Abstract
This study was designed to investigate the effect and molecular mechanisms of Haishengsu (HSS), a protein extract from a shellfish Tegillarca granosaL., on a drug resistant leukemia cell line. Cultured K562/Adriamycin (ADM) cells were treated with HSS at 10, 20 and 40 μg/mL, respectively. The apoptosis and expression of p-glycoprotein was evaluated by flow cytometry. Expressions of caspase-3 and Bcl-2 were also evaluated. There was a significant dose-dependent increase in the apoptosis in the HSS treated K562/ADM cells (P < 0.05 and 0.01, respectively). The p-glycoprotein expression in the 40 μg/mL HSS group (14.8%) was lower than in the control (16.9%, P < 0.05) and the 10 μg/mL HSS group (7.3%, P < 0.05), but it was similar to the HSS 20 μg/mL group (10.7%, P > 0.05). The expressions of apoptosis-stimulating protein caspase-3 protein were increased, whereas the expressions of apoptosis-suppressing Bcl-2 were decreased in the HSS groups, as compared with the levels in the control group (P < 0.05). We conclude that HSS induces apoptosis of the Adriamycin-resistant K562/ADM cells. The enhanced expressions in caspase-3 and the reduced expressions in Bcl-2 protein may have contributed to the apoptosis-stimulating effect of HSS. The inhibition of p-glycoprotein suggests that HSS may diminish the resistance to Adriamycin and potentially enhance the therapeutic effects.
Introduction
Tegillarca granosaL. (order Arcoida), which is rich in protein and vitamin B12, has been widely used as a traditional Chinese medicine in mainland China to treat cancer for more than a century. However, the pharmacological ingredients responsible for the anticancer effects of Tegillarca granosa have not been investigated with modern scientific approaches.
Several proteins have been purified from Tegillarca granosa in recent years, and their pharmacological actions have been assessed in vitro and in vivo. One protein with a molecular weight of 7.7 kDa was found to be active against blood coagulation factor Va, prolonging thrombin time (CitationJung et al., 2007). Haishengsu (HSS) is another purified protein with a molecular weight of approximately 15 kDa but an unknown chemical structure. HSS had a potent suppressive effect on several types of tumor cells in vivo and in vitro (CitationYao et al., 2005; CitationLiu et al., 2004; CitationZhang et al., 2005; CitationLi et al., 2008). HSS was found to inhibit tumor cell line Ketr-3 and A549, blocking the cell cycle at phase G0/G1 and G2/M (CitationWang et al., 2006). It suppressed the growth of S180 tumor cells in rats by up to 55% (CitationWang et al., 2006). Our recent study found that HSS suppresses the growth of leukemia K562 cell by inhibiting the G0/G1 and S phases of the cell cycle (Li et al., 2007). Use of HSS also induced apoptosis in these leukemia cells by reducing the expression of apoptosis suppressor Bcl-2, and by increasing the expression of apoptosis promoting Bax (Li et al., 2007).
The in vivo anticancer effects of HSS have been investigated in two small clinical trials. Daily intravenous administration of 2.4 mg HSS for 4 weeks resulted in complete or partial remission of non-small-cell lung cancer in 49% of the patients (CitationZhang et al., 2005). Similar remission rate was also found in 27 patients with renal cancer (CitationZhang et al., 2005). There were no significant adverse effects in patients who received the 4-week HSS treatment (CitationZhang et al., 2005). In a more recent randomized, double-blind, placebo-controlled trial, the effects of HSS as an adjunct therapy of conventional chemotherapeutic regimens were assessed in 83 patients with non-small-cell lung cancer. It was found that the remission rate in the HSS group was 23% higher than in the placebo group, and the prevalence of chemotherapy-induced nausea or vomiting was significantly reduced in the HSS-treated patients (CitationLi et al., 2008).
Resistance to chemotherapy is one of the major obstacles to the effective treatment of chronic myeloid leukemia. Patients with advanced chronic myeloid leukemia have been less sensitive to therapy, and responses have been short lived (CitationKantarjian et al., 2006). In addition, treatment resistance is an emerging problem at all disease stages (CitationKantarjian et al., 2006). K562 cell line is a blast crisis cell line of chronic myeloid leukemia (CitationKlein et al., 1976). K562/ADM cells are drug resistant strains of K562 cells induced by doxorubicin (Adriamycin) (CitationTsuruo et al., 1986). The primary purpose of the present study was to investigate whether HSS could inhibit drug resistance in the K562/ADM cells and, if so, what potential molecular mechanisms may be involved in this beneficial effect. The effect of HSS on the rate of apoptosis was also evaluated.
