Abstract
The effects of vitamin E and Hippophae rhamnoides L. (Elaeagnaceae) extract (HRe-1) on nicotine-induced oxidative stress in rat liver were investigated. Four groups, eight rats each, were used in this study, and the supplementation period was 3 weeks. The groups were: nicotine (0.5 mg/kg/day, intraperitoneal (i.p.)); nicotine plus vitamin E (75 mg/kg/day, intragastric (i.g.)); nicotine plus HRe-1 (250 mg/kg/day, i.g.); and the control group. The malondialdehyde and nitric oxide levels, glutathione peroxidase, glutathione S-transferase, glutathione reductase, superoxide dismutase, and total and non-enzymatic superoxide scavenger activities were measured spectrophotometrically in supernatants of the tissue homogenates. Nicotine increased the malondialdehyde level in liver tissue compared with control. This nicotine-induced increase in lipid peroxidation was prevented by both vitamin E and HRe-1. Superoxide dismutase activity was higher in the nicotine plus vitamin E-supplemented group compared with nicotine and control groups. Glutathione reductase activity was higher in the nicotine group compared with the control group. However, glutathione peroxidase activity in the control group was higher than the levels in the nicotine, and the nicotine plus HRe-1 supplemented groups. The nitric oxide level was higher in the nicotine group compared with all other groups. Total and non-enzymatic superoxide scavenger activities and glutathione S-transferase activity were not affected by any of the treatments. Our results suggest that Hippophae rhamnoides extract as well as vitamin E can protect the liver against nicotine-induced oxidative stress.
Introduction
It is well known that nicotine, a major toxic component of cigarette smoke, induces oxidative stress in vitro (CitationYildiz et al., 1999) and in vivo (CitationHelen et al., 2000; CitationKalra et al., 1991). Increased lipid peroxidation has been reported in Chinese hamster ovary cells (CitationYildiz et al., 1999), pancreatic tissue of rats (CitationWetscher et al., 1995) incubated with nicotine, and in liver, lung, and heart tissues (CitationHelen et al., 2000), and also in brain tissue (CitationBhagwat et al., 1998) of intraperitoneal nicotine-administered rats. Increased lipid peroxidation is also reported in the blood of smokers (CitationAltuntas et al., 2002; CitationKalra et al., 1991). In addition, transdermal nicotine patches used in the treatment of tobacco dependence in smokers can cause poisoning among children and adults (CitationEbbert et al., 2007; CitationWoolf et al., 1996). It seems that people who smoke are exposed to nicotine-induced oxidative stress. In addition, smokers consume fewer green vegetables and fruits, which are rich in antioxidants, than non-smokers in both sexes (CitationBeser et al., 1995). In a meta-analysis, it has also been found that smokers declare lower intakes of polyunsaturated fat, fiber, vitamin C, vitamin E, and β-carotene than non-smokers (CitationDallongeville et al., 1998). These differences in dietary habits may further exacerbate the nicotine-induced oxidative stress in smokers. Oxidative stress is considered to take part in the pathogenesis of various diseases, including alcoholic and non-alcoholic liver diseases, cancer, and diabetes (CitationGawrieh et al., 2004; CitationGul et al., 2000; CitationLieber, 2004; CitationMessina, 1991; CitationVendemiale et al., 1999).
Vitamin E is an effective lipid-soluble, chain-breaking antioxidant, protecting cell membranes from peroxidative damage (CitationBrigelius-Flohe & Traber, 1999). It is shown that vitamin E can also counteract against nicotine-induced lipid peroxidation in animals (CitationHelen et al., 2000) and in humans (CitationBrown et al., 1996). On the other hand, Hippohae rhamnoides L. (Elaeagnaceae) is a perennial plant native to Europe and Asia (CitationRousi, 1971) and also widely distributed in the fields of North and East Anatolia (CitationGuliyev et al., 2004). Fruits of Hippophae rhamnoides have been used extensively in traditional medicine in Turkey as well as China and former Soviet republics (CitationGuliyev et al., 2004). It contains tocopherols and tocotrienols (CitationAndersson et al., 2008), and vitamin C (CitationGutzeit et al., 2008). It has antioxidant and various other pharmacological effects including antiulcerogenic, antimicrobial, radioprotective, antitoxic, and anticoagulant effects (CitationGuliyev et al., 2004). For example, it has been reported that Hippophae rhamnoides extract (HRe-1) has antioxidant activity in vitro (CitationGao et al., 2000) and also in vivo (CitationCheng, 1992).
