Abstract
Herba Cistanche, a Chinese herb derived from the whole plant of Cistanche deserticola Y.C. Ma (Orobanchaceae), has been shown to enhance mitochondrial ATP generation and to protect against myocardial ischemia/reperfusion (I/R) injury ex vivo in rats. To define the role of mitochondria in the cardioprotective action of Herba Cistanche, we investigated the effect of Herba Cistanche treatment on mitochondrial glutathione status and functional parameters in rat hearts. Treatment with a methanol extract of Herba Cistanche enhanced mitochondrial glutathione status, decreased mitochondrial Ca2+ content, and increased mitochondrial membrane potential. In addition, an increase in State 4 respiration, indicative of uncoupled respiration, was observed in mitochondria isolated from Herba Cistanche-treated rat hearts. The enhancement of mitochondrial glutathione status and functional ability, as well as the putative induction of uncoupling proteins, may be related to cardioprotection afforded by Herba Cistanche treatment protecting against I/R injury.
Introduction
Reperfusion of a previously ischemic myocardium causes a series of mitochondrial changes, including increased reactive oxygen species (ROS) production, Ca2+ overload, and a decline in adenosine triphosphate (ATP) production, all of which can lead to necrosis- and/or apoptosis-mediated cell death (CitationRedegeld et al., 1992; CitationLemasters et al., 1998). As mitochondria play a crucial role in determining the fate of cardiomyocytes following ischemia/reperfusion (I/R) challenge, protection against myocardial I/R injury should therefore be targeted toward preservation of mitochondrial structural and functional integrity. Maintenance of mitochondrial energy metabolism after I/R challenge is particularly important because heart contractile function is almost entirely dependent on mitochondria-generated ATP. Cardiomyocytes that are alive but not contracting are of very little use.
Herba Cistanche, the whole dried plant of Cistanche deserticola Y.C. Ma (Orobanchaceae), is a parasitic plant growing mainly in the desert areas of north and northeast China. Herba Cistanche, classified as a “Yang-invigorating” herb in Chinese medicine, is prescribed for kidney deficiency, female sterility, and constipation arising from bowel dryness in senile patients (CitationChen, 1998). Previous studies in our laboratory have shown that a methanol extract of Herba Cistanche increases myocardial ATP generation capacity (CitationLeung & Ko, 2008) and protects against myocardial I/R injury in rats (CitationLeung, 2006). Whereas stimulation of ATP generation capacity was paralleled by enhancement in mitochondrial electron transport (CitationLeung & Ko, 2008), cardioprotection against I/R injury afforded by Herba Cistanche treatment was associated with a decrease in myocardial ATP depletion (CitationLeung, 2006). In the present study, to define the role of mitochondria in the cardioprotective action of Herba Cistanche, we investigated the effect of Herba Cistanche treatment on mitochondrial glutathione status, Ca2+ content, membrane potential, and respiration rate, in rat hearts.
Materials and methods
Herbal material
Herba Cistanche was purchased from a local (Hong Kong-based) herbal dealer (Lee Hoong Kee). The herb was authenticated by the supplier and a voucher specimen (HKUST00301) was deposited in the Department of Biochemistry, the Hong Kong University of Science & Technology (HKUST). Methanol extraction of the herb was conducted, based on previous studies indicating that such an extract enhanced mitochondrial ATP generation and protected against I/R injury in rat hearts (CitationLeung, 2006; CitationLeung & Ko, 2008). In brief, Herba Cistanche (400 g) was cut into small pieces and extracted by heating under reflux in 300 mL of methanol at 60°C for 2 h, as previously described (CitationLeung & Ko, 2008). The procedure was repeated twice. The pooled extract was dried by evaporating the solvent under reduced pressure; the yield was 42% (w/w) with respect to the amount of crude herb. The extract was stored at 4°C until use. Although Chinese herbs are traditionally water-extracted for oral consumption, methanol was used in our earlier study for convenience in processing and storage of samples (CitationYim & Ko, 2002), and we found methanol extraction to be satisfactory in all respects.
