fatty acid (1.47 and 3.35-fold) while the levels of phospholipids were found to be reduced (0.64 and 0.712-fold) significantly in serum and liver, respectively compared to control group 1. Pretreatment of A. spinosus extract (400 mg/kg) showed significant (p < 0.05) protection in the levels of cholesterol (0.78 and 0.83-fold), triglycerides (0.54 and 0.69-fold), free fatty acids (0.83 and 0.63-fold) and phospholipids (1.35 and 1.29-fold) in serum and liver, respectively compared to intoxicated group 3 ( and ). Pretreatment of A. spinosus extract afforded a significant protection against d-GalN/LPS-induced liver injury.
Table 1. Levels of serum cholesterol, triglycerides, free fatty acids and phospholipids in control and experimental groups of rats.
Table 2. Levels of cholesterol, triglycerides, free fatty acids and phospholipids in the liver of control and experimental groups of rats.
There were no significant changes in the activities of marker enzymes, bilirubin level and lipids in the rats treated with A. spinosus extract (400 mg/kg) alone as compared to the control, thereby showing the absence of any adverse toxic effects of A. spinosus extract.
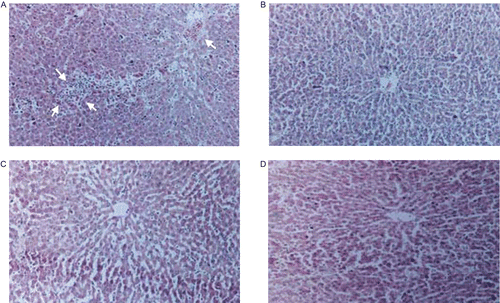
Diffused areas of hepatitis, especially in the perivenular region which extends to the central zone with inflammatory collections, were observed in d-GalN/LPS-induced rats () when compared to control (). Pretreatment with A. spinosus extract reversed to a large extent the hepatic lesions produced by d-GalN/LPS as it was evident from the absence of cellular necrosis and inflammatory infiltrate around the central zone () which further confirms the hepatoprotective potential of the A. spinosus extract. The rats, given A. spinosus extract alone, did not show any abnormal change in the architecture of the liver () as compared to the control rats.
Figure 3. (A) Photomicrograph of d-GalN/LPS intoxicated rat liver section which shows loss of architecture and cell necrosis (perivenular) extending to the central zone. The cell necrosis with inflammatory collections is more prominent in the central zone than around central vein. Hematoxylin-eosin stain (100×). (B) Photomicrograph of control rat liver section showing normal architecture. Hematoxylin-eosin stain (100×). (C) Photomicrograph of liver section of rat pre-treated with ASE prior to d-GalN/LPS challenge showing central vein surrounded by hepatocytes with sinusoidal dilatation with occasional inflammatory cells. No hepatic necrosis was seen around central vein or in the central zone. Hematoxylin-eosin stain (100×). (D) Photomicrograph of liver section of rat treated with ASE alone for 10 days showing normal liver parenchyma with central vein and cords of hepatocytes. Hematoxylin-eosin stain (100×).
