Abstract
Several drugs of herbal origin are known to possess anxiolytic and antidepressant effects. In a recent study, we showed that extracts from Euphorbia hirta L. (Euphorbiaceae) (Eh) demonstrated anxiolytic effects in rats subjected to chronic immobilization stress (CIS) but not in rats that underwent forced swim stress (FSS). Acetylcholine and the cholinergic system are known to be involved in anxiety. However, whether the cholinergic system is involved in the anxiolytic actions of Eh are not known. In the current study, we evaluated the effects of Eh treatment of rats subjected to either CIS or FSS on acetylcholinesterase (AChE) activity in the frontal cortex, hippocampus, and septum. CIS increased the AChE activity in all three regions, while Eh treatment restored it to normal levels. FSS increased the AChE activity only in the septum, and Eh treatment marginally restored this to normal levels. Thus, these results indicate the involvement of the cholinergic system in the behavioral effects of Euphorbia hirta.
Introduction
A broad range of findings obtained during the past several decades support the view that forebrain acetylcholine (ACh) modulates several cognitive functions (CitationGold, 2003). Particularly, ACh is thought to play a role in anxiety. In models of experimental anxiety, physostigmine, an acetylcholinesterase (AChE) inhibitor, demonstrated anxiolytic properties (CitationSienkiewicz-Jarosz et al., 2000). Further, muscarinic antagonists increase, while nicotinic agonists decrease anxiety (CitationBrioni et al., 1993; CitationRodgers & Cole, 1995). In clinical studies, AChE inhibitors decreased anxiety in Alzheimer’s patients (CitationWeiner et al., 1997) and a cholinesterase-inhibiting sage (Salvia officinalis L., Lamiaceae) demonstrated anti-anxiety effects in human subjects (CitationKennedy et al., 2006). These several lines of evidence show that ACh or the cholinergic system plays a role in mediating anxiety.
Stress is increasingly being recognized as the precipitant of several psychiatric illnesses including anxiety and depression (CitationMcEwen, 2000). In earlier studies, rats subjected to chronic immobilization stress (CIS) or forced swim stress (FSS) showed anxiety in the elevated plus maze (EPM) and the open field test (OFT) (CitationAnuradha et al., 2008; CitationGovindarajan et al., 2006; CitationVyas et al., 2002). In addition to anxiety, stress is also known to produce learning and memory deficits. For example, chronic stress impaired learning in the T-maze and radial arm maze (CitationRamkumar et al., 2008; CitationSrikumar et al., 2006, Citation2007) or in other paradigms such as the Barnes maze and Morris water maze (CitationBodnoff et al., 1995; CitationMcLay et al., 1998). Several morphological, neurochemical, and neurogenic mechanisms have been proposed to underlie the stress-induced cognitive deficits (CitationMcEwen, 2000). Detailed evaluation of the hippocampal morphology over the past several years clearly indicates atrophy of the hippocampal CA3 neurons following stress (CitationMagarinos & McEwen, 1995; CitationRamkumar et al., 2008). Further, the dopaminergic and cholinergic neurotransmitter systems have been shown to be involved in mediating the stress-induced deficits (CitationSrikumar et al., 2006, Citation2007; CitationSunanda et al., 2000).
Several studies have examined the possible amelioration of stress-induced cognitive deficits or other types of anxiety (CitationBarros et al., 2007; CitationRajarao et al., 2007; CitationSrikumar et al., 2006, Citation2007). In this context, drugs of natural origin are being evaluated in pursuit of better molecules or targets to treat anxiety disorders (CitationCarlini, 2003; CitationKienzle-Horn, 2002). In clinical studies, Kava-kava (Piper methysticum G. Forst., Piperaceae) demonstrated effective anti-anxiety properties in several randomized controlled trials. St. John’s wort (Hypericum perforatum L., Clusiaceae) and sympathyl were found to possess anti-anxiety properties in a single double-blind, placebo-controlled trial (CitationSaeed et al., 2007).
Chrysin, an extract of Passiflora incarnata L. (Passifloraceae) showed anxiolytic properties in the EPM (CitationBrown et al., 2007). Several tests of experimental anxiety indicated the anti-anxiety effects of the inflorescences of Tilia americana L. (Tiliaceae), and β-sitosterol was identified as one of the active constituents (CitationAguirre-Hernandez et al., 2007). In a recent study, Euphorbia hirta L. (Euphorbiaceae) (Eh) was an effective anxiolytic in CIS-induced anxiety in the EPM and OFT. It is found to mediate its action through the benzodiazepine–γ-aminobutyric acid A (GABAA) receptor–Cl− channel complex (CitationAnuradha et al., 2008).
