Abstract
Morinda elliptica Ridley (Rubiaceae) has been used traditionally as a medicine to treat various diseases in Malaysia and southeast Asia. In the present study we investigated the immunomodulatory effects of damnacanthal isolated from the roots of Morinda elliptica. The immunomodulatory effect of this compound was evaluated by using the lymphocyte proliferation assay with mouse thymocytes and human peripheral blood mononuclear cells (PBMC). In addition, the effect of the compound on PBMC cell cycle progression was studied by using flow cytometry. The production of human interleukin-2 and human inteleukin-12 cytokines was also assessed using the enzyme linked immunosorbent assay (ELISA) technique. The lymphocyte proliferation assay showed that damnacanthal was able to activate mouse thymocytes and PBMC at a low concentration (0.468 μg/mL). Moreover, the production of human interleukin-2 and human interleukin-12 cytokines in the culture supernatant from damnacanthal activated lymphocytes was markedly up-regulated at 24 h and sustained until 72 h with a slight decrease with time. A positive correlation was found between the level of these two cytokines and the MTT-based proliferation assay. Based on the above results, damnacanthal can act as an immunomodulatory agent which may be very useful for maintaining a healthy immune system.
Introduction
Morinda species are popular herbs worldwide. One of these species is Morinda elliptica Ridley (Rubiaceae) which is widely distributed in peninsular Malaysia. It is commonly known as “mengkudu kecil” in Malaysia, “noni” in Hawaii and as “yoo-thuean” in Thailand (CitationAli et al., 2000). This herb has been used traditionally as a medicine to treat various diseases such as headache, cholera, diarrhea, gastric ulcers and fever (CitationOng & Norzalina, 1999; CitationWang et al., 2002). In Malaysia the crushed leaves of this herb are commonly added to rice to increase people’s appetite (CitationJasril et al., 2003). The roots and leaves of this herb are also used as a topical treatment for hemorrhoids and as a tonic after childbirth (CitationJasril et al., 2003). In addition, this herb is also claimed to be able to stimulate the immune system and thus prevent the formation and proliferation of cancers such as Lewis lung cancer (LLC) (CitationHirazumi et al., 1996; Citation1999).
Morinda elliptica has long been known to contain substantial amounts of anthraquinones such as damnacanthal. Damnacanthal has some unique chemical and biological properties. In 1993, Hiramatsu and colleagues reported that damnacanthal isolated from noni roots (Morinda citrifolia) acted as an inhibitor of Ras function which is believed to be associated with signal transduction in several human cancers such as those of the lungs and colon and in leukemia. Damnacanthal had been shown to exert a potent inhibitory activity towards tyrosine kinase so affecting the Lck, Scr, Lyn and EGF receptors (CitationHiwasa et al., 1999). Furthermore, damnacanthal which was solubilized in DMSO also affected intracellular Ca2+ mobilization, as demonstrated in cultured bovine coronary endothelial cells (CitationAoki et al., 2000) and showed an intense inhibitory effect against DNA topoisomerase 11 with an IC50 of 20 μg/mL (CitationTosa et al., 1998). Previously, CitationAli et al. (2000) reported that damnacanthal isolated from Morinda elliptica was cytotoxic for MCF-7 (breast carcinoma) and CEM-SS (T-lymphoblastic leukemia) cell lines. This compound was also found to have a strong antimicrobial activity and moderate activity against HIV (CitationAli et al., 2000). More recently, CitationMasakazu et al. (2006) discovered that damnacanthal is an inhibitor of viral protein R (Vpr) which is important in the development of anti-HIV therapy.
Undoubtedly, damnacanthal possesses various pharmacological activities. Although comprehensive tests have been carried out to study the cytotoxic, antimicrobial and antiviral effects of this compound, no specific study had been reported addressing the immunomodulatory activity of damnacanthal. Thus, the present study was carried out to investigate the immunomodulatory effect of damnacanthal on mouse thymocytes and human peripheral blood mononuclear cell (PBMC) proliferation and also on the induction of human PBMC interleukin 2 and interleukin 12.
