Abstract
The present study evaluated the in vivo hepatoprotective activity of two medicinal plants, namely, Justicia schimperiana (Hochst. ex Nees) (Acanthaceae) and Verbascum sinaiticum Benth. (Scrophulariaceae) used in Ethiopian traditional medical practices for the treatment of liver diseases. The levels of hepatic marker enzymes, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) were used to assess their hepatoprotective activity against carbon tetrachloride (CCl4)-induced hepatotoxicity in Swiss albino mice. The results revealed that pretreating mice with the hydro-alcoholic extracts of both plants significantly suppressed the plasma AST ((P < 0.01) J. schimperiana; (P < 0.05) V. Sinaiticum) and ALT ((P < 0.05) J. schimperiana) activity when compared with the CCl4 intoxicated control. Among the Soxhlet extracts of each of the plants, the methanol extract of J. schimperiana showed significant hepatoprotective activity. Further fractionation of this extract using solid phase extraction and testing them for bioactivity indicated that the fractions did not significantly reverse liver toxicity caused by CCl4. However, the percentage hepatoprotection of the distilled water fraction was comparable with that of the standard drug silymarin at the same dose (50 mg/kg) as evidenced by biochemical parameters. Histopathological studies also supported these results. In vitro DPPH assay conducted on the water fraction of J. schimperiana and the Soxhlet methanol fraction of V. sinaiticum showed that they possess moderate radical scavenging activity (IC50 = 51.2 and 41.7 μg/mL, respectively) which led to the conclusion that the hepatoprotective activity of the plants could be in part through their antioxidant action.
Introduction
Liver diseases remain serious health problems and are caused, among others, by drugs, chemicals, and alcohol. Chronic liver disease is a major cause of morbidity and mortality throughout the world. Conventional medical therapy for many common liver disorders, including non-alcoholic fatty liver disease and viral hepatitis, has limited efficacy and potentially life-threatening side effects. This has increased dependence on complementary and alternative medicine (CAM), especially herbal therapy. Various medicinal plants and their formulations are used in traditional medicine for their hepatoprotective effects and a number of herbal preparations are available on the market. The most common medicinal plants and herbal preparations used for the management of liver diseases include Phyllanthus spp. (Euphorbiaceae), Silybum marianum (L.) Gaertn (milk thistle) (Asteraceae), glycyrrhizin (licorice root extract), and Liv 52 (a mixture of herbs). Most of these herbal drugs were shown to speed up the natural healing process of the liver (CitationDhiman & Chawla, 2005; CitationMani Senthilkumar et al., 2005).
The biodiversity of the Ethiopian flora offers great possibilities in the search for natural products with novel structures that have a range of biological activities including treatment of various liver disorders (CitationAsres et al., 2001a).
Justicia schimperiana (Hochst. ex Nees) (syn. Adhatoda schimperiana (Hochst. ex Nees) Andreson) (Acanthaceae), is an erect shrub up to 4 m high with woody and internodal stem (CitationAbebe et al., 2003). The plant is very common in villages and towns as well as in cities growing on waste places or grown as a hedge and used as a boundary marker around native compounds. The plant is relatively fast growing and prefers altitudes of 2400 m or above (CitationGetahun, 1976). In Ethiopian traditional medicine, the whole plant is used as a remedy for excessive pellagra, as a laxative (CitationGetahun, 1976), as well as for the treatment of stomach complaints, hepatitis, venereal diseases, malaria, asthma, leishmaniasis and cough (CitationAsres et al., 2001b); jaundice, epilepsy, mental illness and leprosy (CitationAbebe & Ayehu, 1993). CitationGeyid et al. (2005) reported the presence of polyphenols, unsaturated sterol/triterpenes and saponins in the methanol extract of the dried leaves of J. schimperiana.