In the literature, there are studies where Hippophae rhamnoides extracts were used in the prevention or treatment of experimental and clinical liver injuries (CitationCheng, 1992; CitationGao et al., 2003; CitationLiu et al., 2006). For example, the seed oil of Hippophae rhamnoides alleviated liver injury caused by CCl4 in rats (CitationCheng, 1992; CitationLiu et al., 2006). Also, sea buckthorn extract, which was taken for 6 months orally, shortened the duration for normalization of aminotransferases in cirrhotic patients (CitationGao et al., 2003). However, to the best of our knowledge, whether Hippophae rhamnoides extract prevents nicotine-induced lipid peroxidation in liver tissue of rats has not been studied before.
We have previously reported that HRe-1 prevents nicotine-induced lipid peroxidation in blood (CitationSuleyman et al., 2002) but not in brain tissue (CitationGumustekin et al., 2003) of the rat. The aim of this study was to investigate the effects of HRe-1, and also vitamin E, on nicotine-induced oxidative stress in rat liver, specifically alterations in malondialdehyde (MDA) level as an oxidative stress parameter, and the activities of some antioxidant enzymes and also the nitric oxide (NO) level. The results of this study may have clinical implications, since HRe-1 is a non-toxic plant extract that can easily be used as a dietary supplement.
Materials and methods
Animals
Thirty-two rats (Sprague-Dawley strain with a body weight of 225 ± 28 g), fed with standard laboratory chow and water, were used in the study. They were randomly divided into four groups (eight rats per group) and placed in separate cages during the study. The groups were as follows:
Group I: Nicotine [0.5 mg/kg/day, intraperitoneal (i.p.)];
Group II: Nicotine [0.5 mg/kg/day, i.p.) + vitamin E (75 mg/kg/day, intragastric (i.g.)];
Group III: Nicotine (0.5 mg/kg/day, i.p.) + HRe-1 (250 mg/kg/day, i.g.);
Group IV: Control group (received only the same amounts of vehicles, 0.9% NaCl solution, i.p., and corn oil, i.g.).
The supplementation period was 3 weeks. Animal experimentations were carried out in an ethically proper way by following the guidelines as set by the Ethical Committee of Atatürk University.
Preparation and administration of Hippophae rhamnoides extract
The ripe fresh fruits of Hippophae rhamnoides were collected from the Tortum area (altitude 1600 m), a town in Erzurum, Turkey. The fruits of Hippophae rhamnoides were removed from the branches, washed with tap water and dried, then crushed in a mortar and mixed. Fruit mash was placed in a glass jar and hexane was added in an equal volume. Forty-eight hours later, juice was obtained from the mixture by squeezing and centrifuging at 1000 × g for 15 min; clear supernatant was removed by a drip. Hexane was evaporated from the liquid by an evaporator (Rotavapor R110; Büchi, Switzerland). Hippophae rhamnoides extract (HRe-1, 500 mg/mL) was also mixed with corn oil (1/1, v/v), and administered orally by a stomach tube to group III for 3 weeks at 1 mL (250 mg)/kg/day.
Preparation and administration of nicotine
The hydrogen tartrate salt of nicotine (Sigma N-5260) was dissolved in 0.9% NaCl solution to obtain a 0.15 mg/mL concentration of nicotine. Then, the pH of the nicotine solution was adjusted to 7.4 by 0.1 N NaOH. Nicotine (0.5 mg/kg/day) was administered by intraperitoneal injection to groups I, II, and III for 3 weeks.