Materials and methods
Plant material
Roots of Morinda elliptica were collected from Port Dickson, Negeri Sembilan in December 1989 and identified by Anthonysamy Sivarimuthu of the Department of Biology, Faculty of Science, Universiti Putra Malaysia. A voucher specimen (FRI56654) was deposited in the herbarium of the Department of Biology, Faculty of Science, Universiti Putra Malaysia.
Chemicals and compounds
Damnacanthal (C16H10O5) which had been isolated from the roots of Morinda elliptica as described previously (CitationIsmail et al., 1997; CitationAli et al., 2000) was kindly supplied by Nordin Lajis, from the Laboratory of Natural Products, Institute of Bioscience, Universiti Putra Malaysia. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), concanavalin (Con) A, pokeweed mitogen (PWM) Hank’s balanced salts solution (HBSS), dimethyl sulfoxide (DMSO), propidium iodide (PI), Triton X-100 and RNase A were all purchased from Sigma (St Louis, MO, USA). Fetal bovine serum (FBS) was from PAA, Dulbecco’s modified Eagle’s medium (DMEM) from Flowlab (Flowlab, N.S.W, Australia) and Ficoll-Paque plus from Amersham Biosciences, isoflurane, sodium citrate and EDTA were purchased from Merck. Concanavalin A (Con A) (50 µg/mL) and pokeweed mitogen (50 µg/mL) were used as positive controls. These commercial immunomodulators were prepared by dissolving them with DMEM medium (Flowlab, N.S.W, Australia). The stock and substock solutions were prepared as above.
Animals
A total ten male of Imprinting Control Region (ICR) mice, 5-8 weeks old, weight between 18-20g were used in all experiments. The animals were purchased from the Animal House, Universiti Putra Malaysia. The animals were housed under standard conditions at 25° ± 2°C and fed with standard pellets and tap water. This work had been approved by the Animal Care and Use Committee, Universiti Putra Malaysia (UPM), (Ref: UPM/FPV/PS/3.2.1.551/AUP-R2).
Preparation of mouse thymus cell suspensions
The mice were anesthetized with 2% isoflurane (Merck) and sacrificed by cervical dislocation. The thymus which is located above the heart was removed and quickly washed with Hank’s balanced salts solution (HBSS) in the Petri dish. The thymus was minced and pressed through a sterile wire mesh screen with a rubber syringe plunger. The cell suspension was washed with PBS supplemented with 0.1% BSA and 0.06% sodium citrate (PBS-BSA-SC) and spun down at 200 g for 10 min. This step was repeated until the pellet was clean (did not contain any debris or contaminant). The supernatant was discarded and 4 mL of DMEM with 10% heat inactivated serum was added to re-suspend the pellet. Cell counting was then performed to determine the lymphocyte cell number in the suspension. All of the above steps were carried out under sterile conditions in a biological safety cabinet to prevent any contamination.
Isolation of peripheral blood mononuclear cells (PBMC)
Venous blood was collected aseptically from healthy donors in preservative-free heparin tubes. The blood was diluted with phosphate buffered saline (PBS), pH 7.4 and layered onto Ficoll plus. After centrifugation at 400 g for 50 min, the lymphocytes were collected from the interphase and washed three times with PBS. The cells were resuspended in DMEM with 10% fetal bovine serum and antibiotics.