Animal care and drug treatment
Adult female Sprague-Dawley rats (8–10 weeks of age; 200–230 g) were housed in a humidity-controlled room, with a 12 h dark/light cycle, at approximately 22°C, and allowed food and water ad libitum. Animals were randomly assigned to different groups, with five animals in each group. Rats received the Herba Cistanche extract intragastrically (0.2 g/mL in water) at 0.5 g/kg or 1.0 g/kg for 3 consecutive days. Control (untreated) animals received water only. Twenty-four hours after the last dosing, heart ventricular tissue was obtained from anesthetized animals for biochemical analysis. All experimental protocols were approved by the Research Practice Committee, HKUST.
Preparation of mitochondrial fractions
Minced heart ventricular tissues (~0.6 g) were homogenized in a 10-fold (w/v) excess of ice-cold sucrose buffer [0.32 M sucrose, 1 mM ethylene diamine tetraacetic acid (EDTA), 50 mM Tris/HCl; pH 7.4] using a Teflon–glass homogenizer, at 4000 rpm, with 25–30 strokes. Mitochondrial pellets were prepared from tissue homogenates by centrifugation at 800 × g at 4°C for 30 min, and purity was determined by measurement of the relative specific activities of succinate dehydrogenase and lactate dehydrogenase in the supernatant and pellet, respectively, as previously described (CitationEvans, 1992). Mitochondrial pellets were suspended in 1 mL of homogenizing buffer.
Measurement of mitochondrial reduced glutathione and oxidized glutathione levels
Mitochondrial reduced glutathione (GSH) levels were determined enzymatically using 5,59-dithiobis(2-nitrobenzoic acid) (DTNB) and glutathione reductase (GR), in a protocol modified from CitationGriffith (1980). An aliquot (210 µL) of the mitochondrial fraction was mixed with 90 µL of 10% (v/v) 5-sulfosalicyclic acid (SSA) and the mixture was centrifuged at 600 × g for 10 min. To measure oxidized glutathione (GSSG) levels, 100 µL amounts of SSA supernatants were mixed with 10 µL of 20% (w/v) 2-vinylpyridine and 10 µL 60% (v/v) triethanolamine in microfuge tubes. Each tube was allowed to stand at room temperature for at least 1 h. A reaction mixture containing 0.63 mM DTNB and 0.053 mM NADPH (reduced nicotinamide adenine dinucleotide phosphate) in phosphate buffer (0.1 M, with 5 mM Na2EDTA; pH 7.5) was pre-incubated at 30°C for 2 min. An aliquot (30 µL) of either an SSA sample supernatant (total glutathione) or a GSSG sample was added to a well of a 96-well microtiter plate, and 180 µL of pre-warmed reaction mixture, containing 0.525 U/mL GR, was next added. Absorbance changes at 412 nm were monitored spectrophotometrically for 5 min. The concentrations of GSH and GSSG were estimated from calibration curves using GSH and GSSG [dissolved in 3% (w/v) SSA] as standards, and expressed as nmol/mg protein. GSH levels were estimated by subtracting twice the amount of GSSG from that of total GSH.
Measurement of mitochondrial Ca2+ content
Mitochondrial Ca2+ content was measured using a Ca2+-sensitive fluorescence probe, Fluo-5N AM ester (Molecular Probes, Eugene, OR) employing the Victor2 Multi-Label Counter (Model 1420; PerkinElmer, Turku, Finland), as described in CitationMenze et al. (2005). Ca2+ dissociation constants (Kd values) were determined using a Ca2+ calibration kit valid in the concentration range 1–1000 µM; estimated Kd values were 90–100 µM, with high reliability, according to the kit manufacturer’s data. An aliquot (25 µL) of the mitochondrial fraction (0.5 mg protein/mL final concentration) was mixed with 25 µL incubation buffer {100 mM KCl and 30 mM MOPS [3-(N-morpholino)propanesulfonic acid]; pH 7.2} in a 96-well black microtiter plate. The mixture was incubated at 25°C for 15 min; 25 µL digitonin (50 µg/mL) and 25 µL Fluo-5N AM ester (1 µM in 0.005% (w/v) Pluronic F-127) were then added. By facilitating membrane permeation, digitonin mediated mitochondrial entry of fluorescent dye. We found that Fluo-5N levels in rat heart mitochondrial preparations were increased 20-fold in the presence of digitonin. Reaction mixtures were incubated at 25°C for 30 min, and fluorescence readings were measured at an excitation wavelength of 488 nm and an emission wavelength of 532 nm. Mitochondrial Ca2+ contents were estimated using a calibration curve and expressed as µmol/mg protein.