Thus, it is known that stress can precipitate anxiety, and cholinergic mechanisms are involved in both anxiety and in mediating the effects of stress, and Eh is effective in CIS-induced anxiety. However, whether changes in AChE activity occur in response to CIS or FSS, and the effects of Eh treatment on stressed animals are unknown. Accordingly, in the present study, we estimated AChE activity in the frontal cortex, hippocampus, and septum following Eh treatment to rats subjected to either CIS or FSS.
Materials and methods
Experimental animals
Male Wistar rats weighing 200–225 g (2–2.5 months old) obtained from the Central Animal Research Facility at the National Institute of Mental Health and Neuro Sciences (NIMHANS), Bangalore were used for the experiments. The rats were housed and maintained in standard laboratory conditions. Food and water were provided ad libitum, unless specified otherwise in stress protocols. The experimental protocols were approved by the institutional animal ethics committee. All efforts were made to minimize both the suffering and the number of animals used. Rats were randomly assigned to the following groups consisting of six rats each: normal control (NC), chronic immobilization stress (CIS), CIS + vehicle (Veh), CIS + Eh, forced swim stress (FSS), FSS + Veh, FSS + Eh. NC rats did not undergo any stress or treatments and were housed in standard conditions before sacrifice. CIS and FSS groups of rats underwent 10 days of either chronic immobilization stress or forced swim stress, respectively. CIS + Veh, CIS + Eh, FSS + Veh, and FSS + Eh groups of rats underwent 10 days of the respective stress along with either vehicle or Eh treatment as described below.
Induction of stress
Two types of stressors were applied to separate groups of animals: CIS, 2 h daily for 10 days (CitationAnuradha et al., 2008; CitationVyas et al., 2002) and FSS (CitationRoche et al., 2003). All the stress protocols were followed between 10:00 h and 12:00 h. CIS consisted of complete immobilization in rodent immobilization bags as described elsewhere (CitationVyas et al., 2002). For the FSS, swimming apparatus measuring 60 cm in height and 45 cm in diameter was used. Water was filled to a level of 30 cm. The 30 cm depth allowed rats to swim or float without allowing their tails to touch the bottom of the tank. The temperature was maintained at 23 ± 1°C. On the first day of stress, each animal was forced to swim for a period of 15 min. Later, individual animals were forced to swim for a period of 5 min regularly for 10 days. Immediately after the swim session, rats were removed from the tank, dried, and put in a warming cage that was covered with towels for 10 min. Rats were then returned to their respective home cages (CitationAnuradha et al., 2008; CitationRoche et al., 2003).
Drug treatment
Hydroalcoholic extract of the whole plant of Euphorbia hirta was supplied by the Himalaya Drug Company, Bangalore, India. Eh was dissolved in distilled water and was administered at a dose of 200 mg/kg orally, for 7 days, i.e., from the 5th through the 10th day of the stress, 1 h prior to stress, and on the 11th day. Since stress-induced anxiety was decreased by this dose of Eh in the earlier study (CitationAnuradha et al., 2008), we used the same dose in the current study. On the 11th and 13th days, they were exposed to a session of anxiety tests in the elevated plus maze and open field test as reported earlier (CitationAnuradha et al., 2008), and the animals were sacrificed on the 14th day for the AChE assay.
Assay of acetylcholinesterase activity
Acetylcholinesterase activity was measured by modified Ellman’s method (CitationEllman et al., 1961; CitationRamkumar et al., 2008; CitationSrikumar et al., 2006; CitationSunanda et al., 2000). At the end of experimentation, rats were decapitated; the frontal cortex, hippocampus, and septum were dissected quickly. The tissues were homogenized in 0.1 M phosphate buffer, pH 8.0. The reaction mixture consisted of 2.6 mL of phosphate buffer (0.1 M, pH 8.0), 0.4 mL aliquot of homogenate, and 0.1 mL of 0.01 M dithiobisnitrobenzoic acid (DTNB). After addition of the substrate, acetylthiocholine iodide (0.075 M), the change in absorbance was noted every 2 min for 10 min at 412 nm using an LKB spectrophotometer. The activity was expressed as micromoles hydrolyzed per minute per gram of tissue.