Lymphocyte proliferation assay
Lymphocyte proliferation was tested using the 3-[4,5-dimethylthizol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay (CitationMosmann, 1983). Briefly, 100 μL of DMEM was added to each well of a flat-bottomed 96-well plate except for row A. 100 μL of diluted damnacanthal was added to rows A and B. The solution was mixed by pipetting and starting from row B, 100 μL was taken from each well and added to the next well in row C. A series of two-fold dilutions of the drug down from row B to row G were carried out. The excess (100 μL) was discarded. Row H was left untouched. 100 μL of cell suspension (peripheral blood mononuclear cells or thymic lymphocytes) was added into all the wells and incubated at 37°C, 5% CO2 and 90% humidity for 24, 48, and 72 h. A stock solution of 5 mg/mL MTT in PBS was prepared and 20 μL of MTT was added to each well. The culture medium was aspirated and 100 μL of DMSO was added to each well to dissolve the purple crystals. Finally, the plate was read on an automated spectrophotometric EL 340 μ Quant ELISA Reader (Bio-Tek Instruments, Winooski, USA) using test and reference wavelengths of 570 nm. The percentage of proliferation was calculated by using the following equation:
Flow cytometry analyses
A flow cytometer was used to determine the effects of damnacanthal on human PBMC cell cycle progression. This study was carried out to evaluate the effects of damnacanthal and pokeweed mitogen (PWM) on the alteration of PBMC cell cycle phases at different incubation times. Cell cycle analysis might also allow us to differentiate cell subpopulations, such as viable cells and apoptotic cells without needing an extra separation step (CitationLoken, 1980). The significant phase in evaluating the proliferation of cells is at the G2/mitosis phase. At this phase cells will have two copies of DNA which indicate cell proliferation has occurred. PBMC were chosen in order to evaluate the capacity of damnacanthal to stimulate the proliferation of lymphocytes in vitro. In this study, PWM was used as a positive control. The active concentrations chosen for damnacanthal and PWM in order to stimulate the proliferation of PBMC were 30 and 50 µg/mL, respectively. In this study, 1 mL of PBMC with a density 1 × 106 cells/mL, was treated with 1 mL of damnacanthal and PWM according to their active concentrations as mentioned above. The treatments were carried out in 6-well plates (Nunc, NY, USA) with a total working volume of 2 mL for each well. The treated cells were then incubated for 24, 48, and 72 h and harvested by centrifugation at 1000 rpm (200 g) for 10 min. Subsequently, the treated cells were fixed with 80% ethanol at 4°C for 2 h. Then the cells were spun down and washed twice with PBS at pH 7.5. The cell pellets were finally dissolved and stained in PBS buffer consisting of 0.1% Triton X-100, 10 mM EDTA, 50 µg/mL RNase and 3 µg/mL propidium iodide (PI). This process was done in the dark because PI is sensitive to light. The cells were then incubated for 30 min at 4°C and analyzed using the Coulter EPICS Altra flow cytometer (Beckman Coulter, Miami, USA) at the Biological Laboratory, Faculty of Veterinary Medicine, Universiti Putra Malaysia within 24 h.
Cytokine production by human peripheral blood mononuclear cells
The production of human interleukin-2 and human interleukin-12 was measured by ELISA according to the instructions of the manufacturer (Bendemedsystems, Vienna, Austria). All experiments were carried out in microtiter plates provided in the kit. Briefly, 150 μL of distilled water was added to all the standard wells and blank wells. Then, 100 μL of distilled water was added into the sample wells, followed by adding 50 μL of culture supernatants (PBMC treated with the compound) and the positive control (PWM) into each of the designated wells. The culture supernatants of the control and test compounds were used without dilution. The plate was covered and incubated at room temperature (18°C to 25°C) for 3 h on a microplate shaker. After that, the plate was washed three times with approximately 400 μL of washing buffer per well with thorough aspiration of the microwell contents in between washes. The plate was then tapped onto an absorbent pad to remove the excess washing buffer. After washing, 100 μL of TMB substrate solution was added and incubated at room temperature on a microplate shaker at 100 rpm. After 10 min, 100 μL of stop solution was pipetted into each well and the result was read immediately using a Benchmark microtiter plate ELISA reader (Bio-Tek Instruments, Winooski, USA) at 450 nm with reference wavelength at 620 nm.
Statistical analysis
All experiments were performed in triplicate and the results were expressed as mean ± SE. Student’s t-test was used to analyze the statistical significance of the differences between the control and the treated values.
