Abstract
Fructose feeding induces a rise in blood pressure in normal rats and is associated with insulin resistance, hyperinsulinemia, and hypertriglyceridemia. We have examined the effect of myricetin (100 and 300 mg/kg, p.o. for 6 weeks) isolated from Vitis vinifera Linn. (Vitaceae) on systolic blood pressure (SBP), vascular reactivity, serum glucose, triglycerides, cholesterol, and insulin in fructose-induced hypertension. Myricetin reduced systolic blood pressure and vascular reactivity changes to catecholamines and reversed the metabolic alterations induced by fructose. The cumulative concentration–response curve (CCRC) of Ang II was shifted toward the right in rats treated with myricetin, using isolated strips of ascending colon. The results suggest that myricetin could prevent the development of high blood pressure induced by a diet rich in fructose, probably by reversing the metabolic alterations induced by fructose. In conclusion, myricetin has antihypertensive action in the fructose model.
Introduction
Vitis vinifera Linn. (Vitaceae) is known as the common grape (vine or raisins). It is used in the Ayurvedic and Unani systems of medicine. Raisins possess laxative, cooling, expectorant, antioxidant, diuretic, aphrodisiac, and stomachic properties (CitationWarrier et al., 1996). Vitis vinifera seeds contain flavonoids, mainly myricetin. The fructose hypertensive model represents an acquired form of systolic hypertension, wherein feeding normal Wistar rats a fructose-enriched diet results in hyperinsulinemia, insulin resistance, hypertriglyceridemia, and consequently hypertension (CitationVerma et al., 1997). Insulin resistance and hyperinsulinemia are frequently associated with both clinical and experimental hypertension (CitationVerma et al., 1994; CitationMadar et al., 1997). If these metabolic abnormalities were responsible for the development of hypertension, then drug interventions which combat these effects may also decrease high blood pressure. Flavonoid intake appears to be inversely related to mortality from coronary heart disease in epidemiological studies (CitationHertog et al., 1993). Red wine extracts which are rich in flavonoids strongly inhibit the synthesis of endothelin-1 (CitationCorder et al., 2001), a vasoactive peptide that is linked to the development of coronary arteriosclerosis. Based on these findings we speculated that myricetin, a natural flavonoid obtained from Vitis vinifera, may show antihypertensive activity. The present work was therefore undertaken to evaluate the effect of myricetin on systolic blood pressure and some metabolic parameters in the fructose-induced hypertensive rat.
Materials and methods
Preparation of extract
Vitis vinifera raisins (2 kg), purchased locally, were authenticated by Dr. S. C. Pal, NDMVP Samaj’s College of Pharmacy, Nashik, India. Cut pieces of the raisins were hydrolyzed with 2 M HCl for 30 min at 100°C. Fractionation of the residue was carried out by column chromatography (silica 60–120) using ethyl acetate as the mobile phase (yield 0.5%, w/w) (CitationHarborne, 2007). The presence of myricetin in the extract was confirmed by matching thin layer chromatography (TLC) of myricetin provided by Professor S. C. Pal (Department of Pharmacognosy, College of Pharmacy, Nashik), using a solvent system comprising toluene:ethyl acetate:methanol (5:3:3), with retention factor (Rf) value of 0.62 (CitationRusjan & Zora, 2007), and by high performance liquid chromatography (HPLC) using acetonitrile in pyrophosphate buffer, pH 2.4 (1:3), the flow rate being 1.2 mL/min at 20°C, scanned at wavelength 266 nm. The retention time of myricetin was observed to be 5.76 min (CitationTokusoglu et al., 2003). The myricetin obtained was 95% pure.
Animals
Male albino rats (Wistar strain), weighing between 150 and 200 g, were obtained from the Serum Institute, Pune. Animals were housed in groups of five under standard laboratory conditions of temperature 25 ± 1°C with free access to food (Hindustan Lever, India) and water. The experiments were performed during the light portion of the day (09.00–14.00 h). The Institutional Animal Ethical Committee approved the protocol of this study.