Discussion
d-Galactosamine is a well established hepatotoxicant which induces a diffuse type of liver injury closely resembling human viral hepatitis (CitationDecker & Keppler, 1972). The toxicity of d-GalN results from inhibition of RNA and protein synthesis in the liver (CitationKonishi et al., 1974). The metabolism of d-GalN may deplete several uracil nucleotides including UDP-glucose, UDP-galactose and UTP (CitationDecker & Keppler, 1974) which are trapped in the formation of uridine-diphosphogalactosamine. Accumulation of UDP-sugar nucleotides (CitationEndo et al., 1992; CitationManabe et al., 1996) may contribute to the changes in the rough endoplasmic reticulum and to the disturbance of protein metabolism. Further, intense galactosamination of membrane structures is thought to be responsible for loss in the activity of ionic pumps. The impairment in the calcium pump, with consequent increase in the intracellular calcium is considered to be responsible for cell death (CitationTsai et al., 1997). d-Galactosamine/lipopolysaccharide (d-GalN/LPS) (300 mg/kg body weight/30 µg/kg body weight) induced highly elevated levels of serum AST, ALT, ALP, LDH, γ-GT and bilirubin in the untreated toxic control group. Elevated serum enzymes are indicative of cellular leakage and loss of functional integrity of the cell membrane in liver (CitationAmresh et al., 2007b; CitationDrotman & Lawhorn, 1978). Hence, significant rise in the serum enzyme levels could be taken as an index of liver damage (CitationLim et al., 2000). Pretreatment with 50% ethanol extract of whole plant of Amaranthus spinosus attenuated the increased activities of these enzymes in serum caused by d-GalN/LPS recovery towards normalization, and suggests that A. spinosus extract causes parenchymal cell regeneration in liver, thus protecting membrane fragility, thereby, decreasing enzyme leakage. Determination of serum bilirubin represents an index for the assessment of hepatic function, and any abnormal increase in the levels of bilirubin in the serum indicate hepatobiliary disease and severe disturbance of hepatocellular function (CitationMartin & Friedman, 1992). Increased levels of bilirubin in this study is in agreement with previous reports showing that d-GalN-induced hepatitis is characterized by increased levels of bilirubin in serum (CitationSree Rama Murthy & Srinivasan, 1993). The A. spinosus extract-mediated suppression of the increased bilirubin level suggests the possibility of the A. spinosus extract being able to stabilize biliary dysfunction.
Any liver disease will show an increased blood cholesterol level (CitationMcIntyre & Rosalki, 1992). d-GalN/LPS significant increase of cholesterol noted in this study might have been due to the inability of the diseased liver to remove cholesterol from circulation. Pretreatment of A. spinosus extract (400 mg/kg) also showed protection against serum and liver lipid changes caused by d-GalN/LPS evidencing a broad spectrum of hepatoprotective property. This finding could be correlated with the results of the previous studies (CitationBlack et al., 1983).
Hepatocellular damage due to alcohol, virus and drug-induced hepatitis causes modest hypertriglyceridemia (CitationGlickman & Sebesin, 1982), which is due to the biochemical changes interfering with the transport of triglycerides out of liver. Our study also showed an increased accumulation of triglycerides in d-GalN/LPS-induced rats which are in agreement with previous reports (CitationCartwright et al., 1982; CitationBlack et al., 1983). The administration of endotoxic LPS resulted in an increase in the free fatty acid content in rats (CitationDhuley & Naik, 1998). Accumulation of free fatty acids is a consequence of changes in hepatic lipid metabolism. This is well correlated in our study with the increased levels of free fatty acids due to the administration of d-GalN/LPS, which is responsible for the increment of phospholipase A2 activity as a consequence of the high level of intracellular calcium causing the hydrolysis of liver membrane phospholipids and release of arachidonic acid (CitationKramer, 1993). This might account for the decreased levels of phospholipids observed in the serum and liver of d-GalN/LPS challenged rats.
Rats pretreated with A. spinosus extract (400 mg/kg) prior to the induction of hepatic damage showed a restoration of the altered lipid levels induced by d-GalN/LPS towards near normalcy thereby showing the modulating effect of A. spinosus extract against d-GalN/LPS-induced changes in the lipid levels in rats. Histopathological observations supported the protective effect of Amaranthus spinosus in d-GalN/LPS administered rats. There were no significant changes in the activities of marker enzymes, bilirubin level and lipids in the rats treated with A. spinosus extract alone (400 mg/kg) thereby showing the absence of any adverse toxic effects of A. spinosus extract. The hepatic lesion-produced central zone with inflammatory collections induced by d-GalN/LPS was prevented by treatment with A. spinosus extract. This can be well correlated with the protective actions of picroliv, which showed lipid changes against hepatotoxicity induced by paracetamol and d-GalN (CitationAnsari et al., 1991).