Results
Mitogenic activity of damnacanthal on mouse thymocytes
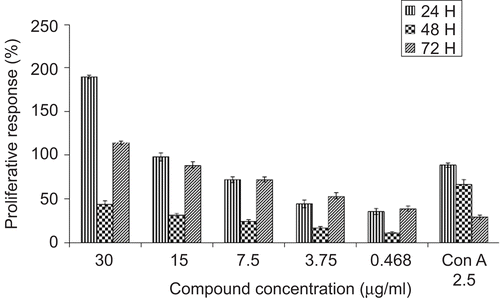
Damnacanthal was found to stimulate the proliferation of mouse thymocytes significantly in a dose- and time-dependent manner (). Damnacanthal initiated much better proliferation of mouse thymocytes than Con A since 30 µg/mL of damnacanthal induced 189% thymocyte proliferation while 2.5 µg/mL of Con A only induced 88% proliferation and this effect was significantly reduced after 48 and 72 h incubation. Maximal growth was found after 24 h incubation with 30 μg/mL of damnacanthal, achieving a two-fold increase compared to the positive control (Con A). Con A, used as a positive control at its optimal concentration (2.5 μg/mL) was found to markedly promote lymphocyte growth in a time-dependent manner with maximal growth after 24 h treatment. However, this proliferation decreased significantly after 48 and 72 h with values of 66.12% and 28.86%, respectively. The results also suggested that this compound was not toxic to mouse thymocytes even at a high concentration of 30 μg/mL.
Figure 1. Mitogenic activity of damnacanthal on mouse thymocytes was determined by using MTT assay. Mouse thymocytes were isolated and incubated with increasing concentrations (0.468 μg/mL–30 μg/mL) of damnacanthal or Con A (2.5 μg/mL, as positive control) in culture medium for 24, 48, and 72h. Results were expressed as mean percentage ratio of MTT absorbance in damnacanthal-treated and control well ± standard error of three independent experiments with three wells each.
Mitogenic activity of damnacanthal on human peripheral blood mononuclear cells
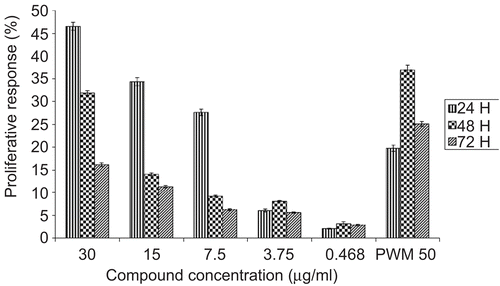
Damnacanthal was found to significantly stimulate the proliferation of PBMC in a dose- and time-dependent manner (). It stimulated the proliferation of human lymphocytes throughout the treatment period but did not inhibit human lymphocyte proliferation at any of the tested concentrations. Damnacanthal was able to induce the highest proliferation of PBMC at 24 h treatment with a value of 46.55% at 30 μg/mL. At this incubation period it showed significantly better proliferation of PBMC when compared to the positive control PWM. PWM stimulated the proliferation of PBMC in a time-dependent manner. It induced better proliferation of PBMC after 48 h with a value of 36.87%. It was also clear that the proliferative effects of PBMC treated with PWM were much higher than damnacanthal after 48 and 72 h treatments. Obviously, damnacanthal seemed to stimulate PBMC at an early incubation time (24 h) whereas PWM seemed to stimulate PBMC after a longer incubation period.
Figure 2. Mitogenic activity of damnacanthal on PBMC was tested by using MTT assay. PBMC were isolated and incubated with increasing concentrations (0.468 μg/mL–30 μg/mL) of damnacanthal or PWM (50 μg/mL, as positive control) in culture medium for 24, 48, and 72h. Results were expressed as mean percentage ratio of MTT absorbance in damnacanthal-treated and control well ± standard error of three independent experiments with three wells each.
Flow cytometry analysis of cell cycle distributions on PBMC based on the proliferation effects of damnacanthal and PWM at 24, 48, and 72 h incubation time
The changes in the cell cycle distribution of PBMC treated with damnacanthal and PWM at different incubation times (24, 48, and 72 h) are shown in .
Table 1. Flow cytometry analysis of cell cycle distribution on PBMC based on proliferation effect of damnacanthal and PWM. The values were the means ± SE of three experiments.