Verbascum sinaiticum Benth (Scrophulariaceae) is an erect herb reaching up to 2 m high. The whole plant is densely shaggy, grayish and its leaves are simple and broadly ovate-oblong (CitationAbebe et al., 2003). In Ethiopian traditional medicine, the plant is used in the treatment of syphilis, tumor, liver diseases, stomach troubles, diabetes, scabies, amoebiasis, diarrhea, epilepsy, and cough (CitationAbebe et al., 2003). CitationAfifi et al. (1993) reported the presence of two flavonolignans, hydrocarpin and sinaiticin, as well as two flavones, chrysoeriol and luteolin, in the leaves of V. sinaiticum. All these compounds were shown to have dose-dependent cytotoxic effect when tested against cultured P-388 cells (a murine leukemia cell line). Phytochemical and biological studies on V. sinaiticum growing in Egypt have revealed the presence two iridoids: ajugol and aucubin in the aerial parts of the plant. Moreover, investigation of the flavonoid constituents led to the isolation and identification of luteolin and chrysoeriol-7-glucoside. Both the alcohol and methylene chloride/methanol (1:1) extracts were shown to have hepatoprotective effect against carbon tetrachloride (CCl4) induced cytotoxicity at 25 and 10 μg/mL for the alcoholic and CH2Cl2/MeOH extracts, respectively. The study attributed hepatoprotective activity to the presence of iridoid compounds which constitute the major components of the CH2Cl2-MeOH (1:1) extract (CitationMahmoud et al., 2007).
Despite the significant popularity of several herbal medicines in general, and those used for the management of liver diseases in particular, they have not yet become acceptable treatment modalities. The present study was therefore conducted to test the hepatoprotective activities of the aforementioned two medicinal plants used in Ethiopian traditional medical practices for the treatment of liver diseases.
Materials and methods
Plant materials and extraction
The leaves of J. schimperiana and V. sinaiticum were collected in October 2005 from Yeka subcity, around Bella, Addis Ababa, Ethiopia. The identity of each plant specimen was confirmed by Melaku Wondafrash at the National Herbarium, Department of Biology, Addis Ababa University, Ethiopia, where voucher specimens were deposited (J. schimperiana, US 001 and V. sinaiticum, US 004).
The required plant parts were shade-dried and powdered. The powder of each plant (100 g) was separately extracted with 80% methanol by maceration for 72 h (three times) with frequent agitation and the resulting liquid was filtered (Whatman No. 3 filter paper). The combined extracts were concentrated under reduced pressure at a temperature not exceeding 40°C. The concentrated extract was then dried in an oven at 40°C for about 48 h. The resulting dried mass of each plant (yield: 15.8% for J. schimperiana, and 21.1% for V. sinaiticum) was then powdered, packed into a glass vial, properly labeled and stored in a desiccator over silica gel until use.
J. schimperiana and V. sinaiticum powders (20 g each) were separately and sequentially extracted with petroleum ether, chloroform, acetone and methanol using Soxhlet apparatus. The solvents were evaporated under reduced pressure and the fractions were then placed in a vacuum oven at 30°C for about 24 h to remove any residual solvent. The resulting semisolid mass of each fraction was stored in a desiccator until use under the same conditions as the crude extracts.
Fractionation by solid-phase extraction
Further fractionation of the methanol extract of J. schimperiana was carried out by solid phase extraction on Isolute C18 (10 g, IST, Hengoed, Wales) using MeOH-H2O gradient. The methanol extract (500 mg) obtained from Soxhlet extraction was eluted first with 50 mL of distilled water followed by the same amount of 25%, 50%, and 75% methanol solutions in water and 100% methanol, successively to give fractions designated A0, A1, A2, A3 and A4, respectively. Each fraction was concentrated and dried following the same procedure as in the crude extracts and kept in a desiccator for future use.
Animals
Swiss albino mice of either sex, weighing 22–30 g and aged 6–8 weeks were used for the experiments. The animals were purchased from the Ethiopian Health and Nutrition Research Institute (EHNRI), Addis Ababa, and the National Veterinary Institute, Debrezeit. All animals were maintained at a temperature of 22° ± 2°C and with a 12 h light/12 h dark cycle and given pellet diet and water ad libitum. The animals were acclimatized to their new environment for at least one week prior to the experiments. All procedures performed were reviewed and approved by the Institutional Review Board and conform to internationally accepted principles.