Thus, the present study confirms the hepatoprotective action of A. spinosus extract against d-GalN/LPS-induced hepatitis in rats. The curative efficacy of A. spinosus extract was very promising as evidenced by the reversal of the altered values following the administration probably by promoting regeneration of hepatocytes. The hepatoprotective action may be mediated through the inhibition of UDP sugar derivatives, enhancement of glycoprotein biosynthesis, stabilization of cell membrane and inhibition of lipid accumulation by its hypolipidemic property. Preliminary qualitative phytochemical screening of 50% ethanol extract of whole plant of Amaranthus spinosus has given the positive tests for alkaloids, glycosides, flavonoids, saponins, carbohydrates, protein, amino acids, lipids, steroids, phenolic acid and tannins (CitationTrease & Evans, 1983). Previous phytochemical investigations of Amaranthus spinosus showed that the presence of polyphenolic compounds such as betalains, quercetin, rutin, kaempferol glycosides, β-sitosterol and stigmasterol which may account for the hepatoprotective action of A. spinosus extract against d-GalN/LPS-induced hepatitis in rats (CitationBerghofer & Schoenlechner, 2002; Suryavanshi et al., 2007).
References
- Al-Mahtab M, Rahman S, Khan M (2008): Occult hepatitis B virus related decompensated cirrhosis of liver in young males: First report of two cases from Bangladesh. Hep Mon 8: 147–150.
- Aghel N, Rashidi I, Mombeini A (2007): Hepatoprotective activity of Capparis spinosa root bark against CCl4 induced hepatic damage in mice. Iranian J Pharm Res 6: 285–290.
- Amresh G, Rao CV, Singh PN (2007a): Antioxidant activity of Cissampelos pareira on benzo (a) pyrene induced mucosal injury in mice. Nutr Res 27: 625–632.
- Amresh G, Rao CV, Singh PN (2007b): Evaluation of Cissampelos pareira on gastric cancer and enzymes associated with carcinogen metabolism. Pharm Biol 45: 595–603.
- Ansari RA, Tripathi SC, Patnaik GK, Dhawan BN (1991): Antihepatotoxic properties of picroliv: An active fraction from rhizomes of Picrorhiza kurrooa. J Ethnopharmacol 34: 61–68.
- Azhar-ul-Haq Mailk, A, Afza N, Khan SB, Muhammad P (2006): Coumaroyl adenosine and lignan glycoside from Amaranthus spinosus Linn. Pol J Chem 80: 259–263.
- Azhar-ul-Haq Malik, A., Khan AU, Shah MR, Muhammad P (2004): Spinoside, new coumaroyl flavone glycoside from Amaranthus spinosus. Arch Pharm Res 27: 1216–1219.
- Berghofer E, Schoenlechner R (2002): Grain amaranth, in:Belton PS, Taylor JRN, eds, Pseudocereals and Less Common Cereals. Grain Properties and Utilisation Potential. Berlin, Heidelberg, New York, Springer, pp. 219–260.
- Black DD, Tso P, Weidman S, Sabesin SM (1983): Intestinal lipoproteins in the rat with d-galactosamine hepatitis. J Lipid Res 24: 977–992.
- Blunden G, Yang M, Janicsak G, Mathe I, Carabot Cuervo A (1999): Betaine distribution in the Amaranthaceae. Biochem Syst Ecol 27: 87–92.
- Cartwright CK, Ragland JB, Weidman SW, Sabesin SM (1982): Alterations in lipoprotein composition associated with galactosamine induced rat liver injury. J Lipid Res 23: 667–679.
- Decker K, Keppler D (1972): Galactosamine induced liver injury, in:Popper H, Schaffner F, eds, Progress in Liver Disease. New York, Grune & Stratton, pp. 183–199.