Preparation and administration of vitamin E
Vitamin E (Ephynal 300 capsule; Roche, France) was dissolved in corn oil (30 mg/mL) and administered orally by a stomach tube (approximately 75 mg/kg/day) to group II for 3 weeks.
Sample collection
At the end of the experiment, the animals were anesthetized with ketamine-HCl (Ketalar, 20 mg/kg, i.p.). The animals were killed by exsanguination by cardiac puncture after thoracotomy. Then, liver tissue was carefully removed, rinsed in saline, and stored at −80°C until homogenization.
Preparation of supernatants for enzymes and MDA measurements
Homogenization of the liver tissue for enzyme activities and tissue MDA level
A piece of liver tissue (approximately 300 mg) was homogenized using an OMNI TH International, model TH 220 (Warrenton, VA, USA) homogenizer in 20 mM Tris-HCl, pH 7.4 (1/10 w/v) on ice for 10 s at the first speed level. Then, the homogenate was centrifuged at 10,000 × g for 15 min at 4°C.
The supernatant was stored at −80°C in aliquots until biochemical measurements. Activities of the antioxidant enzymes, superoxide dismutase (SOD) and glutathione peroxidase (GPX), and NO and MDA levels were determined from these supernatants spectrophotometrically.
Assays of the enzyme activities
Glutathione peroxidase (GPX) activity was measured by coupled spectrophotometric assay at 340 nm from the oxidation of NADPH (reduced nicotinamide adenine dinucleotide phosphate) in the presence of H2O2 used as substrate (CitationPaglia & Valentina, 1967). Glutathione S-transferase (GST) activity of the supernatant was measured by using 1-chloro-2,4-dinitrobenzene (CDNB) and glutathione (GSH) as described (CitationHabig et al., 1974). Glutathione reductase (GR) activity was determined by coupled spectrophotometric registration at 340 nm, using glutathione disulfide (GSSG) as substrate and NADPH (CitationCarlberg & Mannervik, 1985).
Determination of total superoxide scavenger activity (TSSA), non-enzymatic superoxide scavenger activity (NSSA), and superoxide dismutase (SOD) activity
TSSA and NSSA assays, as indicators of tissue antioxidant capacity, were performed in the samples before and after adding trichloroacetic acid (TCA, 20%), as described (CitationDurak et al., 1998). First, TSSA is measured. In this method, the xanthine–xanthine oxidase complex produces superoxide radicals that react with nitroblue tetrazolium (NBT) to form a farmazone compound. TSSA activity is measured at 560 nm by detecting inhibition of this reaction. By using a blank reaction in which all reagents are present except the supernatant sample, and by determining the absorbance of the sample and blank, TSSA activity is calculated. Second, NSSA activity is measured in TCA-treated fractions prepared by treating part of the sample with a final concentration of 20% (w/v) TCA solution (to remove all enzymes and proteins), and centrifuging at 5000 × g for 30 min. After the elimination of proteins by this procedure, the NSSA activity assay is performed in the supernatant fraction. SOD activity is calculated as the difference between TSSA and NSSA (CitationDurak et al., 1998).
Determination of the nitric oxide (NO) level
Liver tissue NO levels were measured using Griess reagent as previously described (CitationBories & Bories, 1995; CitationMoshage et al., 1995). Griess reagent consists of sulfanilamide and N-(1-naphthyl) ethylenediamine. First, nitrate is converted to nitrite using nitrate reductase. The second step is the addition of Griess reagent, which converts nitrite to a deep purple azocompound; photometric measurement of the absorbance at 540 nm determines the nitrite concentration (sodium nitrate is used as a standard). NO levels are expressed as nmol/mg protein.