Drugs and chemicals
Adrenaline (Adr), noradrenaline (NA), phenylephrine (PE), angiotensin II (Ang II), and urethane were purchased from Sigma, Mumbai. Fructose was obtained from Merck (India). Urethane and fructose (10%) were freshly prepared in distilled water, and all drug solutions were freshly prepared in saline before each experiment. Myricetin was dissolved in distilled water and administered orally.
Fructose-induced hypertension
Hypertension was induced experimentally in male Wistar rats (150–200 g) by a high fructose diet (fructose 10%, w/v) ad libitum for 6 weeks. Fructose solution was prepared every 2 days by dissolving the fructose in distilled water (CitationVogel & Wolfgang, 2002).
A total of 30 male Wistar rats (150–200 g) were randomized and divided into six groups of five each:
Group I. Control: Animals received no medication but were given distilled water for drinking.
Group II. Animals received myricetin (100 mg/kg/day, p.o.) and distilled water for drinking for 6 weeks.
Group III. Animals received myricetin (300 mg/kg/day, p.o.) and distilled water for drinking for 6 weeks.
Group IV. Fructose 10%: Animals received 10% fructose solution instead of drinking water ad libitum for 6 weeks.
Group V. Fructose 10% + myricetin (100): Animals received 10% fructose solution instead of drinking water ad libitum with myricetin (100 mg/kg/day, p.o.) for 6 weeks.
Group VI. Fructose 10% + myricetin (300): Animals received 10% fructose solution instead of drinking water ad libitum with myricetin (300 mg/kg/day, p.o.) for 6 weeks.
Measurement of blood pressure by non-invasive and invasive (direct) methods
During the course of treatment, the change in body weight was recorded and the change in blood pressure was monitored once a week for 6 weeks by the tail-cuff method (non-invasive blood pressure technique, NIBP) (CitationVogel & Wolfgang, 2002). After completion of the treatment schedule, rats from each group were anesthetized with urethane (120 mg/100 g). The femoral vein was cannulated with a fine polyethylene catheter for administration of the drug. Tracheostomy was performed and blood pressure was recorded from the left common carotid artery using a pressure transducer by a direct method onto a chart data system (PowerLab/4SP; ADInstruments, Australia). Heparinized saline (100 IU/mL) was used to fill the transducer and the fine polyethylene catheter cannulated to the carotid artery to prevent clotting. After 30 min of stabilization, heart rate, basal blood pressure, and vascular reactivity to NA (1 μg/kg), Adr (1 μg/kg), PE (1 μg/kg), and Ang II (25 ng/kg) were recorded.
Biochemical analysis
At the end of the experiment, blood was collected from the retro-orbital plexus (under light ether anesthesia) using capillary tubes in fresh vials. Serum was separated in a centrifuge (Remi Special Centrifuge Types R-23 and R-24; Mumbai) maintained at 3000 rpm for 2 min. The fresh serum was used for assaying glucose, cholesterol, and triglycerides using standard biochemical kits (Span Diagnostics, Surat). Serum insulin was determined by the radioimmunoassay method (Ashirwad Pathology, Nashik) (CitationPratt & Wolding, 1977).
In vitro study
After completion of the treatment schedule, rats from individual groups were sacrificed. The cumulative concentration–response curve (CCRC) of Ang II was obtained using strips of isolated rat ascending colon (CitationRegoli & Vane, 1964) in physiological salt solution with the following composition (mM): NaCl (118); KCl (4.7); CaCl2 (2.5); MgSO4 (1.2); NaHCO3 (25); KH2PO4 (1.2); and glucose (11); pH 7.4. This was warmed to 37°C and aerated using carbogen (95% oxygen and 5% carbon dioxide). One end of the strip was tied to an aerator tube and other end to a lever. Each strip was placed under optimum resting tension (1 g) and allowed to equilibrate for 30 min.
The contractile response to each dose of Ang II was recorded for 60 s.
Statistics
Mean ± SEM values were calculated for each group. One-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test was used for statistical analysis. Values of p < 0.05 were considered statistically significant.