- Decker K, Keppler D (1974): Galactosamine hepatitis: Key role of the nucleotide deficiency period in the pathogenesis of cell injury and cell death. Rev Physiol Biochem Pharmacol 71: 78–105.
- Dhuley JN, Naik SR (1998): Effect of rhinax on bacterial lipopolysaccharide induced endotoxemia in rats. Indian J Exp Biol 36: 315–317.
- Drotman RB, Lawhorn GT (1978): Serum enzymes are indicators of chemical induced liver damage. Drug Chem Toxicol 1: 163–171.
- Elsheikh RM, Daak AA, Elsheikh MA, Karsany MS, Adam I (2007): Hepatitis B virus and hepatitis C virus in pregnant Sudanese women. Virol J 104.
- Endo Y, Kikuchi T, Nakamura M (1992): Ornithine and histidine decarboxylase activities in mice sensitized to endotoxin, interleukin-1 or tumor necrosis factor by d-galactosamine. Br J Pharmacol 107: 888–894.
- Falholt K, Lund B, Falholt W (1973): An easy colorimetric micromethod for routine determination of free fatty acids in plasma. Clinica Chimica Acta 46: 105–111.
- Folch J, Less M, Solane SGH (1957): A simple method for isolation and purification of total lipids from animal tissues. J Biol Chem 226: 497–509.
- Foster LB, Dunn RT (1973): Stable reagents for determination of serum triglycerides by colorimetric Hantzsch condensation method. Clin Chem 19: 338–340.
- Glickman RM, Sebesin SM (1982): Lipid metabolism, in:Asias IM, Schachter D, Popper H, Shafritz DA, eds, Liver Biology and Pathobiology. New York, Raven Press, pp. 123–142.
- Hema ES, Sivadasan M, Anil KN (2006): Studies on edible species of Amaranthacea and Araceae used by Kuruma and Paniya tribes in Wayanad district, Kerala, India. Ethnobotany 18:122–126.
- Hilou A, Nacoulma OG, Guiguemde TR (2006): In vivo antimalarial activities of extracts from Amaranthus spinosus L. and Boerhaavia erecta L. in mice. J Ethnopharmacol 103: 236–240.
- Kapadia GJ, Balasubramanian V, Tokuda H, Iwashima A, Nishino H (1997): Inhibition of 12-O-tetradecanoylphorbol-13-acetate induced Epstein-Barr virus early antigen activation by natural colorant. Cancer Lett 115: 173–178.
- King EJ, Armstrong, AR (1934): A convenient method for determining serum and bile phosphatase activity. Can Med Assoc J 31: 376–381.
- King J (1965): The dehydrogenase of oxidoreductase lactate dehydrogenase, in:King JC ed., Practical Clinical Enzymology. London, Van Nostrand, pp. 83–93.
- Kirtikar KR, Basu BD (2001): Indian Medicinal Plants, Vol. 9, second edition. Dehradun, India, Oriental Enterprises, pp. 2832–2836.
- Konishi T, Shinozuka H, Faber JL (1974): The inhibition of rat liver nuclear ribonucleic acid synthesis by galactosamine and its reversal by uridine. Lab Invest 30: 751–756.
- Kramer KM (1993): Structure and function of cytosolic phospholipase A2. Exp Med 11: 1467–1474.
- Lim HK, Kim HS, Choi HS, OhS Jang, CG, Choi J, Kim SH, Chang MJ (2000): Effects of acetylbergenin against d-galactosamine induced hepatotoxicity in rats. Pharmacol Res 5: 471–474.
- Lin BF, Chiang BL, Lin JY (2005): Amaranthus spinosus water extract directly stimulates proliferation of B lymphocytes in vitro. Int Immunopharmacol 5: 711–722.
- Malloy HT, Evelyn KA (1937): The determination of bilirubin with the photometric colorimeter. J Biol Chem 119: 481–490.