Measurement of MDA level
MDA, which is the final product of lipid peroxidation, was determined spectrophotometrically according to a similar method described (CitationOhkawa et al., 1979). Briefly, a mixture of 8.1% sodium dodecyl sulfate (SDS) (0.2 mL), 20% acetic acid (1.5 mL), and 0.9% thiobarbituric acid (1.5 mL) was added to the mixture to bring the total volume up to 4 mL. This mixture was incubated at 95°C for 1 h. After incubation, the tubes were left to cool under cold water, then 5 mL n-butanol/pyridine (15:1, v/v) was added, followed by mixing. The samples were centrifuged at 4000 rpm for 10 min. The organic phase, which accumulated at the top of the tube, was sampled, and sample absorbances were measured with respect to the blank at 532 nm. The concentration of MDA was calculated using 1.56 × 105 M−1 cm−1 as molar extinction coefficient.
Protein measurement
Protein concentration of the supernatant was measured by the Bradford method (CitationBradford, 1976). Biochemical measurements were carried out at room temperature using a spectrophotometer (Cecil CE 3041; Cambridge, UK).
Statistical analysis
The results given are the mean ± SD. One-way analysis of variance (ANOVA) with post hoc least significant difference (LSD) test was used to compare the group means, and p < 0.05 was considered statistically significant. SPSS for Windows (version 10.0.0) was used for statistical analysis.
Results
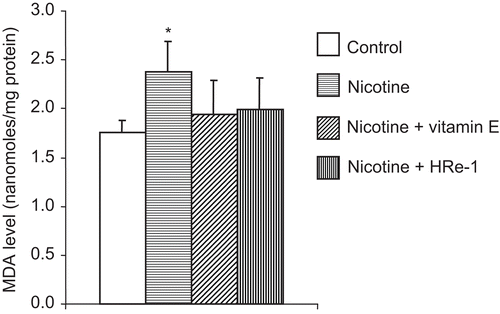
Nicotine increased the MDA level in liver tissue when compared with that of the normal control group. This nicotine-induced increase in lipid peroxidation was prevented by both vitamin E and HRe-1 ().
Figure 1. The effects of nicotine, and nicotine plus vitamin E or Hippophae rhamnoides L. extract (HRe-1) on malondialdehyde level in liver tissue of the rat. *Nicotine group vs. control (p < 0.01), nicotine + vitamin E (p < 0.05), and nicotine + HRe-1 (p < 0.05) groups. The results are mean ± SD. The group means were compared with one-way ANOVA with post hoc LSD test.
The SOD activity was higher in the nicotine plus vitamin E supplemented group compared with the nicotine only and control groups. GR activity was higher in the nicotine group compared with the control group. However, GPX activity in the control group was higher than the levels in the nicotine, and the nicotine plus HRe-1 supplemented groups. The NO level was higher in the nicotine group compared with all other groups. Total and non-enzymatic superoxide scavenger activities and GST activity were not affected by any of the treatments ().
Table 1. The effects of nicotine, and nicotine plus vitamin E or Hippophae rhamnoides L. extract (HRe-1) on total and non-enzymatic superoxide scavenger activities (TSSA and NSSA), antioxidant enzymes superoxide dismutase (SOD), glutathione reductase (GR), glutathione S-transferase (GST), and glutathione peroxidase (GPX) activities, and also nitric oxide (NO) level in liver tissue of the rat.
Discussion
Recent studies have reported decreased SOD, catalase, and GPX activities and also glutathione levels in liver tissue of nicotine-administered rats (s.c., 2.5 mg/kg, 5 days a week for 22 weeks) (CitationSudheer et al., 2008). On the other hand, the levels of inflammatory markers (nuclear factor κB (NF-κB) and cyclooxygenase-2 (COX-2) expression) were also found to be significantly increased in that study, which may be due to the excessive production of reactive oxygen species by nicotine (CitationSudheer et al., 2008). Reactive oxygen species are already known to upregulate the expression of NF-κB (CitationChen et al., 2001), which in turn is involved in regulation of the expression pattern of COX-2 mRNA. CitationEl-Sokkary et al. (2007) also reported decreased SOD activity and glutathione levels in liver of rats due to nicotine administration.
