Abstract
Herbal medicines have been used since prehistoric times by different cultures worldwide for the treatment of diabetes. The present investigation evaluated the effect of Ficus racemosa Linn. (Moraceae) stem bark on carbohydrate hydrolyzing enzymes, viz., porcine pancreatic α-amylase, rat intestinal α-glucosidase, sucrase, and almond β-glucosidase, using in vitro model systems. In addition, the effect of heat treatment was also studied. Untreated F. racemosa bark (FRB) significantly inhibited (p ≤ 0.05) α-amylase, α-glucosidase, β-glucosidase, and sucrase in a dose-dependent manner. Heat treatment of the sample comparably increased α-amylase, α-glucosidase, and sucrase inhibitory activities, while a marginal decrease in β-glucosidase inhibitory activity was observed; however, no statistical differences were noted. Untreated FRB showed IC50 values of 0.94% and 280, 212, and 367 μg/mL for α-amylase, α-glucosidase, β-glucosidase, and sucrase, respectively, while the IC50 values for heat treated FRB were 0.58% and 259, 223, and 239 μg/mL, respectively. Further, a significant correlation (p ≤ 0.01; r = 0.791) was observed between α-amylase, α-glucosidase, β-glucosidase, and sucrase inhibitory activities of both untreated and heat treated FRB. The results clearly demonstrate that inhibition of carbohydrate hydrolyzing enzymes is one mechanism through which F. racemosa stem bark exerts its hypoglycemic effect in vivo. Therefore, the potential exists to explore the utilization of F. racemosa stem bark in the development of nutraceuticals and functional foods for the management of diabetes and related symptoms/disorders.
Introduction
Diabetes mellitus is a metabolic disorder of multiple etiology characterized by chronic hyperglycemia with a disturbance of carbohydrate, fat, and protein metabolism resulting from defects in insulin secretion, insulin action, or both (CitationWHO, 1999). Recent studies suggest that postprandial hyperglycemia could induce non-enzymatic glycosylation of various proteins, resulting in the development of chronic complications such as micro- and macrovascular diseases (CitationBaron, 1998), and has been proposed as an independent risk factor for cardiovascular disease (CitationCeriello, 1998). Therefore, control of postprandial plasma glucose levels is critical in the early treatment of diabetes mellitus (CitationShim et al., 2003). This can be done by retarding the absorption of glucose through the inhibition of carbohydrate hydrolyzing enzymes such as α-amylase, α-glucosidase, β-glucosidase, and sucrase, in the digestive tract. Inhibitors of these enzymes delay carbohydrate digestion and prolong overall carbohydrate digestion time, causing a reduction in the rate of glucose absorption and, consequently, blunting postprandial hyperglycemia (CitationRhabasa-Lhoret & Chiasson, 2004). Several α-glucosidase inhibitors have recently been developed from natural sources (CitationAsano et al., 2001; CitationHiroyuki et al., 2001; CitationLee & Lee, 2001; CitationMatsui et al., 2001). Examples of such inhibitors that are in clinical use are acarbose, miglitol, and voglibose (CitationBailey, 2003). In the present investigation, Ficus racemosa stem bark (FRB), a proven hypoglycemic agent (CitationRao et al., 2002; CitationVasudevan et al., 2007) being used by various cultures across India since ancient times for the treatment of diabetes (CitationAnonymous, 1952), was studied for its ability to inhibit carbohydrate hydrolyzing enzymes using in vitro model systems. Further, the effect of heat treatment on the enzyme inhibitory activities was also studied.
Materials and methods
Chemicals and reagents
p-Nitrophenyl-α-d-glucopyranoside, p-nitrophenyl-β-d-glucopyranoside, β-glucosidase from almonds, and 3,5-dinitrosalisylic acid were purchased from Sisco Research Laboratory, India. A glucose oxidase/peroxidase assay kit was purchased from Aggappe Diagnostics, India. α-Amylase (23 u/mg solid) was purchased from Sigma Aldrich, India. All the chemicals and reagents used in the study were of extra pure analytical grade.