Measurement of mitochondrial membrane potential
Membrane potential (ΔΨm) was assessed by a method modified from that of CitationBonavita et al. (2003), using a fluorescent dye, the lipophilic cationic probe 5,596,69-tetrachloro-1,193,39-tetraethylbenzimidazolylcarbocyanine iodide, commonly termed JC-1. Aliquots (50 µL) of mitochondrial fractions (adjusted to 1 mg protein/mL) were incubated at 37°C with 50 µL of substrate solution (containing 6 mM pyruvate and 6 mM malate), 25 µL of pretreated adenosine diphosphate (ADP) (30 mM) solution, and 25 µL of 3 µM JC-1, in the dark. The ΔΨm values were obtained by measuring fluorescence at 535 nm (FL1) versus 580 nm (FL2), using the Victor2 Multi-Label Counter. JC-1 forms aggregates in mitochondria, resulting in high FL2 fluorescence values, and indicating normal mitochondrial potential. Loss of ΔΨm leads to a reduction in FL2 fluorescence (JC-1 aggregates to a lesser extent) and a concomitant increase in FL1 fluorescence (from JC-1 monomers). Data were expressed as ratios of FL1/FL2 fluorescence values. Carbonyl cyanide m-chlorophenylhydrazone (CCCP), a ΔΨm-collapsing agent, was used to validate the assay.
Measurement of mitochondrial respiration
Mitochondrial respiration rate was measured at 37°C using a Hansatech Oxygraph-Plus instrument equipped with a Clark-type oxygen electrode (Sarasota, FL, USA). Mitochondrial fractions (0.6 mg protein/mL) were suspended in respiration buffer containing 125 mM KCl, 20 mM MOPS, 10 mM Tris, 0.5 mM ethylene glycol tetraacetic acid (EGTA), and 2 mM KH2PO4; pH 7.2. After incubation at 37°C for 2 min, each sample received 50 µL substrate solution (5 mM pyruvate and 5 mM malate). Respiration rates were measured in the presence (State 3) of 1 mM ADP and after phosphorylation of all ADP to ATP (State 4). The ratio of the State 3:State 4 respiration rates is the respiratory control index, which indicates the tightness of coupling between respiration and phosphorylation (CitationJavadov et al., 2005; CitationKuwabara et al., 1997). Prior to the addition of substrate solution, State 1 respiration rate was measured until the commencement of State 2 respiration, and State 2 respiration rate was estimated before the addition of ADP.
Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA) for multiple group comparisons. Least significant difference (LSD) values were used to identify significant differences between two groups when p values were < 0.05.
Results
As shown in and , treatment with a methanol extract of Herba Cistanche (0.5 or 1.0 g/kg/day × 3) significantly enhanced mitochondrial glutathione status in rat hearts, as evidenced by dose-dependent increases in GSH levels (11–30%) and decreases in GSSG levels (31%).
Figure 1. A methanol extract of Herba Cistanche affects mitochondrial reduced glutathione levels in rat hearts. Animals were orally treated with a methanol extract of Herba Cistanche at the indicated daily doses for 3 consecutive days. Mitochondrial reduced glutathione (GSH) levels were measured as described in “Materials and methods.” Each bar represents mean ± SEM, with five animals/group. *Significantly different from control group; asignificantly different from group pretreated with 0.5 g/kg extract.
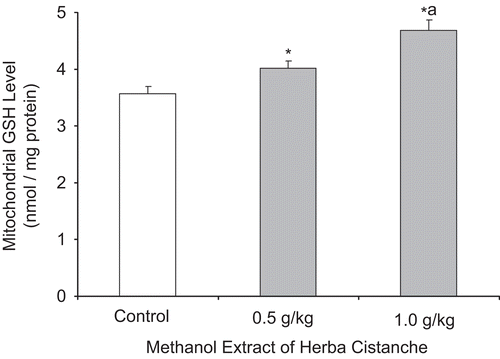
Figure 2. A methanol extract of Herba Cistanche affects mitochondrial oxidized glutathione levels in rat hearts. Animals were orally treated with a methanol extract of Herba Cistanche at the indicated daily doses for 3 consecutive days. Mitochondrial oxidized glutathione (GSSG) levels were measured as described in “Materials and methods.” Each bar represents mean ± SEM, with five animals/group. *Significantly different from untreated control group.
