Abstract
Tanshinone IIA (Tan IIA), one of the key components of Salvia milthorrhiza Bunge (Lamiaceae), is used to treat liver disease. The present study was carried out to investigate the possible mechanisms involved in the hepatoprotective effects of Tan IIA on carbon tetrachloride (CCl4)-induced hepatocyte toxicity. In cultures treated with 1 or 2 μM CCl4, Tan IIA (10–75 μM) significantly increased hepatocyte survival rates. However, only at a concentration of 75 μM could Tan IIA partially reverse the CCl4 (3 μM)-induced decrease of survival rate (34 ± 3% vs. 18 ± 3%, n = 8, p < 0.01). In isolated mitochondria energized with succinate, Tan IIA could inhibit the large swelling effect induced by CCl4 (1 and 2 μM). Base on these results, Tan IIA could protect rat primary cultured hepatocytes from CCl4-induced toxicity partially by the inhibitory effect on the opening of mitochondrial permeability transition (MPT).
Introduction
Tanshinone IIA (Tan IIA), a derivative of phenanthrene-quinone isolated from Danshen (Salvia milthorrhiza Bunge (Lamiaceae)), a widely used Chinese herbal medicine, has antioxidant properties. Tan IIA inhibits alcoholic liver disease (CitationYin et al., 2008), inhibits the inflammatory response of colitis (CitationBai et al., 2008), and suppresses the inflammation in atherosclerotic lesions (CitationFang et al., 2008) by means of antioxidant activity. Meanwhile, the effects of Tan IIA have been elucidated against carbon tetrachloride (CCl4)-induced hepatotoxicity (CitationLiu et al., 2001, Citation2002, Citation2003). However, the mechanisms underlining the hepatoprotective effects of Tan IIA have not been reported.
CCl4 was found to induce severe oxidative stress in the liver more than 40 years ago (CitationFriede, 1960). Numerous studies have show evidence that antioxidative supplements could rescue CCl4-induced hepatocyte toxicity (CitationQiusheng et al., 2004; CitationWang et al., 2008), which triggers apoptosis via a mitochondria-initiated pathway (CitationCai et al., 2005). Moreover, an increase in the resistance of hepatic mitochondria to Ca2+-stimulated permeability transition (mitochondrial permeability transition, MPT) protected mouse livers from CCl4 toxicity (CitationChiu et al., 2007).
All of these data suggest that Tan IIA might have a protective role against CCl4-induced hepatocyte toxicity by inhibition of MPT. In the present study, we investigated the involvement of MPT in CCl4-induced hepatocyte toxicity.
Materials and methods
Chemicals
All chemicals and solvents were from Sigma (St. Louis, MO, USA) and of analytical grade unless otherwise stated. Tanshinone IIA (Tan IIA) was dissolved in dimethylsulfoxide (DMSO) to obtain the stock solution, which was further diluted to achieve the final concentration. DMSO (≤0.1%) was used as a control.
Animals and isolation of rat primary hepatocytes
Sprague-Dawley male rats with an average weight of 200 ± 30 g were supplied by Shanghai Slac Laboratory Animal Co. Ltd. (Shanghai, China). The rats were housed in cages under controlled conditions at 20 ± 3°C and 45–65% humidity with a 12 h light–dark cycle (lights on 06:00 h). Drinking water and food were provided ad libitum throughout the study. All procedures were performed in accordance with guidelines on the care and use of experimental animals set by Fudan University Cancer Hospital.
Hepatocytes were isolated from Sprague-Dawley male rats by two-step collagenase perfusion as described previously (CitationCai et al., 2005; CitationZhai et al., 2008) with collagenase IV. Monolayer hepatocytes were cultured in Ham’s F-12/Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad, CA, USA) (1:1) supplemented with 15% fetal bovine serum (PAA Laboratories GmbH, Linz, Austria), 100 U/mL penicillin, and 70 μg/mL streptomycin. Hepatocytes provided with fresh medium throughout each test were referred to as the control cultures. In the present tests, Tan IIA was added to the culture medium 1 h prior to CCl4 and cultures treated with Tan IIA alone were referred to as the vehicle control.