In this study, it is confirmed that nicotine induces oxidative stress in liver tissue, reflected as increased MDA level. Increased TBARS (thiobarbituric acid reactive substances), conjugated dienes, and hydroperoxide levels have been reported in heart, liver, and lung tissues of intraperitoneal nicotine-administered rats (CitationHelen et al., 2000). Increased MDA (CitationEl-Sokkary et al., 2007), TBARS, and hydroperoxides (CitationSudheer et al., 2008) in liver tissue of nicotine-administered rats have also been reported. The possible mechanisms whereby nicotine increases oxidative stress include disruption of the mitochondrial respiratory chain, leading to leakage from the electron transport chain in cardiomyocytes of rabbits (CitationGvozdjakova et al., 1992), depletion of cellular glutathione level in Chinese hamster ovary cells (CitationYildiz et al., 1999), and decreased activities of catalase and SOD in various tissues of the rat (CitationHelen et al., 2000). It seems that superoxide anions and hydrogen peroxide are the main source of nicotine-induced free radical production, which depletes the cellular glutathione level, which has a central role in antioxidant defense in the cell (CitationGul et al., 2000).
Both vitamin E and HRe-1 protect the liver against nicotine-induced oxidative stress in our present study. Prevention of the increase in lipid peroxidation in liver tissue of intraperitoneal nicotine-injected rats by vitamin E supplementation has been reported (CitationHelen et al., 2000). However, to the best of our knowledge, prevention of nicotine-induced lipid peroxidation in liver tissue by HRe-1 extract is reported for the first time in this study. It was also reported that seed oil of Hippophae rhamnoides markedly inhibited MDA formation in the liver induced by CCl4, acetaminophen, and ethyl alcohol toxicity (CitationCheng, 1992). It also prevented the depletion of GSH in damaged liver induced by acetaminophen in the same study (CitationCheng, 1992). This glutathione-preserving effect of Hippophae rhamnoides could have taken part in the prevention of nicotine-induced oxidative stress in liver tissue of the rat in our present study. Flavonols such as quercetin and isorhamnetin, tocopherols such as α-tocopherol and β-tocopherol, carotenoids such as α-carotene and β-carotene, and vitamin C present in Hippophae rhamnoides could have taken part in the antioxidant effects of this plant extract (CitationAndersson et al., 2008; CitationGuliyev et al., 2004; CitationGutzeit et al., 2008).
Increased NO levels in the liver might have taken part in nicotine-induced oxidative stress in this study. Increased GR activity may be a compensatory increase against nicotine-induced oxidative stress. Increased SOD activity would have taken part in the prevention of nicotine-induced oxidative stress in the vitamin E supplemented group. Although not reaching statistical significance (p = 0.094), this increase might also have taken part in the prevention of nicotine-induced oxidative stress in the HRe-1 supplemented group.
Our results demonstrate that both vitamin E and HRe-1 can attenuate nicotine-induced oxidative stress in the liver. Ethanol and water extracts of sea buckthorn (Hippophae rhamnoides) were found to be non-toxic, and the LD50 value was reported to be more than 5 g/kg (CitationVijayaraghavan et al., 2006) and 10 g/kg (CitationSaggu et al., 2007). Therefore, it is suggested that Hippophae rhamnoides extract as well as vitamin E can be used as a dietary supplement in order to prevent nicotine-induced oxidative stress in smokers.
Declaration of interest
This study has been partly supported by The Research Foundation of the Atatürk University, Erzurum, Turkey.
References
- Altuntas I, Dane S, Gumustekin K (2002): Effects of cigarette smoking on lipid peroxidation. J Basic Clin Physiol Pharmacol 13: 69–72.
- Andersson SC, Rumpunen K, Johansson E, Olsson ME (2008): Tocopherols and tocotrienols in sea buckthorn (Hippophae rhamnoides L.) berries during ripening. J Agric Food Chem 56: 6701–6706.