Collection of plant material
Ficus racemosa stem bark was collected from Mukkadahally, Chamarajanagar district of Karnataka, India during September 2007, and was subsequently identified by Dr. Shivprasad Hudeda, JSS Ayurvedic Medical College, Mysore; a voucher specimen (BOT-001/2008) was deposited at the herbarium of the Department of Studies in Botany, University of Mysore, Mysore, India. The bark was cut into small pieces, dried (50°C) and powdered, passed through a 60 mesh sieve (BS), and stored in an airtight container at 4°C till further use.
Heat treatment
The bark powder was subjected to heat treatment in a vacuum oven at 100°C for 60 min, cooled in a desiccator, and used for the preparation of emulsion.
Preparation of the sample emulsion
Amounts of 100 mg of both untreated and heat treated FRB were triturated with two drops of Tween 20 in a glass mortar and pestle and emulsified with maleate buffer (0.1 M, pH 6.0). The volume was made up to 10 mL using a volumetric flask so as to obtain an emulsion containing 10 mg of the sample/mL of emulsion.
Preparation of crude enzyme solution of α-glucosidase and sucrase from rat intestinal brush border
The crude enzyme solution containing α-glucosidase and sucrase was prepared by slight modification of the procedure of CitationDahlqvist (1964). Male healthy rats of Wistar strain maintained on standard laboratory chow, weighing 140–160 g, were obtained from the Central Animal House, Department of Zoology, University of Mysore. The rats were fasted overnight, sacrificed by cervical dislocation, and the small intestines immediately excised. The intestines were washed with ice-cold maleate buffer and the brush border was carefully removed using glass slides and homogenized with maleate buffer (1:5, w/v) in ice-cold condition. The homogenate was then centrifuged (10,000 g; 15 min) at 4°C. The supernatant was collected and used as a crude source of α-glucosidase and sucrase. All animal procedures were approved by the Animal Ethical Committee of the University of Mysore in accordance with animal experimentation and care.
Assay of α-amylase inhibitory activity
The effect of FRB on α-amylase activity was studied using an enzyme–starch system (CitationOu et al., 2001). FRB (1–5%) was mixed by stirring with 25 mL of 4% potato starch in a beaker; 100 mg of α-amylase was added to the starch solution, stirred vigorously, and incubated at 37°C for 60 min. After the incubation period, 0.1M NaOH was added to terminate enzyme activity. The mixture was centrifuged (3000 g; 15 min) and the glucose content in the supernatant was determined.
Assay of α-glucosidase inhibitory activity
α-Glucosidase inhibitory activity was assayed according to the method of CitationHonda and Hara (1993). Enzyme solution (10 µL) and varying concentrations of sample emulsion (10–50 µL) were incubated together for 10 min at 37°C and the volume was made up to 210 µL with maleate buffer, pH 6.0. The enzyme reaction was started by adding 200 µL of 2 mM p-nitrophenyl-α-d-glucopyranoside solution and further incubated at 37°C for 30 min. The reaction was terminated by treating the mixture in a boiling water bath for 5 min. After the addition of 1.0 mL of 0.1 M disodium hydrogen phosphate solution, absorption of the liberated p-nitrophenol was read at 400 nm.
Assay of β-glucosidase inhibitory activity
Various concentrations of sample emulsion (10–50 μL) were pre-incubated with β-glucosidase (5 μL; 380 U/mL) and the volume was made up to 210 μL with phosphate buffer (10 mM; pH 7.0). The enzyme reaction was started by adding 200 µL of p-nitrophenyl-β-d-glucopyranoside solution (10 mM) and the mixture incubated (37°C, 30 min). After incubation, distilled water (850 μL) was added, the solution heated at 100°C for 3 min to stop the reaction, and the absorbance of the solution read at 440 nm (CitationKawai et al., 2006).