Acute toxicity testing
Thirty-five Swiss albino mice were divided into seven groups (I-VII) of five animals each. Group I received distilled water 10 mL/kg orally and animals from Groups II to VI were given hydro-alcoholic extracts of J. schimperiana at doses of 200, 300, 400, 500, 1000 mg/ kg orally, respectively. Group VII received 2000 mg/kg hydro-alcoholic extracts of V. sinaiticum p.o. Symptoms of toxicity and mortality were observed for 24 h. The behavioral and CNS profiles such as spontaneous rearing and grooming, evidence of calmness and sedation, and loss of writhing reflex were also observed.
In vivo hepatoprotective activity testing
In vivo hepatoprotective activity was evaluated on the basis of CCl4-induced liver damage in mice as previously described by CitationMansour (2000). The animals were divided into six groups of six animals (). Group I was kept on normal diet and served as normal control and received distilled water (10 mL/kg, p.o.) daily for 5 days and then given olive oil (3 mL/kg, i.p.) on day 5, 30 min after receiving distilled water; Group II served as CCl4 control group, and received distilled water (10 mL/kg, p.o.) daily for 5 days and then received CCl4: olive oil (1:120; 3 ml/kg, i.p.) on day 5, 30 min after administration of distilled water. Groups III and IV, V and VI were treated with total extracts of the study plants at a dose of 200 mg/kg body weight daily for 5 days. Groups III and V further received olive oil (3 mL/kg, i.p.) on day 5, 30 min after receiving extract (CitationWilliamson et al., 1998) whereas Groups IV and VI, received CCl4: olive oil (1:120; 3 mL/kg, i.p.) on day 5, 30 min after administration of the extract. All of the animals were restricted to water after administration of CCl4 or olive oil on day 5. Twenty-four h after the i.p. administration of CCl4 in olive oil or olive oil alone, the mice were anesthetized with ether, and their blood was collected directly from the heart and allowed to clot for 30 min at room temperature.
Table 1. Effects of the hydro-alcoholic extracts of Justicia schimperiana and Verbascum sinaiticum at a dose of 200 mg/kg on activities of serum enzymes in mice injected with CCl4 in olive oil (25 µL/kg), i.p.
Biochemical assays
Serum was separated by centrifugation at 3000 rpm for 15 min and liver damage was assessed by estimation of serum activities of alanine aminotransferase (ALT) (CitationBergmeyer, 1980), aspartate aminotransferase (AST) (CitationBergmeyer et al., 1976) and alkaline phosphatase (ALP) (CitationTietz et al., 1983), using commercially available test kits and an automatic blood biochemical analyzer (Bioanalyzer, SABA PM 4000; Rome, Italy).
The chloroform (Group C & F), acetone (Group D & G) and methanol (Group E & H) extracts from the Soxhlet extraction of J. schimperiana and V. sinaiticum, at a dose of 200 mg/kg, and silymarin (Group I) (standard drug) at a dose of 50 mg/kg, were tested for hepatoprotective activity following the above procedure (). Similarly, the 100% water fraction of J. schimperiana at doses of 50 and 100 mg/kg; 25% methanol fraction at a dose of 50 mg/kg; 100% methanol fraction at a dose of 50 mg/kg; and silymarin at a dose of 50 mg/kg, were also tested for hepatoprotective activity following the above procedure. In addition, in this case, the livers were harvested, grossly examined and preserved in 10% formalin solution for histological examination.
Histopathological study
Small pieces of the liver, fixed in 10% buffered formalin, were processed for embedding in paraffin. Sections of 5–6 µm were cut and stained with hematoxylin and eosin (H&E) and examined under the microscope for histopathological changes. Images were captured using an Olympus DP12 CCD camera at original magnification of 10 × (Olympus DP12 Microsystems Digital Imaging Olympus, Japan).