Materials and methods
Cell lines and cell culture
This study was approved by the institutional review board of Liaocheng People’s Hospital, Taishan Medical College. K562/ADM cells were obtained from the Institute of Hematology, Chinese Academy of Medical Science (Tianjin, China). These cells were cultured in Roswell Park Memorial Institute (RPMI 1640) culture medium containing 10% fetal calf serum (Gibco, Beijing, China; SANYO, Wood Dale, IL, USA) at 37°C in an incubator (SANYO) of saturated humidity. This drug-resistant leukemia cell line was derived from the parental K562 cell line by continuous exposure to increasing concentrations of adriamycin (up to 20 μg/mL). The multi-drug resistant phenotype is not expressed by K562 but is over expressed in K562/ADM (CitationYang et al., 1995). Drug resistance of the K562/ADM cells was maintained by adding 1 μg/mL of Adriamycin. Adriamycin was ceased three days before the cell line was used for this study.
Apoptosis in the K562/ADM cells
Annexin-V assay was used to detect apoptosis in the K562/ADM cells. This protocol involved the following groups: control (K562/ADM cells only) and three HSS groups (10, 20, and 40 μg/mL). The three HSS doses were chosen after a pilot study found that the minimum effective concentration was approximately 10 μg/mL. HSS was obtained from its manufacturer, Haisheng Oncology Hospital of Qingdao City, China. The authenticity of HSS was verified by the investigators and a voucher specimen number (990211) was recorded. K562/ADM cells were washed twice with ice cold phosphate-buffered saline (PBS) at 4°C. The washed cells were re-suspended with 250 μL of Annexin-V buffer. The cell concentration was adjusted to 1 × 106/mL. The cell solution (100 μL) was added into a 5 mL centrifuge tube, which was supplemented by 5 μL of Annexin-V-Fluorescein isothiocyanate (FITC) (Jingmei Biological, Shengzhen, China) and 10 μL iodized aziridine. The tube was incubated for 15 min before adding 400 μL PBS into the tube. The apoptosis rate was examined by flow cytometry (Beckman-XL, Shanghai, China).
The expression of p-glycoprotein in K562/ADM cells
K562 and K562/ADM cells were seeded in 96-well flat bottom plates (5~10 × 105 cells per well) and were incubated for four hours at 37°C in culture medium (Gibco), in the absence (control group) or in the presence of HSS at three concentrations (10, 20, and 40 μg/mL). p-Glycoprotein monoclonal antibody (1:200, 10 μL, Institute of Hematology, Chinese Academy of Medical Science) was subsequently added to each well. After 30 min at 4°C, cells were washed with PBS and the medium were removed. The second antibody (fluorescence-labeled rabbit anti-mouse antibody, 1:200, 20 μL, Institute of Hematology, Chinese Academy of Medical Science) was added to each well. After 30 min at 4°C, cells were washed twice with PBS; 200 μL of the cell solution was examined by flow cytometry for p-Glycoprotein expression, using the method reported by CitationFord et al. (2003). p-Glycoprotein expression was quantified by the percentage of p-glycoprotein-positive cells and the absolute number of cells per μL.
Examination for apoptosis-related protein caspase-3 and Bcl-2
To measure caspase-3, after washing the cells from the control and three HSS groups with PBS, 0.5 mL of ice-cold cell lysis buffer (Beyotime, Shanghai, China) containing 1 mM phenylmethyl sulfonyl fluoride (PMSF) was added to the plate (100 mm) on ice. After 5 min, cells were scraped off the plate, sonicated on ice and microcentrifuged for 10 min at 4°C. The supernatant was collected and applied to cleaved caspase-3 sandwich enzyme linked immunosorbent assay (ELISA) kit (Beyotime, Shanghai) according to the manufacturer’s instructions. Bcl-2 expression was examined by using an immunocytochemical kit (SP-20002, Zhongshan Golden Bridge, China).
Statistical analysis
Data were expressed as means ± SD. SAS6.12 software was used for data analysis. Numerical data were analyzed with one-way ANOVA. Categorical data were analyzed with Chi-square test. P < 0.05 and < 0.01 was considered statistically significant for numerical data, and P < 0.05 was considered statistically for categorical data.
Results
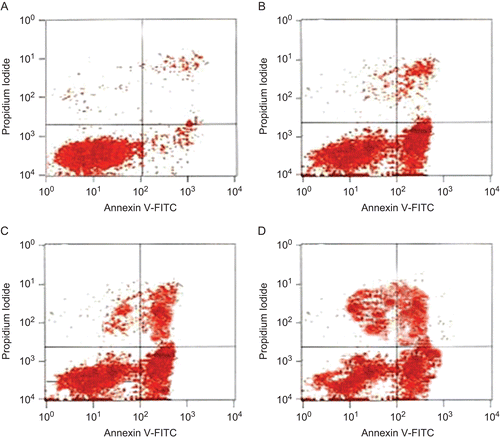
Inducing apoptosis of K562/ADM cells
At 12 h, apoptosis in the HSS 40 μg/mL group was significantly higher than in the control group and in the 10 or 20 μg/mL HSS groups (P < 0.05, ). The apoptosis increased with the extension of time and at 36 h, the apoptosis in the three HSS groups was higher than in the control group (P < 0.05 and 0.01, respectively, , ).