Analysis of the cell cycle profiles as shown in revealed that cells treated with damnacanthal underwent higher proliferation at 24 h with a percentage of 57.42% compared to untreated cells with a value of 4.63% at the G2/M phase. The percentage of cell proliferation was 12.4-fold higher in treated than in untreated cells. However, the percentage of cells treated with damnacanthal entering into G2/M decreased gradually after 48 h and 72 h with values of 40.26% and 43.46%, respectively. Nevertheless, the percentage of cell proliferation after 48 h and 72 h treatment were 1-fold and 2.7-fold higher when compared to untreated cells. The reduction in the percentage of cell proliferation was due to the higher percentages of cells entering the Sub G1 phase, as shown by the apoptotic rates of 15.89% and 4.5% after 48 h and 72 h treatments, respectively.
As demonstrated in , the proliferation of PBMC treated with pokeweed mitogen at 24 h incubation was significantly higher when compared to untreated cells with values of 9.26% and 4.63%, respectively. Yet the percentage of cells treated with pokeweed mitogen entering into G2/M increased gradually at 24, 48 and 72 h with values of 9.26%, 30.48% and 41.91%, respectively. At early incubation times, the low percentage of cells that entered the G2/M phase corresponded to an increase of cells that entered the sub G1 phase, thus indicating that PWM may cause higher apoptosis at earlier incubation times, particularly at 24 h and 48 h, with values of 18.84% and 13.25%, respectively. However, PWM elicited better proliferation of PBMC being 2.6-fold higher when compared to the control after 72 h treatment.
The production of human interleukin-2 and human interleukin-12
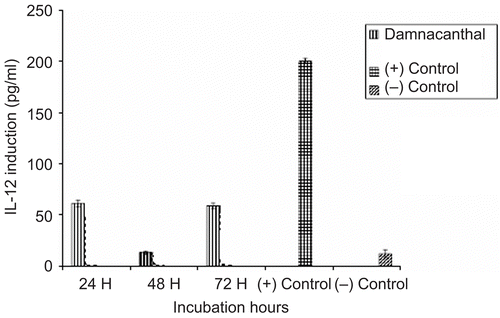
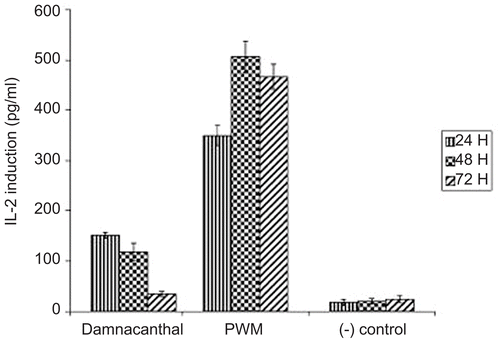
As is shown in , damnacanthal had significantly increased the production of human interleukin-2 in a time-dependent manner. Interestingly, damnacanthal promoted higher induction of human IL-2 after 24 h incubation with a concentration of 151.66 picogram (pg)/mL which was 7.5-fold higher when compared to the negative control with a value of 20 pg/mL. However, this value decreased significantly after 48 h and 72 h treatments with 5.9-fold and 1.75 fold reduction, respectively. In contrast, the induction of human IL-2 was increased by PWM in a time-dependent manner. PWM induced higher production of human IL-2 at 48 h of incubation with a 25.33-fold higher induction when compared to the negative control. In addition, it also demonstrated a steady induction of human IL-2 throughout the treatment period.
Figure 3. The production of human interleukin-2 in culture supernatants upon stimulation of PBMC by damnacanthal, and PWM. PBMC were isolated and incubated at 24, 48, and 72 h with active concentrations of damnacanthal at 30 μg/mL, while PWM at 50 μg/mL and interleukin-2 induction was specifically determined by ELISA.
As is shown in , the production of human interleukin-12 by damnacanthal was high (61.33 pg/mL) when compared to the negative control interleukin-12 with a value of 12 pg/mL at 24 h. Damnacanthal induced an equal amount of human interleukin-12 at 24 h and 72 h treatments with values of 61.33 and 59 pg/mL, respectively. The results indicated that it had significantly induced the production of human IL-12 at 24 and 72 h treatment with 5.1-fold and 4.9-fold increases, respectively when compared to the negative control. Damnacanthal exhibited a sharp reduction in the production of human IL-12 after 48 h treatment with a value only 1.1-fold higher when compared to the negative control.