Dose-dependent activity study
The methanol fraction obtained from the Soxhlet extraction of J. schimperiana was tested against CCl4-induced liver injury in mice at dose levels of 100 (Group b), 200 (Group c), 300 (Group d), and 400 mg/kg (Group e) following the above procedure.
In vitro DPPH assay
The hydrogen-donating or radical-scavenging capacities of the 100% water fraction of J. schimperiana and the methanol fraction of V. sinaiticum were measured using the stable radical, DPPH. following the method given by CitationBlois (1958). The samples were diluted with methanol to prepare solutions equivalent to 2,000, 1,000, 500, 250, and 125 μg of dried sample per mL of solutions. An aliquot of 0.004% DPPH solution (5 mL) in methanol was pipetted into a test tube followed by the addition of 50 μL of a sample solution. The mixture was incubated at 37°C for 30 min in the dark and its absorbance at 517 nm was measured (Jenway Model 6500 spectrophotometer). The percentage inhibition of DPPH radicals was calculated using the following equation:
where, Ao is absorbance of the control, and As is absorbance of the sample at 517 nm.
The IC50 values were calculated by linear regression of the plots, where the x-axis represented the concentration of the test plant extracts and the y-axis represented the average percentage of radical-scavenging activity from three replicates.
Statistical analysis
All values are expressed as mean ± SEM. The data were analyzed by one-way analysis of variance (ANOVA) and Tukey post test. The hepatoprotective activity of the test material was calculated using the following formula:
where, a is the mean value of the marker produced by hepatotoxin; b is the mean value of the marker produced by toxin plus test material; and c is the mean value produced by the vehicle control (CitationGarg et al., 1994). P-values < 0.05 were considered significant.
Results and discussion
CCl4 is one of the oldest and most widely used toxins for experimental induction of liver fibrosis in laboratory animals. CCl4 requires bioactivation by the cytochrome P450 system of phase I in liver and yields the reactive metabolite trichloromethyl radical (CCl3˙) and peroxy trichloromethyl radical (CCl3O˙) Peroxidation of membrane lipids secondary to the formation of the CCl˙ and/or CCl3OO˙ radicals is believed to be the basis for the toxic effect of CCI4 (CitationBahcecioğlu et al., 1999). Both trichloromethyl and its peroxy radical are capable of binding to proteins or lipids, or of abstracting hydrogen atoms from polyunsaturated fatty acid (PUFA), initiating lipid peroxidation thus causing damage to cell membrane, changing enzyme activity and finally inducing hepatic injury or necrosis (CitationKumar et al., 2005; CitationHung et al., 2006). The method was chosen for this study because CCl4-induced liver injury resembles the damage caused by acute viral hepatitis, and also evaluation of the preventive action in liver damage induced by CCl4 has been widely used for hepatoprotective drug screening. Damage to the structural integrity of the liver by CCl4 is reflected by an increase in the activities of AST, ALT, ALP, and LDH, which are released into the circulation after cellular damage. CCl4 also induces ample infiltration of the lymphocytes and Kupffer cells, massive centrilobular necrosis, sinusoidal dilatation and congestion (CitationGalati et al., 2005).
Hepatoprotective activity of the hydro-alcoholic extracts
In the present study, treating the mice with CCl4 at a dose of 25 µL/kg i.p. caused hepatocellular damage as indicated by an increase in plasma AST and ALT activity (). Pretreating mice with the hydro-alcoholic extracts of J. schimperiana significantly suppressed the plasma AST (P < 0.01) and ALT (P < 0.05) activity when compared with the CCl4 intoxicated control. Similarly pretreating mice with the hydro-alcoholic extracts of V. sinaiticum also significantly suppressed the plasma AST (P < 0.05) activity when compared with the CCl4-intoxicated control.