Table 1. Haishengsu (HSS) induced apoptosis in the K562/ADM cells.
The expression of p-glycoprotein in K562/ADM cells
The positive p-glycoprotein expression in the control group was 16.9%. p-Glycoprotein expression in the 10, 20, and 40 μg/mL HSS group was 14.8, 10.7, and 7.3%, respectively. The p-glycoprotein expression in the 40 μg/mL HSS group was lower than in the control (P < 0.05) and the 10 μg/ml HSS group (P < 0.05).
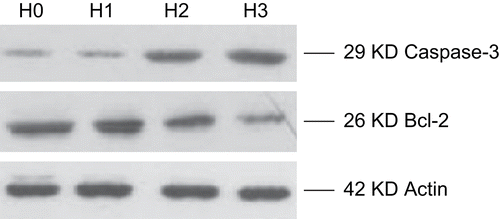
Effect of HSS on the expression of caspase-3 and Bcl-2 protein
As shown in , the relative optical density value of caspase-3 in the HSS group was higher than in the control group (P < 0.05). The relative optical density value of Bcl-2 in the HSS group was lower than in the control group (P < 0.05).
The expression of caspase-3 and Bcl-2 in the HSS and control groups is shown in .
Table 2. Optical density value of control and study groups.
Discussion
The major findings of this study are: 1) HSS treatment induced the apoptosis of K562/ADM cells in a dose-dependent manner; 2) After HSS treatment, caspase-3 expression in the K562/ADM cells was increased, whereas Bcl-2 expression was decreased; 3) HSS led to a significant reduction in p-glycoprotein expression in the K562/ADM cells.
To the best of our knowledge, this is the first study to demonstrate that HSS, a seashell protein, is able to facilitate the apoptosis of adriamycin-resistant leukemia cell line K562/ADM, and to suppress the expression of p-glycoprotein, a drug-resistance inducing protein in cancer cells. These findings may have significant clinical implications, because if these in vitro therapeutic benefits are also present in vivo, HSS may be used as an adjunct therapy to enhance the pharmacological effects of the conventional anticancer drugs on leukemia.
How HSS induced apoptosis in the drug-resistant K562/ADM cells is not entirely clear. Apoptosis is a complex process involving a cascade of reactions and multiple genes. Caspases are a group of protease involved in the regulation of apoptosis. Caspase-3 is one of the key executioners of apoptosis. It is responsible for the proteolytic cleavage of many key proteins during apoptosis, activating the apoptotic processes (CitationCohen, 1997). The proteolytic activities of caspase-3 control the occurrence and development of apoptosis, and are considered the central part of apoptosis (CitationBoatright & Salvesen, 2003). In the present study, HSS dose-dependently increased the expression of caspase-3 in the K562/ADM cells. At 40 μg/mL, HSS almost doubled the expression of caspase-3 in comparison with the caspase-3 expression in the control group. These results suggest that stimulation of the caspase-3 expression pathway may have contributed to the apoptosis increment seen in the HSS groups.
In addition to caspase-3, Bcl-2 gene family also plays an important role in the regulation of apoptosis. Bcl-2 and Bcl-xl are apoptosis suppressors, whereas Bax and Bak are apoptosis triggers (CitationGupta et al., 2002). In the present study, increasing concentrations of HSS were associated with a decreasing optical density of Bcl-2 (), indicating a reduced Bcl-2 expression in the K562/ADM cells. This reduced Bcl-2 expression may have alleviated some of the apoptosis inhibition, contributing to the increased rate of apoptosis in the HSS treatment groups.
p-Glycoprotein is a 160-kDa adenosine triphosphate (ATP)-dependent efflux transporter, belonging to the ATP-binding cassette transporter super-family (CitationHiggins, 1992). p-Glycoprotein alters the pharmacokinetics of numerous structurally and pharmacologically diverse substrate drugs (CitationHiggins, 1992). In addition, this protein has the ability to confer the multi-drug resistance phenotype to cancer cells (CitationHiggins, 1992). Therefore, modulation of p-glycoprotein expression and/or activity is potentially a useful strategy to improve the pharmacological effects of anticancer drugs (CitationHiggins, 1992). It may also help to overcome the intrinsic or acquired resistance against chemotherapy in chronic myeloid leukemia and other forms of cancer (CitationHiggins, 1992).