- Beser E, Baytan SH, Akkoyunlu D, Gul M (1995): Cigarette smoking, eating behaviour, blood haematocrit level and body mass index. Ethiop Med J 33: 155–162.
- Bhagwat SV, Vijayasarathy C, Raza H, Mullick J, Avadhani NG (1998): Preferential effects of nicotine and 4-(N-methyl-N-nitrosamine)-1-(3-pyridyl)-1-butanone on mitochondrial glutathione S-transferase A4-4 induction and increased oxidative stress in the rat brain. Biochem Pharmacol 56: 831–839.
- Bories PN, Bories C (1995): Nitrate determination in biological fluids by an enzymatic one-step assay with nitrate reductase. Clin Chem 41: 904–907.
- Bradford MM (1976): A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254.
- Brigelius-Flohe R, Traber MG (1999): Vitamin E: function and metabolism. FASEB J 13: 1145–1155.
- Brown KM, Morrice PC, Arthur JR, Duthie GG (1996): Effects of vitamin E supplementation on erythrocyte antioxidant defence mechanisms of smoking and non-smoking men. Clin Sci (Lond) 91: 107–111.
- Carlberg I, Mannervik B (1985): Glutathione reductase. Methods Enzymol 113: 484–490.
- Chen F, Castranova V, Shi X, Demers LM (2001): New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clin Chem 45: 7–17.
- Cheng TJ (1992): [Protective action of seed oil of Hippophae rhamnoides L. (HR) against experimental liver injury in mice]. Zhonghua Yu Fang Yi Xue Za Zhi 26: 227–229.
- Dallongeville J, Marecaux N, Fruchart JC, Amouyel P (1998): Cigarette smoking is associated with unhealthy patterns of nutrient intake: a meta-analysis. J Nutr 128: 1450–1457.
- Durak I, Canbolat O, Kacmaz M, Ozgen G, Ozturk HS (1998): Antioxidant interferences in superoxide dismutase activity methods using superoxide radical as substrate. Clin Chem Lab Med 36: 407–408.
- Ebbert JO, Post JA, Moyer TP, Dale LC, Schroeder DR, Hurt RD (2007): Nicotine percentage replacement among smokeless tobacco users with nicotine patch. Drug Alcohol Depend 89: 223–226.
- El-Sokkary GH, Cuzzocrea S, Reiter RJ (2007): Effect of chronic nicotine administration on the rat lung and liver: Beneficial role of melatonin. Toxicology 239: 60–67.
- Gao X, Ohlander M, Jeppsson N, Bjork L, Trajkovski V (2000): Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J Agric Food Chem 48: 1485–1490.
- Gao ZL, Gu XH, Cheng FT, Jiang FH (2003): Effect of sea buckthorn on liver fibrosis: a clinical study. World J Gastroenterol 9: 1615–1617.
- Gawrieh S, Opara EC, Koch TR (2004): Oxidative stress in nonalcoholic fatty liver disease: pathogenesis and antioxidant therapies. J Investig Med 52: 506–514.
- Gul M, Kutay FZ, Temocin S, Hanninen O (2000): Cellular and clinical implications of glutathione. Indian J Exp Biol 38: 625–634.
- Guliyev VB, Gul M, Yildirim A (2004): Hippophae rhamnoides L.: chromatographic methods to determine chemical composition, use in traditional medicine and pharmacological effects. J Chromatogr B Analyt Technol Biomed Life Sci 812: 291–307.
- Gumustekin K, Altinkaynak K, Timur H, Taysi S, Oztasan N, Polat MF, Akcay F, Suleyman H, Dane S, Gul M (2003): Vitamin E but not Hippophea rhamnoides L. prevented nicotine-induced oxidative stress in rat brain. Hum Exp Toxicol 22: 425–431.
- Gutzeit D, Baleanu G, Winterhalter P, Jerz G (2008): Vitamin C content in sea buckthorn berries (Hippophae rhamnoides L. ssp. rhamnoides) and related products: A kinetic study on storage stability and the determination of processing effects. J Food Sci 73: C615–C620.