The results of the present study indicated that there were no significant changes in the activities of serum AST and ALT levels in mice which were given hydro-alcoholic extracts of J. schimperiana and V. sinaiticum, at a dose of 200 mg/kg p.o. for 5 days without the hepatotoxin CCl4 (). However, a significant increase (P < 0.001) in the activities of serum enzymes occurred 24 h after exposure to CCl4 in the hepatotoxin only groups, which was not observed when the total extracts of J. schimperiana and V. sinaiticum were administered. As shown in , the hydro-alcoholic extracts of J. schimperiana and V. sinaiticum displayed significant protective effects on CCl4-induced liver damage in mice. Therefore, the total extracts of these plants were subjected to solvent fractionation by Soxhlet extraction.
Hepatoprotective activity of Soxhlet extracts
As shown in , mice treated with a single dose of CCl4 in olive oil developed significant hepatic damage as observed from elevated serum levels of hepatospecific enzymes. However, pretreatment of mice with different Soxhlet extracts of J. schimperiana revealed that the methanol extract significantly (P < 0.05) suppressed the plasma AST, ALT and ALP activities when compared with the CCl4-intoxicated control. Similar effects were observed for the methanol extract of V. sinaiticum on plasma AST level. It can, therefore, be said that the active constituents of the plants reside mainly in the methanol extract and that the compounds are polar in nature. Moreover, the application of heat might have facilitated the solubility of these compounds (CitationVontagu et al., 2004).
Table 2. Effects of the different Soxhlet extracts of Justicia schimperiana and Verbascum sinaiticum at a dose of 200 mg/kg on activities of serum enzymes in mice injected with CCl4 in olive oil (25 µL/kg), i.p.
As can be seen from the overall percentage hepatoprotection, the methanol extract of J. schimperiana has a statistically significant hepatoprotective activity compared to the methanol extract of V. sinaiticum. As shown in , the results of the present study revealed that the methanol extract of J. schimperiana possesses somewhat comparable hepatoprotective activity with that of the standard drug silymarin and was therefore, selected for further fractionation using solid phase extraction.
Hepatoprotective activity of the solid phase fractions of J. schimperiana
The different fractions obtained from solid phase extraction of J. schimperiana did not show significant protective activity compared to the CCl4 control (). The results on the hepatoprotective activity of the fractions from solid phase extraction of J. schimperiana clearly showed that preadministration of A0 (distilled water fraction) at doses of 50 and 100 mg/kg did not significantly reverse the increase in serum enzymes AST, ALT and ALP caused by administration of CCl4. As can be seen from the percentage protection data, the plant extract displayed better activity at a dose of 50 mg/kg than at 100 mg/kg. This could be due to intrinsic toxicity of the compound(s) responsible for hepatoprotective activity at higher doses. A dose-dependent activity study also supported this finding. At an equivalent dose (50 mg/kg), the hepatoprotective activities of A0 and silymarin were shown to be comparable.
Table 3. Effects of fractions from the solid phase extraction of the methanol extract of Justicia schimperiana on activities of serum enzymes in mice injected with CCl4 in olive oil (25 µL/kg), i.p.
Dose-dependent activity study
An experiment was carried out to check whether the hepatoprotective activity of J. schimperiana was dose-dependent or not. Thus, pretreatment of mice with different doses of the methanol fraction obtained by Soxhlet extraction showed that hepatoprotective activity decreased with increasing dose (). But statistical data revealed that there is no significant difference in the hepatoprotective activity among the test groups.
Table 4. Effects of the methanol extracts of Justicia schimperiana on activities of serum enzymes in mice injected with CCl4 in olive oil. (25 µL/kg), i.p.
As shown in , the percent hepatoprotection is relatively low at higher doses. This could be due to inherent toxicity of some components which may be present together with the hepatoprotective principles or the hepatoprotective principles themselves might be toxic at higher doses.