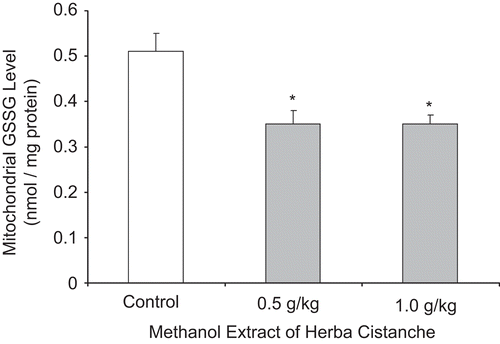
Herba Cistanche treatment dose-dependently decreased mitochondrial Ca2+ levels in rat hearts compared with controls; suppression was 41% at a dose of 1.0 g/kg ().
Figure 3. A methanol extract of Herba Cistanche affects mitochondrial Ca2+ content in rat hearts. Rats were fed a methanol extract of Herba Cistanche at 0.5 g/kg and 1.0 g/kg for 3 days. Mitochondrial Ca2+ contents were measured as described in “Materials and methods.” Each bar represents mean ± SEM, with five animals/group. *Significantly different from untreated control group; asignificantly different from group pretreated with 0.5 g/kg extract.
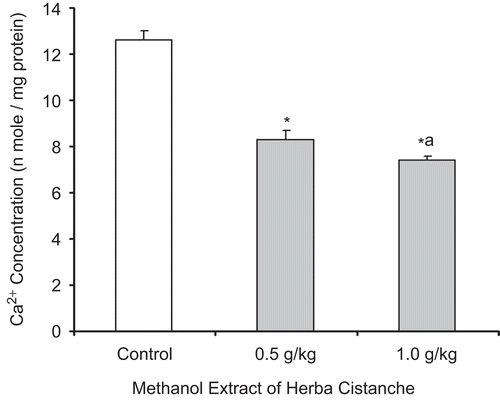
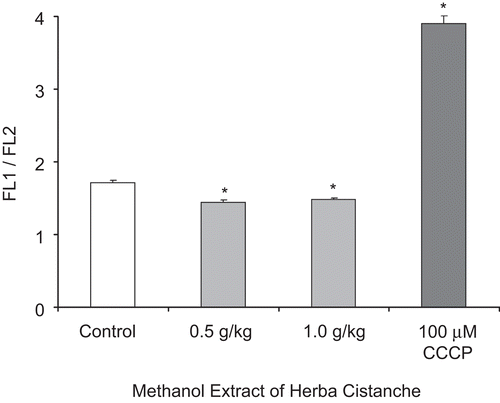
Herba Cistanche treatment increased mitochondrial ΔΨm, as evidenced by a decrease (14–16%) in the FL1/FL2 ratio. CCCP (100 µM) caused ΔΨm collapse, as indicated by an increase in the FL1/FL2 ratio ().
Figure 4. A methanol extract of Herba Cistanche affects mitochondrial membrane potential in rat hearts. Rats were fed a methanol extract of Herba Cistanche at 0.5 g/kg and 1.0 g/kg for 3 days. Mitochondrial ΔΨm values were measured as described in “Materials and methods.” Each bar represents mean ± SEM, with five animals/group. *Significantly different from untreated control group.
Herba Cistanche treatment enhanced mitochondrial respiration, as evidenced by a significantly higher rate of State 3 respiration (84%) in test animals compared to controls (). State 2 and State 4 respiration levels were increased by 47 and 80%, respectively, but the stimulation of State 4 respiration was completely suppressed by 1 mM guanosine-5′-diphosphate, an inhibitor of uncoupling protein 2/3. Herba Cistanche treatment did not affect the respiratory control index, as estimated by the ratio of State 3/State 4 respiration rates.
Table 1. A methanol extract of Herba Cistanche affects mitochondrial respiration in rat hearts.