Discussion and conclusion
Damnacanthal is an anthraquinone produced mainly by plants of the Rubiaceae family. It is commonly isolated from Morinda citrifolia L. var. citrifolia (Rubiaceae), Morinda elliptica Ridley (Rubiaceae), Morinda lucida Benth. (Rubiaceae), Prismatomeris fragrans E.T. Geddes (Rubiaceae) and others (CitationIsmail et al., 1997; CitationTosa et al., 1998; CitationKwanjai et al., 2005). Damnacanthal has been known to possess some unique chemical and biological properties (CitationHiramatsu et al., 1993). The present immunomodulatory studies revealed that damnacanthal significantly enhanced the proliferation of mouse thymocytes and PBMC without leading to inhibition at any of the concentrations tested. This result was in agreement with the results published by CitationWang et al. (2002) who demonstrated that the thymus in animals treated with a juice extract obtained from Morinda citrifolia was enlarged. The chemical constituents of a Morinda citrifolia extract including damnacanthal may enhance immune function by stimulating thymus growth thus exhibiting anti-ageing and anti-cancer activities (CitationWang et al., 2002).
Previously, CitationHirazumi et al. (1996) reported that the juice extracted from Morinda citrifolia was found to inhibit the growth of Lewis lung tumors in mice (LLC), an effect associated with the stimulation of T cell immune response and thymocyte proliferation. In a different experiment, they inoculated mice with LLC, and surprisingly those mice ingesting a daily dose of 15 mg of noni juice showed a significant increase (119%) in life span (CitationHirazumi et al., 1996; Citation1999). In addition, they also showed that the ingestion of noni combined with conventional chemotherapy in the treatment of mice with cancer, could increase life span (CitationHirazumi et al., 1994). Hence, the studies by CitationHirazumi et al. (1994, Citation1996, Citation1999) and CitationWang et al. (2002) supported the capacity of damnacanthal to enhance the immune system through the modulation of thymus proliferation and regeneration. Their data showed that the proliferative effect of damnacanthal on PBMC was less than that of PWM after 48 h and 72 h treatments. This might be due to the ability of PWM to stimulate the proliferation of both T and B cells (CitationStanilove et al., 2005). Nevertheless, the proliferation effects of damnacanthal were sustained even 72 h after treatment.
Damnacanthal is one of the phenolic group of compounds. Phenolic compounds have been known to possess broad biological activities, including that of immune system enhancer (CitationManuele et al., 2006). According to CitationZhao et al. (2007), a previous study indicated that many phenolic compounds could stimulate the proliferation of splenocytes. The most common phenolic compound which is known to have immunomodulator activities is scopoletin (CitationManuele et al., 2006; CitationWang & Su, 2001). Phenolic compounds could either stimulate or suppress the immune system due to the hydroxyl groups in their structures. The hydroxyl groups can affect enzymes or the electron-transfer system, thus resulting in immunomodulating properties, particularly stimulating proliferation of lymphocytes and phagocytosis (CitationManosroi et al., 2003). However, the mechanism by means of which hydroxyl groups affect proliferation and phagocytosis remain unclear (CitationManosroi et al., 2003).
Since cytokines play an important role in regulating the proliferation and differentiation of lymphocytes, the modulatory effects of damnacanthal on the production of human IL-2 and the production of human IL-12 are of interest. Damnacanthal was found to induce higher production of human interleukin-2 and lower production of human interleukin-12 in a time-dependent manner. The production of human IL-2 by damnacanthal occurred much earlier at 24 h. Meanwhile, PWM was used as a positive control since it had been reported previously to induce IL-2 and IFN-γ production (CitationStanilove et al., 2005). The present results showed that the induction of human IL-2 by damnacanthal was less than by PWM throughout the treatment periods. However, the induction of human IL-2 by damnacanthal was considered to be higher when compared to their capability for inducing the production of human IL-12. It is well documented that T cell proliferation is the result of IL-2 expression, which is dependent on T cell activation (CitationAdam et al., 2003). Thus, this finding suggests that damnacanthal tends to induce T cells rather than B cells, as IL-2 is known as a central cytokine in the regulation of T cell responses (CitationChandok & Farber, 2004).