Hepatocyte survival rates
Rat primary hepatocytes were treated with Tan IIA in the presence or absence of CCl4. The survival rates were estimated by the standard trypan blue exclusion assay. Briefly, rat primary hepatocytes were trypsinized and dyed with 0.4% Trypan blue after being collected by centrifugation. Viabilities were determined by counting an average of 100 cells per field in four different fields per culture.
Preparation of rat liver mitochondria and induction of MPT
Hepatic mitochondria were isolated from rats in a sucrose–HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid] buffer by differential centrifugation (CitationCai et al., 2005). Mitochondria (75 μg mitochondrial protein per mL) were suspended in 1 mL buffer (2 mM HEPES, pH 7.5, 0.25 M sucrose, 10 mM succinate, 1 mM potassium phosphate). MPT was initiated by the addition of CCl4 with calcium (20 μM final concentration) as indicated in the figures. The progression of MPT was monitored by the change in absorbance at 540 nm at room temperature.
Measurement of hepatocyte mitochondrial membrane potential
Mitochondrial membrane potential was evaluated as the accumulation of TMRE (tetramethylrhodamine, ethyl ester, perchlorate; final concentration of 500 nM) according to the method described by CitationWu et al. (1990). CsA (1 μM) was used in this procedure as an inhibitor of mitochondrial permeability transition (MPT). Fluorescence readings were taken on a fluorimeter (NOVOstar; BMG LABTECH, Offenburg, Germany) with the excitation wavelength at 485 nm and the emission wavelength at 520 nm.
Statistical analysis
Group mean values and standard deviations were calculated. Data are expressed as the mean ± SD of three independent experiments unless otherwise stated. Statistically significant differences were determined by Student’s t-test. Differences were considered statistically significant if p < 0.05.
Results
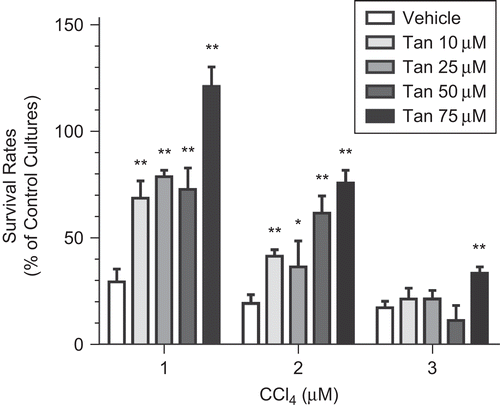
Tanshinone IIA reduces the hepatocytotoxic effect of CCl4
CCl4 (1, 2, and 3 μM) remarkably decreased the survival rates of rat primary hepatocytes to 30.2 ± 6.5%, 20.8 ± 4.3%, and 18.9 ± 3.1%, respectively (n = 8). In cultures treated with 1 or 2 μM CCl4, Tan IIA (10–75 μM) significantly increased the survival rates (). Moreover, Tan IIA (75 μM) successfully protected rat primary hepatocytes against CCl4 (3 μM)-induced hepatocyte death. However, at lower concentrations (10–50 μM), Tan IIA failed to block the toxic effects of 3 μM CCl4.
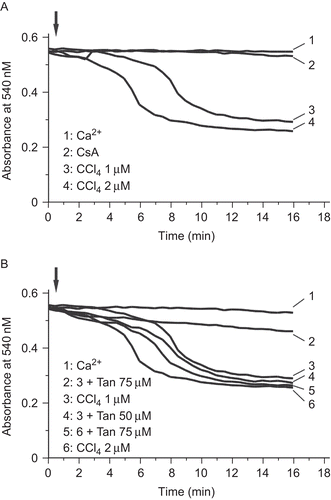
Tanshinone IIA inhibits CCl4-induced mitochondrial permeability transition opening
To further investigate the role of mitochondrial permeability transition (MPT) in the protective effects of Tan IIA against CCl4 hepatocyte toxicity, we monitored the apparent decrease in the absorbance of the mitochondria suspension at 540 nm. CCl4 could induce mitochondrial swelling with a quick onset and large magnitude (). Mitochondrial swelling induced by CCl4 was totally blocked when CsA was present (). In the presence of 75 μM Tan IIA, both the rate of onset and the magnitude of 1 μM CCl4-induced mitochondrial swelling were inhibited (line 2 vs. line 3, ).