In the present study, at 40 μg/mL, HSS reduced the p-glycoprotein expression to about half of the control group’s level. These results suggest HSS may diminish the resistance to chemotherapy, enhancing the cytotoxic effects of anticancer drugs used in the management of leukemia.
It should be noted that the findings on the suppressive effects of HSS on p-glycoprotein expression are preliminary. These findings need to be further studied and verified in an in vivo animal model. In addition, there is a need for more detailed in vitro and in vivo analysis of the signaling pathways by which HSS led to the suppression of p-glycoprotein expression.
In summary, at a concentration between 10-40 μg/ mL, a seashell protein HSS enhances the rate of apoptosis in adriamycin-resistant leukemia cell line K562/ADM. This enhancement in apoptosis appears to be related to the suppression of anti-apoptotic protein Bcl-2. It may also be due to the increase in apoptosis-stimulating protein caspase-3. More importantly, the expression of p-glycoprotein, a drug-resistant protein in the K562/ADM cell line, is suppressed by HSS.
Declaration of interest
This study was supported by Shandong Province’s Science and Technology Development Projects (no. 2007GG20002011).
References
- Boatright KM, Salvesen GS (2003): Mechanisms of caspase activation. Curr Opin Cell Biol 15: 725–731.
- Cohen GM (1997): Caspases: The executioners of apoptosis. Biochem J 326: 1–16.
- Ford JP, Hoggard G, Owen A, Khoo SH, Back DJ (2003): A simplified approach to determining p-glycoprotein expression in peripheral blood mononuclear cell subsets. J Immunol Methods 274: 129–137.
- Gupta S, Afaq F, Mukhtar H (2002): Involvement of nuclear factor-kappa B, Bax and Bcl-2 in induction of cell cycle arrest and apoptosis by apigenin in human prostate carcinoma cells. Oncogene 21: 3727–3738.
- Higgins CF (1992): ABC transporters: From microorganisms to man. Annu Rev Cell Biol 8: 67–113.
- Klein E, Ben-Bassat H, Neumann H, Ralph P, Zeuthen J, Polliack A, Vanky F (1976): Properties of the K562 cell line, derived from a patient with chronic myeloid leukemia. Int J Cancer 18: 421–431.
- Jung WK, Jo HY, Qian ZJ, Jeong YJ, Park SG, Choi IW, Kim SK (2007): A novel anticoagulant protein with high affinity to blood coagulation factor Va from Tegillarca granosa. J Biochem Molecular Biol 40: 832–838.
- Kantarjian HM, Talpaz M, Giles F, O’Brien S, Cortes J (2006): New insights into the pathophysiology of chronic myeloid leukemia and imatinib resistance. Anna Intern Med 145: 913–923.
- Li GY, Liu JZ, Yu XM, Chen SF, Zhang B, Zhang WF, Wang LX (2008): Effect of a seashell protein haishengsu on cell growth and expression of apoptosis genes in leukemia K562 Cells. Clin Invest Med 31: E218–E221.
- Li GY, Yu XM, Zhang HW, Zhang B, Wang CB, Xin YC, Yang CZ, Zhou RX, Wang LX (2008): Haishengsu as an adjunct therapy to conventional chemotherapy in patients with non-small cell lung cancer: A pilot randomized and placebo-controlled clinical trial. Complement Ther Med 2009; 17: 51–55.
- Liu ZX, Zhan SM, Yao RY, Fang LH, Yang X, Wang CB (2004): An experimental study on anti-tumor effect of Haishengsu injection. Chin J Mar Drugs 23: 35–37.
- Tsuruo T, Iida-Saito H, Kawabata H, Oh-hara T, Hamada H, Utakoji T (1986): Characteristics of resistance to adriamycin in human myelogenous leukemia K562 resistant to adriamycin and in isolated clones. Jpn J Cancer Res 77: 682–692.
- Wang ZL, Chu X, Yang X, Chen SQ, Wang CB (2006): Antitumor effects of extractive from Arca granosa Linnaeus. Shandong Med J 46: 69–73.
- Yang CZ, Luan FJ, Xiong DS, Liu BR, Xu YF, Gu KS (1995): Multidrug resistance in leukemic cell line K562/A02 induced by doxorubicin. Acta Pharmacol Sin 16: 333–337.
- Yao RY, Chu X, Zhang YJ, Yang X, Liu XR, Wang CB (2005): Anti-tumor effect of Haishengsu extracted from Tegillarca granosa in vitro and in vivo. Chin J Mar Drugs 24: 33–36.
- Zhang CX, Li GY, Han CS, Ye XQ, Chen HY, Zhou HJ, Chen SG (2005): Effect of Haishengsu preparation extracted from Tegillarca granosa in treatment of intermediate and late non-small-cell lung cancer (NSCLC) and late renal cancer. Chin J Mar Drugs 24: 55–58.