Discussion
Mitochondria isolated from Herba Cistanche-treated rat hearts showed enhanced glutathione status, as indicated by increased GSH levels and decreased GSSG levels. Maintenance of mitochondrial glutathione redox status is critical for cardioprotection against I/R injury (CitationChiu & Ko, 2003). Cytosolic Ca2+ content increases during myocardial ischemia, via uptake by the inner membrane Ca2+ uniporter (CitationAtaka et al., 1992; CitationGrover et al., 1990), leading to mitochondrial Ca2+ accumulation. Our results indicate that treatment with Herba Cistanche extract enhanced mitochondrial glutathione redox status and decreased mitochondrial Ca2+ level, which may afford protection against myocardial I/R injury (CitationLeung, 2006). The inability of Herba Cistanche treatment, at a higher dose of 1.0 g/kg, to further decrease GSSG and Ca2+ levels, may be related to the existence of a self-limiting regulatory mechanism affecting glutathione redox potential and calcium status in mitochondria.
Mitochondria generate an electrochemical proton gradient across the inner membrane, by electron transport. This gradient is used by ATP synthase to phosphorylate ADP to ATP. Decreased mitochondrial membrane potential, as in hypoxia/reoxygenation after injury of cultured cardiomyocytes (CitationChiu et al., 2008), results in a reduction in ATP generation. Herba Cistanche treatment, which may increase myocardial mitochondrial membrane potential, possibly enhances ATP generation, particularly during post-ischemic reperfusion. Again, the existence of a self-limiting regulatory mechanism may explain why Herba Cistanche treatment effects on mitochondrial membrane potential are not dose-dependent.
Herba Cistanche treatment greatly increased the State 3 respiration rate in rat heart mitochondria, as assessed by oxygen consumption. This finding is consistent with results obtained from in vitro and in situ measurements of mitochondrial ATP generation capacity (CitationLeung & Ko, 2008). Intriguingly, although Herba Cistanche treatment reduced the steady-state level of myocardial ATP (data not shown), the treatment enhanced mitochondrial ATP generation. The involvement of uncoupled respiration may explain these apparently discrepant observations. Uncoupled oxidative phosphorylation occurs when protons are moved across the mitochondrial inner membrane back into the mitochondrial matrix, thus dissipating the membrane potential formed by the electron transport chain. This results in reduction of the proton-motive force that drives ATP formation (CitationGarvey, 2003). As a consequence, Ca2+ concentration and ROS production in mitochondria are dramatically attenuated (CitationTeshima et al., 2003). This suggestion is consistent with results of the present study, which showed a decrease of mitochondrial Ca2+ levels in Herba Cistanche-treated hearts. With respect to uncoupled respiration, synthesis of uncoupling proteins (UCPs) may be induced by Herba Cistanche treatment. UCPs are known to uncouple oxidative phosphorylation, and the expression of UCP1 in transgenic mice hearts protected against I/R-induced myocardial damage (CitationHoerter et al., 2004). The uncoupling effect of UCPs was inhibited by purine nucleotide diphosphates and triphosphates (such as ADP, ATP, guanosine diphosphate (GDP), and GTP) (CitationEchtay et al., 2002). In the present study, mitochondrial parameters such as membrane potential and respiration rate were measured in the presence of ADP (as substrate); the uncoupling effect of UCPs was therefore inhibited. However, when mitochondrial State 4 respiration rate was measured in the absence of ADP, this rate was higher in mitochondria prepared from Herba Cistanche-treated hearts than in hearts of untreated controls. The State 4 respiration rate reflects oxygen consumption by mitochondria when protons leak back into the mitochondrial matrix via a mechanism that does not involve F1F0-ATPase (CitationGarvey, 2003). Possible involvement of UCPs in the Herba Cistanche-induced increase in State 4 respiration is supported by the finding that this enhancement was suppressed by GDP (CitationBento et al., 2007).
In conclusion, treatment with Herba Cistanche may enhance mitochondrial glutathione status, decrease mitochondrial Ca2+ level, and increase mitochondrial membrane potential and respiration rate in rat hearts. The enhancement of mitochondrial glutathione status and functional ability, and the putative induction of UCPs, may be related to the cardioprotection afforded by Herba Cistanche treatment after I/R injury.
Declaration of interest
The authors report no conflict of interest. The authors are responsible for the content and writing of the paper.
References
- Ataka K, Chen D, Levitsky S, Jimenez E, Feinberg H (1992): Effect of aging on intracellular Ca2+, pHi, and contractility during ischemia and reperfusion. Circulation 86: II371–II376.