Figure 2. Induction of mitochondrial swelling by CCl4 in isolated hepatic mitochondria. (A) Swelling effect monitored as the decrease of absorbance at 540 nm in energized mitochondria, CCl4 was added where indicated. Note that 20 μM Ca2+ alone could not induce mitochondrial permeability transition (MPT). Mitochondrial swelling was inhibited by adding 1 μM CsA. (B) Swelling effect monitored at A540 as in (A). Ca2+ (20 μM final concentration) was used as the control. 75 μM Tan IIA could inhibit 1 μM CCl4-induced mitochondrial swelling. The data represent a typical experiment conducted at least three times with similar results.
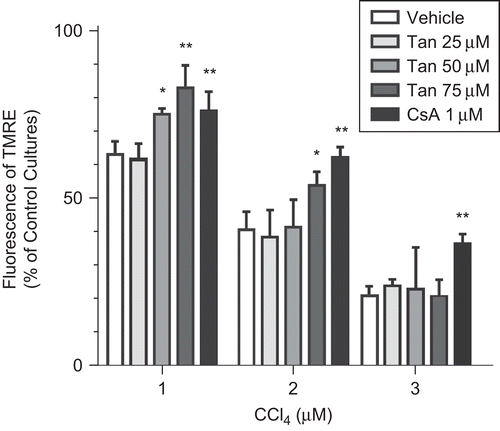
Tanshinone IIA reverses CCl4-induced decrease of mitochondria membrane potential
The mitochondria membrane potential (MMP) was evaluated in hepatocytes treated with increased concentrations of CCl4 in the absence or presence of cyclosporine A (CsA), a selective mitochondrial permeability transition (MPT) inhibitor.
As shown in , MMP dropped to 63.72 ± 3.07%, 41.14 ± 8.02%, and 22.77 ± 1.97% of the control cultures after 24 h exposure to 1, 2, and 3 μM CCl4, respectively. This effect also was reversed by the addition of Tan IIA or CsA.
Discussion
At least two different apoptosis pathways are involved in CCl4-induced apoptosis, the mitochondrial pathway and the death-receptor pathway (CitationAraragi et al., 2003). MPT, a typical character of mitochondrial dysfunction, is a major controlling mechanism in certain apoptotic systems that also contributes to the release of cytochrome c (CitationCai & Jones, 1998), which was detected in CCl4-treated rat primary hepatocytes. At the same time, caspase 3 activation as well as a decrease in cellular glutathione (GSH) content and an increase in malondialdehyde level was observed (CitationCai et al., 2005). However, its pathologic role in CCl4-induced liver injury needs to be further clarified.
MPT could be induced by CCl4 in isolated rat hepatic mitochondria, and the addition of cyclosporine A (CsA), a selective MPT inhibitor, could block this phenomenon (). Tan IIA was able to decrease the mitochondrial sensitivity to Ca2+-induced MPT (). A similar effect led to the protective influence of Schisandrin B against CCl4 toxicity in mouse livers (CitationChiu et al., 2007). Our results as shown in also confirmed the hypothesis that the inhibitory effect of Tan IIA on MPT opening might result in increased viability.
Tan IIA at concentrations lower than 75 μM failed to block CCl4 (1 and 2 μM)-increased mitochondria sensibility to Ca2+-induced MPT () while successfully inhibiting CCl4 (1 and 2 μM)-induced hepatocyte toxicity and reversing the decrease of mitochondria membrane potential ( and ). Moreover, 75 μM Tan IIA failed to block 3 μM CCl4-induced MPT or the decrease of MMP while showing an ability to inhibit 3 μM CCl4-induced hepatocyte toxicity (). These inconsistencies might result from the activities of Tan IIA on other cellular pathways, such as inhibition of JNK activation (CitationPark et al., 2007; CitationYang et al., 2008) or p-ERK1/2 expression (CitationLi et al., 2008), which were absent in cell-free conditions.
The most important findings of the present study were that Tan IIA could increase rat primary hepatocyte viabilities in the presence of CCl4 and that the inhibitory effect of Tan IIA on CCl4-induced mitochondria dysfunction might be involved in the protective effect against CCl4 toxicity.
Declaration of interest
This work was supported by a grant from the Shanghai Municipal Health Bureau (2006Y002A) to one of the authors (Q.Z.).