In addition, the unequal populations of T and B lymphocytes in human peripheral blood lymphocytes namely 90% T cells and only 10% B cells (CitationCerqueira et al., 2004) may contribute to a higher production of human interleukin-2 in the culture supernatant treated with damnacanthal. The capability of damnacanthal in stimulating the proliferation of T cells and in inducing the production of human IL-2 could play an important role in the killing and suppression of cancer cells. CitationLi et al. (2008) reported that a stimulation of the immune system and an increase of the number of CD4 positive T cells could result in massive necrosis in tumor tissues. Induction of IL-2 could play an essential role in triggering an increase of the NK cell population, a heterogeneous group of granular lymphocytes that appear to be very effective in lysing target cancer cells through the activation of lymphokine-activated killer cells (LAK) (CitationManuele et al., 2006). Moreover, tumor cells of various types possess unique sets of tumor markers that can be recognized by NK cells (CitationFarag & Caliguiri, 2006). In addition, the lytic process mediated by NK cells involves the release of cytotoxic factors for which there seem to be more receptors on the surface of the target cancer cells than on the malignant self cells.
Therefore, the results of this study suggested that damnacanthal has a huge potential in stimulating the immune cells with little or no toxicity for healthy cells. However, further studies are needed to elucidate the exact mechanism of the reactions caused by damnacanthal. At the same time, it is vital to utilize multiple assays to allow a comprehensive evaluation of the immunomodulatory efficacy of this compound. In addition, pharmacokinetic studies are also fundamental to further evaluate the effective dose and toxicity effects of damnacanthal. Once the mechanisms of its actions and a comprehensive bioassay are established, damnacanthal could be used as a lead molecule for a new generation of drugs for boosting the immune system.
Declaration of interest
The author state that they have no conflict of interest. The authors thank the Ministry of Science, Technology and the Environment of Malaysia for funding the project under an IRPA grant 09-02-04-10033-EAR.
References
- Adam JK, Odhav B, Bhoola KD (2003): Immune responses in cancer. Pharmacol Ther 99: 113–132.
- Ali AM, Ismail N, Mackeen M, Yazan L, Mohamed S, Ho A, Lajis N (2000): Antiviral, cytotoxic and antimicrobial activities of antharaquinones isolated from the roots of Morinda elliptica. Pharm Biol 38: 3791–3801.
- Aoki K, Parent A, Zhang J (2000): Mechanism of damnacanthal-induced [Ca(2+)] (i) elevation in human dermal fibroblasts. Eur J Pharm 387: 119–124.
- Bryan DL, Forsyth KD, Gibson RA, Hawkes JS (2006): Interleukin-2 in human milk: A potential modulator of lymphocyte development in breastfed infant. Cytokine 33: 289–293.
- Cerqueira F, Cordeiro-Da-Silva A, Gaspar-Marques C, Simoes F, Pinto MMM, Nascimento MSJ (2004): Effect of abietane diterpenes from Plectranthus grandidentatus on T- and B-lymphocytes proliferation. Bioorg Med Chem 12: 217–223.
- Chandok MR, Farber DL (2004): Signalling control of memory T cell generation and function. Semin Immunol 16: 285–293.
- Farag SS, Caliguiri MA (2006): Human natural killer cells development and biology. Blood Rev 20: 123–137.
- Hiramatsu T, Imato M, Koyono T, Umezawa K (1993): Induction of normal phenotypes in Ras transformed cells by damnacanthal from Morinda citrifolia. Cancer Lett 73: 161–166.
- Hirazumi A, Furusawa E, Chou SC, Hokama Y (1994): Anti-cancer activity of Morinda citrifolia on intraperitoneally implanted Lewis lung carcinoma in syngenic mice. Proc West Pharmacol Soc 37: 145–146.