Histopathological study
Histopathological assessment of liver damage was carried out by studying hematoxylin and eosin stained slides of liver tissue. shows the severe hepatic lesions caused by CCl4. Major centrilobular and hepatocellular necrosis as well as major critical disorganization of the complete lobules accompanied by fatty changes and ballooning degeneration were observed in hepatocytes of the livers of mice treated with CCl4 (Group VII). Moreover, the lipid peroxidation and degree of damage is very intense in this group of animals compared to the other groups. As is apparent in , the severe hepatic lesions induced by CCl4 were remarkably lowered by the administration of A0 (distilled water fraction of J. schimperiana, 50 mg/kg), which is in good agreement with the results of the biochemical analysis. The vehicle control group () showed the normal parenchymal architecture with cords of hepatocytes, portal tracts and central veins without noticeable gross alterations except for light congestion of blood vessel compared to the CCl4 control. The toxin mediated changes in livers of mice pretreated with plant extracts Group II (), Group III () and the standard drug silymarin, Group VI (), 24 h after the administration of CCl4, were much less in intensity than those observed in the livers of CCl4-treated mice (). Though the extent of cellular necrosis was less pronounced compared to the CCl4 group, increase in liver size, disorganization of the lobules, reactive inflammatory processes and necrosis with acidophilic cells, were observed in animals of Group III (), Group D () and Group V () compared to groups I, II and VI. In the liver of animals of Group V () there were also intense tumefaction and fatty changes which were an indication of severe damage compared to the other groups with the exception of Group VII animals ().
Figure 1. Representative microphotographs of H&E (×10)-stained histological sections of liver (1A) CCl4 control; (1B) mice treated with fraction A0 (distilled water fraction of Justicia schimperiana), 50 mg/ kg, p.o. and CCl4 (25 µL/kg, i.p.); (1C) vehicle control; (1D) mice treated with A0, 100 mg/kg and CCl4 (25 µL/kg, i.p.); (1E) mice treated with silymarin 50 mg/kg, p.o. and CCl4 (25 µL/kg, i.p.); (1F) mice treated with A1 (25% methanol fraction of Justicia schimperiana), 50 mg/kg and CCl4 (25 µL/kg, i.p.); (1G) mice treated with fraction A4 (pure methanol fraction of Justicia schimperiana) 50 mg/kg, p.o. and CCl4 (25 µL/kg, i.p.).
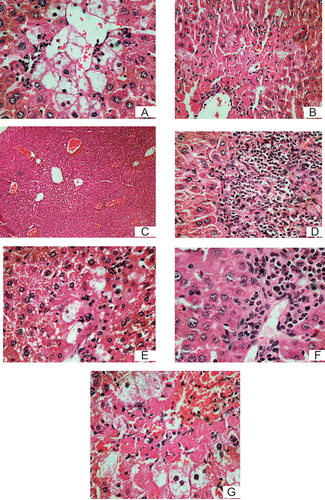
The results of these histopathological findings clearly indicated that the extent of cellular damage was less in animals of Group II and Group VI compared to the CCl4 group and animals of Groups III, IV, and V. This indicates pre-administration of fraction A0 or silymarin at 50 mg/kg has significantly protected the livers of the animals from CCl4 induced damage. Necrosis, which is a more severe form of damage, was also markedly minimized by the pretreatment of the animals with 50 mg/kg of A0 (). In addition, the results of histopathological studies also showed that the degree of protection of A0 is much higher at 50 mg/kg than it is at a dose of 100 mg/kg, which is in good agreement with the results of the serum transferase activities.
In vitro DPPH Assay
Many hepatotoxicants, including CCl4, nitrosamines, and polycyclic aromatic hydrocarbons require metabolic activation, particularly by the liver cytochrome P450 enzymes, to form reactive, toxic metabolites, which in turn cause liver injury in experimental animals and humans. Oxidative damage triggered by CCl4 is believed to be due to the formation of reactive peroxy radicals that are initiators of lipid peroxidation chain reaction, which subsequently provoke inflammatory reactions and hence destruction and damage to cell membrane (CitationShahjahan et al., 2005). Thus, natural compounds that reduce the chemical activating enzymes, or that scavenge free radicals generated, might be good candidates for protection against chemically induced liver toxicity (CitationLee et al., 2004). Also, reports show that many compounds known to be effective against CCl4-mediated liver injury exert their protective action either via a decreased production of CCl4-derived free radicals or through antioxidant activity of the protecting agents themselves (CitationHewawasam et al., 2003).