Statistical analysis
The data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons post hoc test. Values are expressed as mean ± SEM; p < 0.05 was considered statistically significant.
Results and discussion
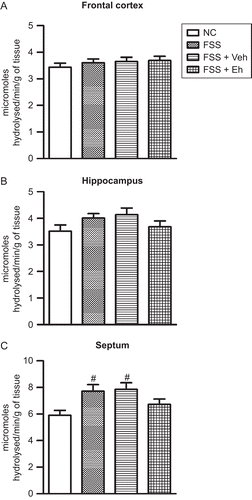
Male Wistar rats were subjected to either CIS or FSS for 10 days and were treated with the hydroalcoholic extract of Euphorbia hirta (200 mg/kg, p.o., for 7 days, i.e. from the 5th through the 10th day of the stress, 1 h prior to stress, and on the 11th day). The animals were sacrificed on the 14th day and the frontal cortex, hippocampus, and septum were obtained. AChE activity was estimated using modified Ellman’s method in tissue homogenates (CitationRamkumar et al., 2008; CitationSrikumar et al., 2006). Ten days of CIS significantly increased the AChE activity in the frontal cortex (F3,20 = 19.14; p < 0.001), hippocampus (F3,20 = 11.11; p < 0.001), and septum (F3,20 = 7.65; p < 0.01) (). Eh treatment significantly reduced the CIS-induced increase in AChE activity in the frontal cortex, hippocampus, and septum (). In contrast, FSS rats demonstrated an increase in AChE activity only in the septum (F3,20 = 4.27; p < 0.05), and it was restored following Eh treatment ().
Figure 1. Restoration of CIS-induced increase in AChE activity in the frontal cortex (A), hippocampus (B), and septum (C) by Euphorbia hirta treatment. NC, normal control; CIS, rats subjected to 10 days of chronic immobilization stress; CIS + Veh, CIS + Eh, rats subjected to CIS followed by vehicle or Euphorbia hirta 200 mg/kg, p.o. treatment, respectively. Values represent mean ± SEM. #p < 0.05, ##p < 0.01, ###p < 0.001 vs. NC; **p < 0.01, ***p < 0.001 vs. CIS; one-way ANOVA followed by Tukey’s post hoc test.
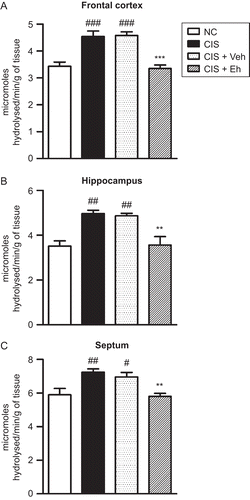
Figure 2. Effect of FSS and Eh treatment on AChE activity in the frontal cortex (A), hippocampus (B), and septum (C). NC, normal control; FSS, rats subjected to forced swim stress; FSS + Veh, rats subjected to forced swim stress and vehicle treatment; FSS + Eh, rats subjected to forced swim stress and Euphorbia hirta treatment. Values represent mean ± SEM. #p < 0.05 vs. NC; one-way ANOVA followed by Tukey’s post hoc test.
Drugs having amnesic or anxiolytic properties are known to influence the septohippocampal cholinergic system (CitationDegroot et al., 2004). Furthermore, several drugs of natural origin have been found to involve the cholinergic system in mediation of the anxiolytic response. Extracts of Hypericum perforatum (St. John’s wort) have been recently demonstrated to mediate the anti-anxiety effect through cholinergic activation (CitationVandenbogaerde et al., 2000). A cholinesterase-inhibiting sage (Salvia officinalis) demonstrated anti-anxiety effects in human subjects (CitationKennedy et al., 2006). In the present study, for the first time, we report that Eh could recruit the cholinergic system in producing anxiolytic activity.