Results
Systolic blood pressure, vascular reactivity, and heart rate
The effects of fructose and myricetin on systolic blood pressure (SBP) and mean change in blood pressure with various drugs are shown in and . Fructose treatment was associated with a significant increase (p < 0.05) in SBP. Chronic treatment with myricetin (100 and 300 mg/kg) completely blocked the elevation of blood pressure in fructose-fed rats in non-invasive and invasive blood pressure measurements. All treatment groups gained weight to a similar extent over the study period without any significant differences. No significant difference in heart rate was observed in the group of animals given fructose or in the myricetin-treated fructose groups, compared with control ().
Figure 1. Effect of myricetin on systolic blood pressure (S.B.P.) in rats. n = 5 rats per group. Data are expressed as mean ± SEM. *p < 0.05 when compared to control and †p < 0.05 when compared to fructose-treated group. F, fructose; M, myricetin.
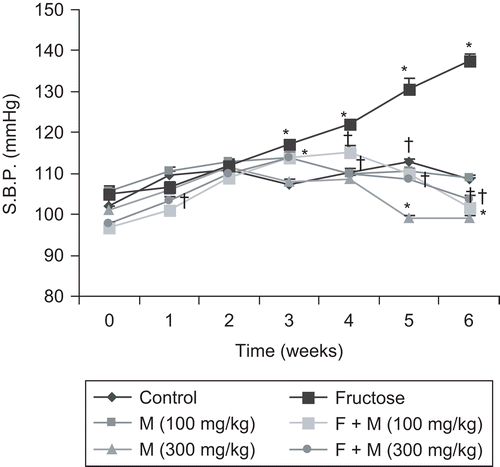
Figure 2. Mean change in blood pressure (B.P.) with various drugs: adrenaline (Adr, 1 µg/kg), noradrenaline (NA, 1 µg/kg), phenylephrine (PE, 1 µg/kg), and angiotensin II (Ang II, 25 ng/kg) in rats. Data are expressed as mean ± SEM. *p < 0.05 when compared to control and †p < 0.05 when compared to fructose-treated group. F, fructose; M, myricetin.
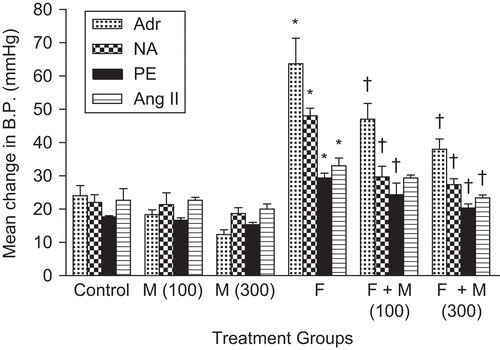
Table 1. Effect of myricetin on gain in body weight, heart rate, and serum concentrations of glucose, cholesterol, triglycerides, and insulin in fructose hypertensive rats.
Biochemical parameters
summarizes the effect of myricetin on serum glucose, cholesterol, triglycerides, insulin, and insulin resistance index in fructose-fed rats. Serum levels of all parameters were significantly higher in animals treated with fructose as compared with the control group. Myricetin treatment decreased all serum parameters in fructose-treated groups. The effect of myricetin was more prominent at the dose of 300 mg/kg.
In vitro study
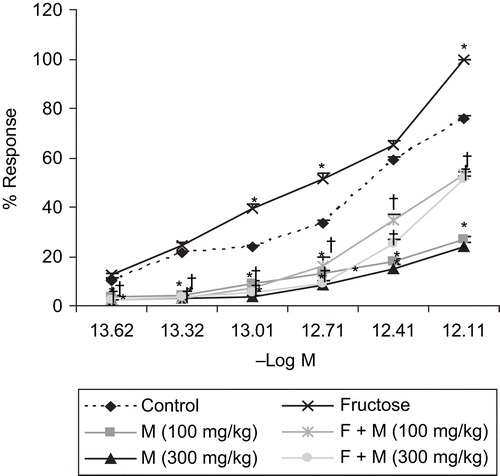
Chronic administration of myricetin (100 and 300 mg/kg/day, p.o.) for 6 weeks in fructose-fed rats significantly (p < 0.05) shifted the cumulative concentration–response curve (CCRC) of Ang II to the right, with suppression of maxima, as compared to the CCRC of fructose-fed rats, for isolated ascending colon ().