Thus, in an attempt to delineate the possible mechanisms involved in the hepatoprotective action of J. schimperiana and V. sinaiticum against CCl4-induced hepatotoxicity in mice, the antioxidant activity of the extracts was investigated using in vitro DPPH assay. The water fraction (A0) obtained by solid phase extraction of J. schimperiana, and the methanol fraction obtained from Soxhlet extraction of V. sinaiticum, displayed dose-dependent free radical scavenging activity with IC50 values of 51.2 and 41.7 µg/mL, respectively. Therefore, the hepatoprotection of J. schimperiana and V. sinaiticum against CCl4-induced hepatotoxicity in mice, in part, could be attributed to the free radical scavenging activity of their extracts.
Acute toxicity study
The results of acute oral toxicity studies in mice indicated that the hydro-alcoholic extract of J. schimperiana cause no death or sign of toxicity up to a dose of 1 g/kg of body weight. One of the animals however, showed symptoms of excitation at a dose of 1 g/kg.
Conclusion
The present study suggests that the hydro-alcoholic extracts and methanol fractions of J. schimperiana and V. sinaiticum, at a dose of 200 mg/kg, possess potent hepatoprotective activity in CCl4-induced liver injury in Swiss albino mice. The methanol extracts of J. schimperiana and V. sinaiticum possess antioxidant activities and may inhibit the deleterious effect of free radicals generated by CCl4. These observations provide biochemical data supporting the traditional uses of J. schimperiana and V. sinaiticum in Ethiopia for the treatment of some hepatic disorders and suggest the possible utilization of these plants as a source of new drugs.
Acknowledgements
The authors would like to express their utmost gratitude to Professor Franklin Perry, Department of Anatomy and Histopathology, Faculty of Medicine, Addis Ababa University, for carrying out the histo-pathological analysis of the liver and to Melaku Wondafrash of the National Herbarium, Department of Biology, Addis Ababa University, for identification of the plant materials.
Declaration of interest
This work was supported by the Office of Graduate Studies and Research of Addis Ababa University.
References
- Abebe D, Ayehu, A (1993): Medicinal Plants and Enigmatic Health Practices of Northern Ethiopía, Addis Ababa, BSPE, pp. 68, 71, 80, 87, 88, 150, 151, 203.
- Abebe D, Debella A, Urga K (2003): Medicinal Plants and Other Useful Plants of Ethiopía, Nairobi, Camerapix, pp. 10, 102, 103.
- Afifi MSA, Ahmed MM, Pezzuto JM, Kinghorn AD (1993): Cytotoxic flavonolignans and flavones from Verbascum sinaiticum leaves. Phytochemistry 34: 839–841.
- Asres K, Bucar F, Edelsbrunner S, Kartnig T, Höger G, Thiel W (2001a): Investigations on antimycobacterial activity of some Ethiopian medicinal plants. Phytother Res 15: 323–326.
- Asres K, Bucar F, Kartnig T, Witvrouw M, Pannecouque C, De Clercq E (2001b): Antiviral activity against human immunodeficiency virus type 1 (HIV-1) and type 2 (HIV-2) of ethnobotanically selected Ethiopian medicinal plants. Phytother Res 15: 62–69.
- Bahcecioğlu H, Ustundag B, Ozercan I, Ercel E, Baydas G, Akdere T, Demir A (1999): Protective effect of Ginkgo biloba extract on CCI4-induced liver damage. Hepatol Res 15: 215–224.
- Bergmeyer HU (1980): IFCC methods for the measurement of catalytic concentrations of enzymes. Part 3. IFCC method for alanine aminotransferase (l-alanine:2-oxoglutarate aminotransferase). Clin Chim Acta 105: 145F–172F.
- Bergmeyer HU, Bowes GN Horder M Moss DW (1976): Provisional recommendations on IFCC methods for the measurement of catalytic concentrations of enzymes. Part 2. IFCC method for aspartate aminotransferase. Clin Chim Acta 70: F19–F29.