The septohippocampal cholinergic pathways are thought to play a pivotal role in mediating the response to novel, stressful, and anxiogenic stimuli (CitationDegroot et al., 2004; CitationLamprea et al., 2003). This pathway is involved in several cognitive functions such as learning, anxiety, motivation, exploratory, and ingestive behaviors (CitationMcNaughton & Gray, 2000). In spite of several studies, the role of ACh in anxiety remains unclear. Low doses of nicotine produce anxiolytic effects, while high doses have been found to be anxiogenic (CitationFile et al., 2000). It has been proposed that the basal level of anxiety and experimental paradigms used are critical in evaluating the effects of cholinergic drugs. At low levels of anxiety, cholinergic antagonists cause anxiety. The endogenous cholinergic tone in the dorsal hippocampus plays a modulatory role in reducing anxiety. It can be speculated that exposure to CIS leads to anxious states that enhance the basal cholinergic tone in the hippocampus as a compensatory mechanism. Our finding that AChE activity in the hippocampus is increased following CIS corroborates this hypothesis. Eh treatment led to decreased anxiety, and, thus, AChE activity was restored. AChE activity in the hippocampus has been shown to correspond to the level of activity of the projections of the medial septum to the hippocampus (CitationLewis et al., 1967). Septal and fimbria–fornix lesions have been shown to decrease AChE activity in the hippocampus (CitationLewis et al., 1967; CitationMellgren & Srebro, 1973).
In contrast to CIS, FSS did not modify the AChE activity except in the septum, where there was a marginal increase in AChE activity. Swim stress has been reported not to change the AChE activity in an earlier study (CitationPung et al., 2006). Further, Eh also did not alter the AChE activity significantly in FSS animals. This lack of action of Eh on AChE activity is similar to the absence of anxiolytic effects of Eh in FSS rats (CitationAnuradha et al., 2008). It has been argued that the serotonergic system plays an important role in FSS-induced anxiety and Eh mediates its action at least in part through the GABAergic system, and thus may be ineffective. The present results show that, to a considerable extent, the cholinergic system also could be involved in the anxiolytic actions of Eh, and FSS might not recruit the cholinergic pathways in a major way, which could explain the ineffectiveness of Eh in FSS-induced anxiety.
In conclusion, the present study demonstrates the involvement of the cholinergic system in mediating the anxiolytic effects of Euphorbia hirta in chronically stressed rats.
Declaration of interest
This study was partially funded by The Himalaya Drug Company, Bangalore, India. We acknowledge Salesworth Education Foundation, Bangalore, India for a scholarship to one of the authors (H.A.).
References
- Aguirre-Hernandez E, Rosas-Acevedo H, Soto-Hernandez M, Martinez AL, Moreno J, Gonzalez-Trujano ME (2007): Bioactivity-guided isolation of beta-sitosterol and some fatty acids as active compounds in the anxiolytic and sedative effects of Tilia americana var. mexicana. Planta Med 73: 1148–1155.
- Anuradha H, Srikumar BN, Shankaranarayana Rao BS, Lakshmana M (2008): Euphorbia hirta reverses chronic stress-induced anxiety and mediates its action through the GABAA receptor benzodiazepine receptor-Cl− channel complex. J Neural Transm 115: 35–42.
- Barros M, Giorgetti M, Souto AA, Vilela G, Santos K, Boas NV, Tomaz C (2007): Persistent anxiety-like behavior in marmosets following a recent predatory stress condition: reversal by diazepam. Pharmacol Biochem Behav 86: 705–711.
- Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ (1995): Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J Neurosci 15: 61–69.
- Brioni JD, O’Neill AB, Kim DJ, Decker MW (1993): Nicotinic receptor agonists exhibit anxiolytic-like effects on the elevated plus-maze test. Eur J Pharmacol 238: 1–8.
- Brown E, Hurd NS, McCall S, Ceremuga TE (2007): Evaluation of the anxiolytic effects of chrysin, a Passiflora incarnata extract, in the laboratory rat. AANA J 75: 333–337.
- Carlini EA (2003): Plants and the central nervous system. Pharmacol Biochem Behav 75: 501–512.
- Degroot A, Wade M, Salhoff C, Davis RJ, Tzavara ET, Nomikos GG (2004): Exposure to an elevated platform increases plasma corticosterone and hippocampal acetylcholine in the rat: Reversal by chlordiazepoxide. Eur J Pharmacol 493: 103–109.
- Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961): A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7: 88–95.
- File SE, Kenny PJ, Cheeta S (2000): The role of the dorsal hippocampal serotonergic and cholinergic systems in the modulation of anxiety. Pharmacol Biochem Behav 66: 65–72.
- Gold PE (2003): Acetylcholine modulation of neural systems involved in learning and memory. Neurobiol Learn Mem 80: 194–210.
- Govindarajan A, Shankaranarayana Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S, Chattarji S (2006): Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci USA 103: 13208–13213.
- Kennedy DO, Pace S, Haskell C, Okello EJ, Milne A, Scholey AB (2006): Effects of cholinesterase inhibiting sage (Salvia officinalis) on mood, anxiety and performance on a psychological stressor battery. Neuropsychopharmacology 31: 845–852.