Assay of sucrase inhibitory activity
The effect of the FRB on sucrase activity was assayed according to the method of CitationHonda and Hara (1993). The enzyme solution (10 µL) and varying concentrations of sample emulsion (10–50 µL) were incubated together for 10 min at 37°C and the volume was made up to 200 µL with maleate buffer (pH 6.0). The enzyme reaction was started by adding 100 µL sucrose solution (60 mM). After 30 min, the reaction was terminated by adding 200 µL of 3,5-dinitrosalicylic acid reagent and treating the mixture in a boiling water bath for 5 min. The absorbance of the solution was read at 540 nm.
The percent inhibitory activities were calculated using the following formula:
where Abs control is the absorbance of the control reaction (containing all reagents except the test sample), and Abs sample is the absorbance of the test sample. An untreated enzyme solution was used as the control. All experiments were carried out in triplicate.
Statistical analysis
The data were analyzed by analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test for significant differences using SPSS 14.0 computer software. The values were considered significant when p ≤ 0.05. IC50 values were calculated by Boltzmann’s dose–response analysis using Origin 6.1 software.
Results
Effect of FRB on α-amylase activity
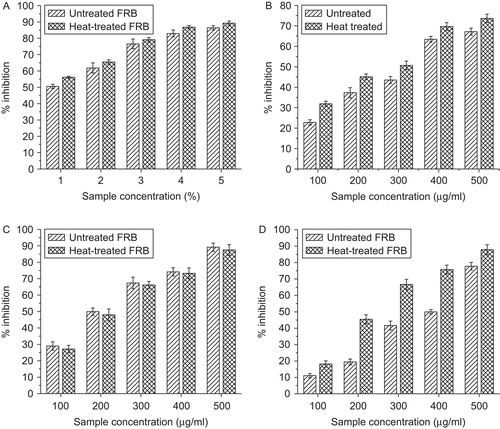
The α-amylase inhibitory activity of FRB was studied using the α-amylase–starch model system and the results are presented in . α-Amylase inhibitory activity of untreated FRB ranged 50–86% and 56–89% for heat treated FRB. Both untreated and heat treated FRB showed dose-dependent inhibition of the enzyme. It was observed that although heat treatment increased the α-amylase inhibitory activity and decreased the IC50 value, this did not reach statistical significance.
Effect of FRB on α-glucosidase and β-glucosidase activity
Both the untreated and heat treated FRB significantly inhibited (p ≤ 0.01) α-glucosidase in a dose-dependent manner (). The inhibitory activities ranged 22–67% and 31–73%, respectively, for untreated and heat treated FRB. An IC50 value of 280 µg/mL was observed for untreated FRB, while a comparably low value of 259 µg/mL was observed for heat treated FRB. However, the difference was statistically insignificant. Similarly, β-glucosidase was inhibited by both untreated and heat treated FRB in a dose-dependent manner () and their inhibitory activities ranged 29–89% and 27–87%, respectively. It is noteworthy here that although heat treatment lead to an increase in α-glucosidase inhibitory activity, a marginal decrease in the β-glucosidase inhibitory activity was observed in heat treated FRB. However, this did not reach statistical significance. The IC50 values for β-glucosidase inhibitory activities were 212 µg/mL and 223 µg/mL, respectively, for untreated and heat treated FRB.
Effect of FRB on sucrase activity
The effect of FRB on sucrase activity is shown in . The sucrase inhibitory activity ranged 12–60% and 15–83% for untreated and heat treated FRB, respectively. A dose-dependent inhibition of rat intestinal sucrase was observed in both untreated and heat treated FRB. However, heat treatment resulted in a significant increase (p ≤ 0.05) in the sucrase inhibitory activity of the sample, and the IC50 value for heat treated FRB was significantly lower (p ≤ 0.05) than that of untreated FRB ().
Table 1. IC50 values of untreated and heat treated Ficus racemosa bark (FRB) for different enzymes.