Figure 3. Effect of myricetin on the cumulative concentration–response curve (CCRC) of angiotensin II on isolated rat ascending colon in rats. n = 5 rats per group. Data are expressed as mean ± SEM. *p < 0.05 when compared to control and †p < 0.05 when compared to fructose-treated group. F, fructose; M, myricetin.
Discussion
It has been shown that sucrose feeding increases norepinephrine excretion and enhances sympathetic nerve responses in rats (CitationGradin et al., 1988). This may cause blood pressure elevation and impairment of blood flow to skeletal muscle, which would favor the development of insulin resistance. Thus, sympathetic hyperactivity is an early and integral part of fructose-induced hypertension, and the metabolic consequences are secondary to sympathetic nervous system-induced insulin resistance. Androgens play a key role in the development of fructose-induced hypertension (CitationSong et al., 2004). We therefore used male rats for our study. In the study, rats maintained on a high fructose diet developed high systolic blood pressure, as compared to control rats, confirming the results of previous studies (CitationHwang et al., 1987; CitationFang & Huang, 1998; CitationCosenzi et al., 1999). Serum insulin and glucose levels were also higher in fructose-treated rats as compared to control.
If fructose-induced hypertension is secondary to increased or augmented plasma insulin levels, then a decrease in plasma insulin levels should also prevent the rise in blood pressure. In our study, this further supports the concept that an increase in blood pressure in fructose-induced hypertension may be due to the direct action of insulin. Myricetin acts by preventing and reversing the development of hyperinsulinemia, which probably explains why hypertension was not maintained and developed in the myricetin-treated fructose group.
CitationSleder et al. (1980) reported that both glucose and fructose feeding led to increased hepatic very-low-density lipoprotein–triglyceride synthesis and output, but their removal from plasma was less efficient in fructose-fed rats. A number of studies favor the hypothesis that hypertriglyceridemia, insulin resistance, or both, contribute to the development of hypertension in these rats (CitationErlich & Rosenthal, 1996). Reports have also shown that reducing insulin levels in these rats leads to a reduction in blood pressure and correction of other metabolic abnormalities (CitationVerma et al., 1994; CitationBhanot & McNeill, 1996). In our study, after drinking a high fructose solution for 6 weeks, normal rats exhibited a significant increase in blood pressure, serum glucose, cholesterol, triglycerides, and insulin, whereas myricetin treatment significantly reduced SBP and all biochemical parameters in fructose-fed animals. The effect seemed to be more pronounced at the higher dose of myricetin (300 mg/kg).
Fructose-induced hypertensive rats are characterized by elevated total mesenteric vascular endothelin-1 (CitationVerma et al., 1995) content and an altered arterial reactivity to endothelin-1 (ET-1). Sympathetic hyperactivity can lead to an increase in blood vessel endothelin-1 levels. Several lines of evidence support the hypothesis that Ang II stimulates the production and release of ET (CitationImai et al., 1992; CitationAlexander et al., 2001). As red wine extracts, which are rich in flavonoids, strongly inhibit the synthesis of ET-1, it is quite possible that myricetin prevents fructose-induced hypertension partly by its property of strongly inhibiting the endothelin-1 receptor. Moreover, the shifting of the CCRC of Ang II toward the right by myricetin (100 and 300 mg/kg/day, p.o.) indicates an inhibitory effect on Ang II receptors.
Thus, myricetin treatment significantly reduced systolic blood pressure and other metabolic parameters in the fructose model of hypertension.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alexander B, Cockrell K, Rinewalt A, Herrington J, Granger J (2001): Enhanced renal expression of preproendothelin mRNA during chronic angiotensin II hypertension. Am J Physiol Regul Integr Comp Physiol 280: R1388–R1392.
- Bhanot S, McNeill J (1996): Insulin and hypertension: A causal relationship? Cardiov Res 31: 212–221.