- Gvozdjakova A, Kucharska J, Gvozdjak J (1992): Effect of smoking on the oxidative processes of cardiomyocytes. Cardiology 81: 81–84.
- Habig WH, Pabst MJ, Jakoby WB (1974): Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249: 7130–7139.
- Helen A, Krishnakumar K, Vijayammal PL, Augusti KT (2000): Antioxidant effect of onion oil (Allium cepa Linn) on the damages induced by nicotine in rats as compared to alpha-tocopherol. Toxicol Lett 116: 61–68.
- Kalra J, Chaudhary AK, Prasad K (1991): Increased production of oxygen free radicals in cigarette smokers. Int J Exp Pathol 72: 1–7.
- Lieber CS (2004): Alcoholic fatty liver: Its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol 34: 9–19.
- Liu C, Xu J, Ye CQ, Huang C (2006): [Effects and comparison of seed oil and sarcocarp oil of Hippophae rhamnoides on rats with experimental hepatocirrhosis]. Zhongguo Zhong Yao Za Zhi 31: 1100–1102.
- Messina MJ (1991): Oxidative stress status and cancer: methodology applicable for human studies. Free Radic Biol Med 10: 175–176.
- Moshage H, Kok B, Huizenga JR, Jansen PL (1995): Nitrite and nitrate determinations in plasma: A critical evaluation. Clin Chem 41: 892–896.
- Ohkawa H, Ohishi N, Yagi K (1979): Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351–358.
- Paglia DE, Valentina WN (1967): Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70: 158–169.
- Rousi A (1971): The genus Hippophae L. A taxonomic study. Ann Bot Fennici 8: 177–227.
- Saggu S, Divekar HM, Gupta V, Sawhney RC, Banerjee PK, Kumar R (2007): Adaptogenic and safety evaluation of sea buckthorn (Hippophae rhamnoides) leaf extract: A dose dependent study. Food Chem Toxicol 45: 609–617.
- Sudheer AR, Muthukumaran S, Devipriya N, Devaraj H, Menon VP (2008): Influence of ferulic acid on nicotine-induced lipid peroxidation, DNA damage and inflammation in experimental rats as compared to N-acetylcysteine. Toxicology 243: 317–329.
- Suleyman H, Gumustekin K, Taysi S, Keles S, Oztasan N, Aktas O, Altinkaynak K, Timur H, Akcay F, Akar S, Dane S, Gul M (2002): Beneficial effects of Hippophae rhamnoides L. on nicotine induced oxidative stress in rat blood compared with vitamin E. Biol Pharm Bull 25: 1133–1136.
- Vendemiale G, Grattagliano I, Altomare E (1999): An update on the role of free radicals and antioxidant defense in human disease. Int J Clin Lab Res 29: 49–55.
- Vijayaraghavan R, Gautam A, Kumar O, Pant SC, Sharma M, Singh S, Kumar HT, Singh AK, Nivsarkar M, Kaushik MP, Sawhney RC, Chaurasia OP, Prasad GB (2006): Protective effect of ethanolic and water extracts of sea buckthorn (Hippophae rhamnoides L.) against the toxic effects of mustard gas. Indian J Exp Biol 44: 821–831.
- Wetscher GJ, Bagchi M, Bagchi D, Perdikis G, Hinder PR, Glaser K, Hinder RA (1995): Free radical production in nicotine treated pancreatic tissue. Free Radic Biol Med 18: 877–882.
- Woolf A, Burkhart K, Caraccio T, Litovitz T (1996): Self-poisoning among adults using multiple transdermal nicotine patches. J Toxicol Clin Toxicol 34: 691–698.
- Yildiz D, Liu YS, Ercal N, Armstrong DW (1999): Comparison of pure nicotine- and smokeless tobacco extract-induced toxicities and oxidative stress. Arch Environ Contam Toxicol 37: 434–439.