- Manabe A, Cheng CC, Egashira Y, Ohta T, Sanada H (1996): Dietary wheat gluten alleviates the elevation of serum transaminase activities in d-galactosamine-injected rats. J Nutr Sci Vitaminol 42: 121–132.
- Mandal M, Mukherji S (2001): A study on the activities of a few free radicals scavenging enzymes present in five roadside plants. J Environ Biol 22: 301–305.
- Martin P, Friedman LS (1992): Assessment of liver function and diagnostic studies, in:Friedman LS, Keeffe EB, eds, Hand Book of Liver Disease. Philadelphia, Churchill Livingstone, pp. 1–14.
- McIntyre N, Rosalki S (1992): Biochemical investigations in the management of liver disease, in:Prieto J, Rodes J, Shafritz DA, eds, Hepatobiliary Diseases. Berlin, Springer Verlag, pp. 39–71.
- Mishra B (1986): Bhavaprakash Nighantu (Hindi), 7th edn.Chunekar K, Pandey G, eds. Chaukhamba Bharati Academy, Varanasi, India, pp. 666–667.
- Mukhopadhya A (2008): Hepatitis C in India. J Biosci 33: 465–473.
- Odhav B, Beekrum S, Akula US, Baijnath H (2007): Preliminary assessment of nutritional value of traditional leafy vegetables in Kwa Zulu-Natal, South Africa. J Food Compost Anal 20: 430- 435.
- Omar HM, Sanders RA, Watkins JB (1996): Minimal effect of acute experimental hepatitis induced by lipopolysaccharide/d-galactosamine on biotransformation in rats. Biochem Pharmacol 52: 1921–1924.
- Parrotta JA (2001): Healing Plants of Peninsular India. New York, CABI Publishing, p. 54.
- Patkai G, Barta J, Varsanyi I (1997): Decomposition of anticarcinogen factors of the beet root during juice and nectar production. Cancer Lett 114:105–106.
- Reitman S, Frankel S (1957): A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28: 53–56.
- Rosalki SB, Rau D (1972): Serum gamma glutamyl transpeptidase activity in alcoholism. Clinica Chimica Acta 39: 41–47.
- Sehrawat A, Khan TH, Prasad L, Sultana S (2006): Butea monosperma and chemomodulation: Protective role against thioacetamide mediated hepatic alterations in Wistar rats. Phytomedicine 13:157–63.
- Sree Rama Murthy M, Srinivasan M (1993): Hepatoprotective effect of Tephrosia purpurea in experimental animals. Indian J Pharmacol 25: 34–36.
- Stintzing FC, Kammerer D, Schieber A, Hilou A, Nacoulma O, Carle R (2004): Betacyanins and phenolic compounds from Amaranthus spinosus and Boerhaavia erecta. Z Naturforsc 59c: 1–8.
- Suryavanshi VL, Sathe PA, Baing MM, Singh GR, Lakshmi SM (2008): Determination of rutin in Amaranthus spinosus Linn. whole plant powder by HPTLC. Chromatographia 67:189–191.
- Teutonico RA, Knorr D (1985): Amaranth: Composition, properties and applications of a rediscovered food crop. Food Technol 39: 49–60.
- Trease GE, Evans WC (1983): Pharmacognosy. London, Baillier Tindall Press, pp. 309, 706.
- Tsai CC, Kao CT, Hsu CT, Lin CC, Lin JG (1997): Evaluation of four prescriptions of traditional Chinese medicine: syh-mo-yiin, guizhi-fuling-wan, shieh-qing-wan and syh-nih-sann on experimental acute liver damage in rats. J Ethnopharmacol 55: 213–222.
- Zilversmit BB, Davis AK (1950): Micro determination of plasma phospholipids by TCA precipitation. J Lab Clin Med 35: 155–161.
- Zlatkis A, Zak B, Boyle AJ (1953): A new method for the direct determination of serum cholesterol. J Lab Clin Med 41: 486–492.