- Bento LM, Fagian MM, Vercesi AE, Gontijo JA (2007): Effects of NH4Cl-induced systemic metabolic acidosis on kidney mitochondrial coupling and calcium transport in rats. Nephrol Dial Transplant 22: 2817–2823.
- Bonavita F, Stefanelli C, Giordano E, Columbaro M, Facchini A, Bonafe F, Caldarera CM, Guarnieri C (2003): H9c2 cardiac myoblasts undergo apoptosis in a model of ischemia consisting of serum deprivation and hypoxia: inhibition by PMA. FEBS Lett 536: 85–91.
- Chen P (1998): Tonic Herbs. Advanced TCM Series. Beijing, Science Press, pp. 233–320.
- Chiu PY, Ko KM (2003): Time-dependent enhancement in mitochondrial glutathione status and ATP generation capacity by schisandrin B treatment decreases the susceptibility of rat hearts to ischemia-reperfusion injury. Biofactors 19: 43–51.
- Chiu PY, Luk KF, Leung HY, Ng KM, Ko KM (2008): Schisandrin B stereoisomers protect against hypoxia/reoxygenation-induced apoptosis and inhibit associated changes in Ca2+-induced mitochondrial permeability transition and mitochondrial membrane potential in H9c2 cardiomyocytes. Life Sci 82: 1092–1101.
- Echtay KS, Roussel D, St Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD (2002): Superoxide activates mitochondrial uncoupling proteins. Nature 415: 96–99.
- Evans WH (1992): Isolation and characterization of membranes and cell organelles. In: Rickwood D, ed., Preparative Centrifugation. A Practical Approach. New York, Oxford University Press, pp. 233–270.
- Garvey WT (2003): The role of uncoupling protein 3 in human physiology. J Clin Invest 111: 438–441.
- Griffith OW (1980): Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106: 207–212.
- Grover GJ, Dzwonczyk S, Sleph PG (1990): Ruthenium red improves postischemic contractile function in isolated rat hearts. J Cardiovasc Pharmacol 16: 783–789.
- Hoerter J, Gonzalez-Barroso MD, Couplan E, Mateo P, Gelly C, Cassard-Doulcier AM, Diolez P, Bouillaud F (2004): Mitochondrial uncoupling protein 1 expressed in the heart of transgenic mice protects against ischemic-reperfusion damage. Circulation 110: 528–533.
- Javadov S, Huang C, Kirshenbaum L, Karmazyn M (2005): NHE-1 inhibition improves impaired mitochondrial permeability transition and respiratory function during postinfarction remodelling in the rat. J Mol Cell Cardiol 38: 135–143.
- Kuwabara M, Takenaka H, Maruyama H, Onitsuka T, Hamada M (1997): Effect of prolonged hypothermic ischemia and reperfusion on oxygen consumption and total mechanical energy in rat myocardium: participation of mitochondrial oxidative phosphorylation. Transplantation 64: 577–583.
- Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, Herman B (1998): The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta 1366: 177–196.
- Leung HY (2006): Effect of Herba Cistanche on mitochondrial ATP generation: a pharmacological basis of “Yang-invigoration”. MPhil Thesis, Hong Kong University of Science and Technology, pp. 67–69.
- Leung HY, Ko KM (2008): Herba Cistanche extract enhances mitochondrial ATP generation in rat hearts and H9C2 cells. Pharma Biol 46: 418–424.
- Menze MA, Hutchinson K, Laborde SM, Hand SC (2005): Mitochondrial permeability transition in the crustacean Artemia franciscana: absence of a calcium-regulated pore in the face of profound calcium storage. Am J Physiol Regul Integr Comp Physiol 289: R68–R76.
- Redegeld FA, Moison RM, Koster AS, Noordhoek J (1992): Depletion of ATP but not of GSH affects viability of rat hepatocytes. Eur J Pharmacol 228: 229–236.
- Teshima Y, Akao M, Jones SP, Marban E (2003): Uncoupling protein-2 overexpression inhibits mitochondrial death pathway in cardiomyocytes. Circ Res 93: 192–200.
- Yim TK, Ko KM (2002): Antioxidant and immunomodulatory activities of Chinese tonifying herbs. Pharm Biol 40: 329–335.