A significant correlation (p ≤ 0.01; r = 0.791) was observed between α-amylase, α-glucosidase, β-glucosidase, and sucrase inhibitory activities of both untreated and heat treated FRB, and the enzyme inhibitory activities of F. racemosa bark were directly proportional to the sample concentration.
Discussion
The development of antidiabetic drugs with complementary mechanisms of action appears more and more necessary in order to achieve durable glycemic control in type 2 diabetes by inhibiting in a reversible way the hydrolysis of disaccharides and the ultimate steps of the digestion of dietary polysaccharides, to reduce the postprandial blood glucose increase in diabetics (CitationBlickle et al., 2008). The present study evaluated the effect of F. racemosa stem bark on glycohydrolase enzymes, viz., α-amylase, α-glucosidase, β-glucosidase, and sucrase.
Several mechanisms have been proposed for the hypoglycemic effect of phytochemicals, such as inhibition of carbohydrate metabolizing enzymes, manipulation of glucose transporters, β-cell regeneration, and enhancement of insulin releasing activity (CitationTiwari & Rao, 2002). In the present investigation, both untreated and heat treated FRB effectively inhibited porcine pancreatic α-amylase. Generally, inhibition of α-amylase activity by medicinal plants is attributed to several possible factors such as fiber concentration, presence of inhibitors on fibers, encapsulation of starch and enzyme by the fibers present in the sample thereby reducing accessibility of starch to the enzyme, and direct adsorption of the enzyme on fibers leading to decreased amylase activity (CitationOu et al., 2001). However, inhibition of α-amylase activity by FRB could be conclusively attributed to the presence of lupeol, a triterpenoid (CitationRahman et al., 1994) reported to inhibit α-amylase (CitationAli et al., 2006).
Glucosidases are crucial in many biological processes including breakdown of edible carbohydrates (CitationMarshall, 1974), and are also involved in a variety of metabolic disorders such as diabetes (CitationRobinson et al., 1991). Thus, potent and selective glucosidase inhibitors have many interesting potential applications, especially in diabetes (CitationRobinson et al., 1991). α-Glucosidase is one among a number of glucosidases located in the brush-border surface membrane of intestinal cells, and is a key enzyme of carbohydrate digestion (CitationCaspary, 1978). α-Glucosidase inhibitors block the actions of the enzyme in the small intestine, which is rate-limiting in the conversion of oligosaccharides and disaccharides to monosaccharides, necessary for gastrointestinal absorption. Postprandial glucose peaks may be attenuated by delayed glucose absorption. The increase in α-glucosidase by heat treatment may be due to possible inactivation of the phytoconstituents that hinder/decrease the inhibition of glucosidases. The main benefits attributable to α-glucosidase inhibitors are reductions in both postprandial glycemic levels and the total range of postprandial glucose levels (CitationLebovitz, 1997).
The inhibition of α-glucosidase by F. racemosa bark can be attributed to the presence of tannins, kaempferol, rutin, bergapten, psoralenes, flavonoids, ficusin, coumarin, phenolic glycosides (CitationBaruah & Gohain, 1992), bergenin, and racemosic acid (CitationLi et al., 2004) that are reported to act as strong antioxidant and anti-inflammatory agents (CitationKhan & Sultana, 2005); reports indicate that phenolic-enriched extracts of Solanum melongena with moderate free radical scavenging linked antioxidant activity have high α-glucosidase inhibitory activity. Inhibition of this enzyme provides a strong biochemical basis for the management of type 2 diabetes by controlling glucose absorption. This phenolic antioxidant-enriched dietary strategy also has the potential to reduce hyperglycemia-induced pathogenesis linked to cellular oxidation stress. These results provide a strong rationale for further animal and clinical studies (CitationKwon et al., 2008). The inhibition of β-glucosidase activity may be exclusively due to the presence of tannins, as reports indicate tannins to be effective and exclusive inhibitors of glycohydrolase enzymes including α- and β-glucosidases (CitationHarnett et al., 2005).