References
- Araragi S, Kondoh M, Kawase M, Saito S, Higashimoto M, Sato M (2003): Mercuric chloride induces apoptosis via a mitochondrial dependent pathway in human leukemia cells. Toxicology 184: 1–9.
- Bai A, Lu N, Guo Y, Fan X (2008): Tanshinone IIA ameliorates trinitrobenzene sulfonic acid (TNBS)-induced murine colitis. Digest Dis Sci 53: 421–428.
- Cai J, Jones DP (1998): Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J Biol Chem 273: 11401–11404.
- Cai Y, Gong LK, Qi XM, Li XH, Ren J (2005): Apoptosis initiated by carbon tetrachloride in mitochondria of rat primary cultured hepatocytes. Acta Pharmacol Sin 26: 969–975
- Chiu PY, Leung HY, Siu AH, Poon MK, Ko KM (2007): Schisandrin B decreases the sensitivity of mitochondria to calcium ion-induced permeability transition and protects against carbon tetrachloride toxicity in mouse livers. Biol Pharm Bull 30: 1108–1112.
- Fang ZY, Lin R, Yuan BX, Yang GD, Liu Y, Zhang H (2008): Tanshinone IIA downregulates the CD40 expression and decreases MMP-2 activity on atherosclerosis induced by high fatty diet in rabbit. J Ethnopharmacol 115: 217–222.
- Friede RL (1960): Inverse histochemical distribution of fat and oxidative enzymes in fatty livers produced by carbon tetrachloride. J Pathol Bacteriol 79: 109–113.
- Li SS, Feng J, Zheng Z, Liang QS (2008): Effect of sodium tanshinone II A sulfonate on phosphorylation of extracellular signal-regulated kinase 1/2 in angiotensin II-induced hypertrophy of myocardial cells. Chin J Int Med 14: 123–127.
- Liu Y, Chen H, Jiang Y (2001): Protective effect of tanshinone IIA on acute hepatic injury in mice. Zhong Yao Cai 24: 588–589.
- Liu Y, Chen H, Jiang Y (2002): Effect of tanshinone IIA on CCl4-induced liver fibrosis in rats. Zhong Yao Cai 25: 31–33.
- Liu Y, Wang X, Liu Y (2003): Protective effects of tanshinone IIA on injured primary cultured rat hepatocytes induced by CCl4. Zhong Yao Cai 26: 415–417.
- Park EJ, Zhao YZ, Kim YC, Sohn DH (2007): PF2401-SF, standardized fraction of Salvia miltiorrhiza and its constituents, tanshinone I, tanshinone IIA, and cryptotanshinone, protect primary cultured rat hepatocytes from bile acid-induced apoptosis by inhibiting JNK phosphorylation. Food Chem Toxicol 45: 1891–1898.
- Qiusheng Z, Xiling S, Xubo Meng S, Changhai W (2004): Protective effects of luteolin-7-glucoside against liver injury caused by carbon tetrachloride in rats. Pharmazie 59: 286–289.
- Wang T, Sun NL, Zhang WD, Li HL, Lu GC, Yuan BJ, Jiang H, She JH, Zhang C (2008): Protective effects of dehydrocavidine on carbon tetrachloride-induced acute hepatotoxicity in rats. J Ethnopharmacol 117: 300–308.
- Wu EY, Smith MT, Bellomo G, Di Monte D (1990): Relationships between the mitochondrial transmembrane potential, ATP concentration, and cytotoxicity in isolated rat hepatocytes. Arch Biochem Biophys 282: 358–362.
- Yin HQ, Kim YS, Choi YJ, Kim YC, Sohn DH, Ryu SY, Lee BH (2008): Effects of tanshinone IIA on the hepatotoxicity and gene expression involved in alcoholic liver disease. Arch Pharm Res 31: 659–665.
- Yang R, Liu A, Ma X, Li L, Su D, Liu J (2008): Sodium tanshinone IIA sulfonate protects cardiomyocytes against oxidative stress-mediated apoptosis through inhibiting JNK activation. J Cardiovasc Pharm 51: 396–401.
- Zhai Q, Lu SR, Li Y, Yang QL, Yu B (2008): Oxidative stress potentiated by diallylsulfide, a selective CYP2E1 inhibitor, in isoniazid toxic effect on rat primary hepatocytes. Toxcol Lett 183: 95–98.