- Blois MS (1958): Antioxidant determinations by the use of a stable free radical. Nature 181: 1199–200.
- Dhiman RK, Chawla YK (2005): Herbal medicines for liver diseases. Dig Dis Sci 50: 1807–1812.
- Galati EM, Mondello MR, Lauriano ER, Taviano MF, Galluzzo M, Miceli N (2005): Opuntia ficus-indica (L.) Mill. fruit juice protects liver from carbon tetrachloride induced injury. Phytother Res 19: 796–800.
- Garg HS, Bhandari SP, Tripathi SC (1994): Antihepatotoxic and immunostimulant properties of iridoid glycosides of Scrophularia koelzii. Phytother Res 8: 224–228.
- Getahun A (1976): Some Common Medicinal and Poisonous Plants Used in Ethiopian Folk Medicine. Addis Ababa, Faculty of Science, Addis Ababa University, pp. 2–3.
- Geyid A, Abebe D, Debella A, Makonnen Z, Aberra F, Teka F, Kebede T, Urga K, Yersaw K, Biza T, Haile Mariam B, Guta M (2005): Screening of some medicinal plants of Ethiopia for their antimicrobial properties and chemical profiles. J Ethnopharmacol 97: 421–427.
- Hewawasam RP, Jayatilak AP, Pathirana C, Mudduwa LK (2003): Protective effect of Asteracantha longifolia extract in mouse liver injury induced by carbon tetrachloride and paracetamol. J Pharm Pharmacol 55: 1413–1418.
- Hung MY, Fu TY, Shih PH, Lee CP, Yen GC (2006): Du-Zhong (Eucommia ulmoides Oliv.) leaves inhibit CCl4-induced hepatic damage in rats. Food Chem Toxicol 44: 1424–1431.
- Kumar RS, Sivakumar T, Sivakumar P, Nethaji R, Vijayabasker M, Perumal Gupta, MP, Mazumder UK (2005): Hepatoprotective and in vivo antioxidant effects of Careya arborea against carbon tetrachloride induced liver damage in rats. Intl J Mol Med Adv Sci 1: 418–424.
- Lee KJ, Woo ER, Choi CY, Shin DW, Lee DG, You HJ, Jeong HG (2004): Protective effect of acetoside on carbon tetrachloride-induced hepatotoxicity. Life Sci 74: 1051–1064.
- Mahmoud SM, Abdel-Azim NS, Shahat AA, Ismail SI, Hammouda FM (2007): Phytochemical and biological studies on Verbascum sinaiticum growing in Egypt. Nat Prod Sci 13: 186–189.
- Mani Senthilkumar KT, Rajkapoor B, Kavimani S (2005): Protective effect of Enicostemma littorale against CCl4-induced hepatic damage in rats. Pharm Biol 43: 485–487.
- Mansour MA (2000): Protective effects of thymoquinone and desferrioxamine against hepatotoxicity of carbon tetrachloride in mice. Life Sci 66: 2583–2591.
- Shahjahan M, Vani G, Devi CS (2005): Protective effect of Indigofera oblongifolia in CCl4-induced hepatotoxicity. J Med Food 8: 261–265.
- Tietz NW, Rinker AD, Shaw LM (1983): IFCC methods for the measurement of catalytic concentration of enzymes. Part 5. IFCC method for alkaline phosphatase (orthophosphoricmonoester phosphohydrolase, alkaline optimum, EC 3. 1. 3. 1). J Clin Chem Clin Biochem 21: 731–748.
- Vontagu HO, Abbah J, Nagazal IE, Kunle OF, Chindo BA, Otsapa PB, Gamaniel KS (2004): Anti-nociceptive and anti-inflammatory activities of the methanolic extract of Parinari polyandra stem bark in rats and mice. J Ethnopharmacol 90: 115–121.
- Williamson EM, Okpako DT, Evans JF (1998): Selection, Preparation and Pharmacological Evaluation of Plant Material. Chichester, John Wiley, pp. 50–51.