Rat intestinal sucrase occurs as a complex of sucrase and isomaltase, which converts sucrose into glucose (CitationHauri et al., 1982). The inhibition of sucrase by F. racemosa bark may also be due to its phenolic compounds. The increase in sucrase inhibitory activity of F. racemosa by heat treatment could be due to the possible inactivation of the antagonists of sucrase inhibitory activity of the sample. Further, the correlation observed between α-amylase, α-glucosidase, β-glucosidase, and sucrase inhibitory activities of both untreated and heat treated FRB represents a parallel and effective inhibition of most of the carbohydrate hydrolyzing enzymes in the digestive tract.
Conclusion
The results of the present study support the usage of F. racemosa bark in traditional medicine for the management of diabetes, and emphasize that inhibition of carbohydrate hydrolyzing enzymes such as α-amylase, α-glucosidase, β-glucosidase, and sucrase is one mechanism through which F. racemosa bark exerts its hypoglycemic effect in vivo. Furthermore, since heat treatment increases the inhibitory activity of F. racemosa bark, it could be used in the formulation of functional foods/nutraceuticals for the effective management of diabetes.
Declaration of interest
The authors acknowledge the University Grants Commission, New Delhi, India, for financial assistance (F.31-278/2005).
References
- Ali H, Houghton PJ, Soumyanath A (2006): α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J Ethnopharmacol 107: 449–455.
- Anonymous (1952): The Wealth of India. New Delhi, Council of Scientific and Industrial Research, pp. 35–36.
- Asano N, Yamashita T, Yasuda K, Ikeda K, Kizu H, Kameda Y, Kato A, Nash RJ, Lee HS, Ryu KS (2001): Polyhydroxylated alkaloids isolated from mulberry trees (Morus alba L.) and silkworms (Bombyx mori L.). J Agric Food Chem 49: 4208–4213.
- Bailey CJ (2003): Textbook of Diabetes, 3rd ed., Vol. 2. Oxford, Blackwell Science Ltd., pp. 73.1–73.21.
- Baron AD (1998): Postprandial hyperglycemia and α-glucosidase inhibitors. Diabetes Res Clin Pract 40: S51–S55.
- Baruah KK, Gohain AK (1992): Chemical composition and nutritive value of Dimaru (Ficus glomerata Roxb.) leaves. Indian J Anim Nutr 9: 107–108.
- Blickle JF, Andres E, Brogard JM (2008): Current status of the treatment of type 2 diabetes mellitus. Alpha-glucosidase inhibitors. Food Chem 106: 247–252.
- Caspary WF (1978): Sucrose malabsorption in man after ingestion of alpha-glucosidehydrolase inhibitor. Lancet 1: 1231–1233.
- Ceriello A (1998): The emerging role of post-prandial hyperglycaemic spikes in the pathogenesis of diabetic complications. Diabetic Med 15: 188–193.
- Dahlqvist A (1964): Method for assay of intestinal disaccharides. Anal Biochem 7: 18–25.
- Harnett SM, Oosthuizen V, van de Venter M (2005): Anti-HIV activities of organic and aqueous extracts of Sutherlandia frutescens and Lobostemon trigonus. J Ethnopharmacol 96: 113–119.
- Hauri HP, Wacker H, Rickli EE, Bigler-Meier B, Quaroni A, Semenza G (1982): Biosynthesis of sucrase-isomaltase. Purification and NH2-terminal amino acid sequence of the rat sucrase-isomaltase precursor (pro-sucrase-isomaltase) from fetal intestinal transplants. J Biol Chem 257: 4522–4528.
- Hiroyuki F, Tomohide Y, Kazunori O (2001): Efficacy and safety of Touchi extract, an alpha-glucosidase inhibitor derived from fermented soybeans, in non-insulin-dependent diabetic mellitus. J Nutr Biochem 12: 351–356.