- Hirazumi A, Furusawa E, Chou SC, Hokama Y (1996): Immunomodulation contributes to the anti-cancer activity of Morinda citrifolia (noni) fruit juice. Proc West Pharmacol Soc 39: 7–9.
- Hirazumi A, Furusawa E, Chou SC, Hokama Y (1999): Immunomodulatory polysaccharide-rich substances from the fruit juice of Morinda citrifolia (noni) with anti-tumor activity. Phytother Res 13: 380–387.
- Hiwasa T, Arase Y, Chen Z, Kita K, Umezawa K, Ito H, Suzuki N (1999): Stimulation of ultraviolet-induced apoptosis of human fibroblast UV-1 cells by tyrosine kinase inhibitors. FEBS Lett 444: 173–176.
- Ismail NH, Ali AM, Aimi N, Kitajima M, Takayama H, Lajis NH (1997): Anthraquinones from Morinda elliptica. Phytochemistry 45: 1723–1725.
- Jasril K, Nordin HL, Lim YM, Abdullah MA, Sukari MA, Ali MA (2003): Antitumor promoting and antioxidant activities of anthraquinones isolated from the cell suspension culture of Morinda elliptica. Asia Pac J Mol Biol Biotechnol 11: 3–7.
- Kwanjai K, Somdej K, Ruchanee P (2005): Biological activity of anthraquinones and triterpenoids from Prismatomeris fragrans. J Ethnopharmacol 100: 184–288.
- Li J, Li Q, Feng T, Li K (2008): Aqueous extract of Solanum nigrum inhibit growth of cervical carcinoma (U14) via modulating immune response of tumor bearing mice and inducing apoptosis of tumor cells. Fitoterapia 79: 548–556.
- Loken MR (1980): Simultaneous quantitation of Hoechst 33342 and immunofluorescence on viable cells using a fluorescence activated cell sorter. Cytometry 1: 136–142.
- Manosroi A, Saraphanchotiwitthaya A, Manosroi J (2003): Immunomodulatory activities of Clausena excavata Burm. f. wood extracts. J. Ethnopharmacol 89: 155–160.
- Manuele MG, Ferraro G, Barreiro Arcos ML, López P, Cremaschi G, Anesini C (2006): Comparative immunomodulatory effect of scopoletin on tumoral and normal lymphocytes. Life Sci 79: 2043–2048.
- Masakazu K, Raymond PW, Dong SA, Jonathan PS, Robert D, Micheal EP, Jing H, Irvin, SYC (2006): Cell-based chemical genetic screen identifies damnacanthal inhibitor of HIV-Vpr induced cell death. Biochem Biophys Res Comm 384: 1101–1106.
- Mosmann T (1983): Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxic assay. J Immunol Methods 65: 55–63.
- Ong HC, Norzalina J (1999): Malay herbal medicine in Gemencheh, Negeri Sembilan, Malaysia. Fitoterapia 70:10–14.
- Stanilove SA, Dobreva ZG, Slavov ES, Mitera LD (2005): C3 binding glycoprotein from Cuscuta europea induced different cytokine profile from human PBMC compared to other plant and bacteria immunomodulators. Int Immunopharmacol 5: 727–734.
- Tosa H, Ilnuma M, Asai F, Tanaka T, Nozaki H, Fujimori S (1998): Anthraquinones from Neonauclea calycina and their inhibitory activity against DNA topoisomerase II. Biol Pharm Bull 21: 641–642.
- Wang MY, Su C (2001): Cancer preventive effect of Morinda citrifolia (noni). Ann NY Acad Sci 952: 161–168.
- Wang MY, West B, Jensen CJ, Nowicki D, Su C, Palu AK, Anderson G (2002): Morinda citrifolia (noni): A literature review and recent advances in noni research. Acta Pharmacol Sin 23: 1127–1141.
- Zhao M, Yang B, Wang J, Liu Y, Yu L, Jiang Y (2007): Immunomodulatory and anticancer activities of flavonoids extracted from litchi (Litchi chinensis Sonn.) pericarp. Int Immunopharmacol 7: 162–177.