- Corder R, Douthwaite J, Lees D, Khan N, Viseu Dos Santos A, Wood E, Carrier M (2001): Endothelin-1 synthesis reduced by red wine. Nature 414: 863–864.
- Cosenzi A, Sacerdote A, Seculin P, Odoni G, Plazzotta N, Bernobich E, Bellini G (1999): Lacidipine prevents the hypertension and renal and cardiac changes induced by high-fructose diet in WKYrats. J Cardiovasc Pharmacol 33: 485–491.
- Erlich Y, Rosenthal T (1996): Contribution of nitric oxide to the beneficial effects of enalapril in the fructose-induced hyperinsulinemic rat. Hypertension 28: 754–757.
- Fang T, Huang W (1998): Angiotensin receptor blockade blunts hyperinsulinemia-induced hypertension in rats. Hypertension 32: 235–242.
- Gradin K, Nissbrand H, Ehrenstom F, Henning M, Persson B (1988): Adrenergic mechanisms during hypertension induced by sucrose and/or salt in the spontaneously hypertensive rat. Naunyn Schmiedebergs Arch Pharmacol 337: 47–52.
- Harborne J (2007): Phytochemical Methods – A Guide to Modern Techniques of Plant Analysis, 3rd ed. New Delhi, Springer (India) Private Limited, pp.75–76.
- Hertog M, Feskens E, Hollman P, Katan M, Krohout D (1993): Dietary antioxidant flavanoids and risk of coronary heart disease: The Zupthen elderly study. Lancet 342: 1007–1011.
- Hwang I, Ho H, Hoffman B, Reaven G (1987): Fructose induced insulin resistance and hypertension in rats. Hypertension 10: 512–516.
- Imai T, Hirata Y, Emori T, Yanagisawa M, Masaki T, Marumo F (1992): Induction of endothelin-1 gene by angiotensin and vasopressin in endothelial cells. Hypertension 19: 753–757.
- Madar Z, Melamed E, Zimlichman R (1997): Acarbose reduced blood pressure in sucrose-induced hypertension in rats. Isr J Med Sci 33: 153–159.
- Pratt J, Wolding M (1977): Recent developments in radioimmunoassay. J Radioanal Chem 35: 45–54.
- Regoli D, Vane J (1964): A sensitive method for the assay of angiotensin. Br J Pharmacol Chemother 23: 351–359.
- Rusjan D, Zora K (2007): A comparison of extraction methods for selected phenolic compounds from grape berry skins using liquid chromatography and spectrophotometry. Acta Chim Slov 54: 114–118.
- Sleder J, Chen Y, Cully M, Reaven G (1980): Hyperinsulinemia in fructose-induced hypertriglyceridemia in the rat. Metabolism 29: 303–305.
- Song D, Arikawa E, Galipeau D, Battell M, McNeill J (2004): Androgens are necessary for the development of fructose induced hypertension. Hypertension 43: 667–672.
- Tokusoglu O, Unal M, Yildirim Z (2003): HPLC-UV and GC-MS characterization of the flavanol aglycones quercetin, kaempferol, and myricetin in tomato pastes and other tomato based products. Acta Chromatogr 13: 196–207.
- Verma S, Bhanot S, Hicke A, McNeill J (1997): Chronic T-type Ca2+ channel blockade with mibefradil in hyperinsulinemic, insulin resistant and hypertensive rats. Cardiovasc Res 34: 121–128.
- Verma S, Bhanot S, McNeill J (1994): Antihypertensive effects of metformin in fructose-fed hyperinsulinemic, hypertensive rats. J Pharmacol Exp Ther 271: 1334–1337.
- Verma, S, Bhanot S, McNeill J (1995): Effect of chronic endothelin blockade in hyperinsulinemic hypertensive rats. Am J Physiol 269: H2017–H2021.
- Vogel G, Wolfgang H, eds. (2002): Drug Discovery and Evaluation – Pharmacological Assays, 2nd ed. Berlin, Springer, pp. 176.
- Warrier P, Nambiar V, Ramankutty C (1996): Indian Medicinal Plants, 1st ed., Vol. 5. Madras, Indcom Press, pp. 396–403.