- Kienzle-Horn S (2002): Herbal medicines for neurological diseases. Curr Opin Investig Drugs 3: 763–767.
- Lamprea MR, Cardenas FP, Silveira R, Walsh TJ, Morato S (2003): Effects of septal cholinergic lesion on rat exploratory behavior in an open-field. Braz J Med Biol Res 36: 233–238.
- Lewis PR, Shute CC, Silver A (1967): Confirmation from choline acetylase analyses of a massive cholinergic innervation to the rat hippocampus. J Physiol 191: 215–224.
- Magarinos AM, McEwen BS (1995): Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience 69: 89–98.
- McEwen BS (2000): The neurobiology of stress: From serendipity to clinical relevance. Brain Res 886: 172–189.
- McLay RN, Freeman SM, Zadina JE (1998): Chronic corticosterone impairs memory performance in the Barnes maze. Physiol Behav 63: 933–937.
- McNaughton N, Gray JA (2000): Anxiolytic action on the behavioural inhibition system implies multiple types of arousal contribute to anxiety. J Affect Disord 61: 161–176.
- Mellgren SI, Srebro B (1973): Changes in acetylcholinesterase and distribution of degenerating fibres in the hippocampal region after septal lesions in the rat. Brain Res 52: 19–36.
- Pung T, Klein B, Blodgett D, Jortner B, Ehrich M (2006): Examination of concurrent exposure to repeated stress and chlorpyrifos on cholinergic, glutamatergic, and monoamine neurotransmitter systems in rat forebrain regions. Int J Toxicol 25: 65–80.
- Rajarao SJ, Platt B, Sukoff SJ, Lin Q, Bender CN, Nieuwenhuijsen BW, Ring RH, Schechter LE, Rosenzweig-Lipson S, Beyer CE (2007): Anxiolytic-like activity of the non-selective galanin receptor agonist, galnon. Neuropeptides 41: 307–320.
- Ramkumar K, Srikumar BN, Shankaranarayana Rao BS, Raju TR (2008): Self-stimulation rewarding experience restores stress-induced CA3 dendritic atrophy, spatial memory deficits and alterations in the levels of neurotransmitters in the hippocampus. Neurochem Res 33: 1651–1662.
- Roche M, Commons KG, Peoples A, Valentino RJ (2003): Circuitry underlying regulation of the serotonergic system by swim stress. J Neurosci 23: 970–977.
- Rodgers RJ, Cole JC (1995): Effects of scopolamine and its quaternary analogue in the murine elevated plus-maze test of anxiety. Behav Pharmacol 6: 283–289.
- Saeed SA, Bloch RM, Antonacci DJ (2007): Herbal and dietary supplements for treatment of anxiety disorders. Am Fam Physician 76: 549–556.
- Sienkiewicz-Jarosz H, Czlonkowska AI, Siemiatkowski M, Maciejak P, Szyndler J, Plaznik A (2000): The effects of physostigmine and cholinergic receptor ligands on novelty-induced neophobia. J Neural Transm 107: 1403–1412.
- Srikumar BN, Raju TR, Shankaranarayana Rao BS (2006): The involvement of cholinergic and noradrenergic systems in behavioral recovery following oxotremorine treatment to chronically stressed rats. Neuroscience 143: 679–688.
- Srikumar BN, Raju TR, Shankaranarayana Rao BS (2007): Contrasting effects of bromocriptine on learning of a partially baited radial arm maze task in the presence and absence of restraint stress. Psychopharmacology (Berl) 193: 363–374.
- Sunanda Shankaranarayana, Rao BS, Raju TR (2000): Restraint stress-induced alterations in the levels of biogenic amines, amino acids, and AChE activity in the hippocampus. Neurochem Res 25: 1547–1552.
- Vandenbogaerde A, Zanoli P, Puia G, Truzzi C, Kamuhabwa A, De Witte P, Merlevede W, Baraldi M (2000): Evidence that total extract of hypericum perforatum affects exploratory behavior and exerts anxiolytic effect in rats. Pharmacol Biochem Behav 65: 627–633.
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S (2002): Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 22: 6810–6818.
- Weiner MF, Svetlik D, Risser RC (1997): What depressive symptoms are reported in Alzheimer’s patients? Int J Geriatr Psychiatry 12: 648–652.