- Honda M, Hara Y (1993): Inhibition of rat small intestinal sucrase and α-glucosidase activities by tea polyphenols. Biosci Biotechnol Biochem 57: 123–124.
- Kawai Y, Kumagai H, Kurihara H, Yamazaki K, Sawano R, Inoue N (2006): β-glucosidase inhibitory activities of phenylpropanoid glycosides, vanicoside A and B from Polygonum sachalinense rhizome. Fitoterapia 77: 456–459.
- Khan N, Sultana S (2005): Chemomodulatory effect of Ficus racemosa extract against chemically induced renal carcinogenesis and oxidative damage response in Wistar rats. Life Sci 29: 1194–1210.
- Kwon YI, Apostolidis E, Shetty K (2008): In vitro studies of eggplant (Solanum melongena) phenolics as inhibitors of key enzymes relevant for type 2 diabetes and hypertension. Bioresource Technol 99: 2981–2988.
- Lebovitz HE (1997): Alpha-glucosidase inhibitors. Endocrinol Metab Clin North Am 26: 539–551.
- Lee DS, Lee SH (2001): Genistein, a soy isoflavone, is a potent alpha glucosidase inhibitor. FEBS Lett 501: 84–86.
- Li RW, Leach DN, Myers SP, Lin GD, Leach GJ, Waterman PG (2004): A new anti-inflammatory glucoside from Ficus racemosa L. Planta Med 70: 421–426.
- Marshall J (1974): Application of enzymic methods to the structural analysis of polysaccharides. Adv Carb Chem Biochem 30: 257–370.
- Matsui T, Ueda T, Oki T, Sugita K, Terahara N, Matsumoto K (2001): alpha-Glucosidase inhibitory action of natural acylated anthocyanins. 1. Survey of natural pigments with potent inhibitory activity. J Agric Food Chem 49: 1948–1951.
- Ou S, Kwok K, Li Y, Fu L (2001): In vitro study of possible role of dietary fiber in lowering postprandial serum glucose. J Agric Food Chem 49: 1026–1029.
- Rahman NN, Khan M, Hasan R (1994): Bioactive components from Ficus glomerata. Pure Appl Chem 66: 2287–2290.
- Rao BR, Murugesan T, Sinha S, Saha BP, Pal M, Mandal SC (2002): Glucose lowering efficacy of Ficus racemosa bark extract in normal and alloxan diabetic rats, Phytother Res 16: 590–592.
- Rhabasa-Lhoret R, Chiasson JL (2004): International Textbook of Diabetes Mellitus, 3rd ed., Vol 1. London, John Wiley & Sons Ltd., pp. 901–914.
- Robinson KM, Begovic ME, Rhinehart BL, Heineke EW, Ducep JB, Kastner PR, Marshall FN, Danzin C (1991): New potent α-glucohydrolase inhibitor MDL 73945 with long duration of action in rats. Diabetes 40: 825–830.
- Shim YJ, Doo HK, Ahn SY, Kim YS, Seong JK, Park IS, Kim BH (2003): Inhibitory effect of aqueous extract from the gall of Rhus chinensis on alpha-glucosidase activity and postprandial blood glucose. J Etnhopharmacol 85: 283–287.
- Tiwari AK, Rao JM (2002): Diabetes mellitus and multiple therapeutic approaches of phytochemicals: present status and future prospects. Curr Sci 23: 30–33.
- Vasudevan K, Sophia D, Balakrishanan S, Manoharan S (2007): Antihyperglycemic and antilipidperoxidative effects of Ficus racemosa (Linn.) bark extracts in alloxan induced diabetic rats. J Med Res 7: 330–338.
- WHO (1999): Definition, diagnosis and classification of diabetes mellitus and its complications, Part 1: Diagnosis and classification of diabetes mellitus. Report of WHO Consultation. Geneva, World Health Organization.