Abstract
Urtica cannabina L. (Urticaceae) is a perennial herb that grows in Xinjiang Uighur Autonomous Region (northwest China). Two megastigmanes (1, 2) and five flavonoid glycosides (3-7) were isolated from its fruit. Compound 1 was determined by spectroscopic analysis to be (+)-blumenol A, and its absolute stereochemistry was determined in detail using chemical conversion and a modification of Mosher’s method. Other compounds were identified as (+)-dehydrovomifoliol (2), isovitexin (3), isoquercitrin (4), astragalin (5), afzelin (6), and quercitrin (7) using spectroscopic (NMR, HMBC, MS) and physical methods (melting point and optical rotation). Compounds 1-7 were isolated from this plant for the first time, while this is the first report of megastigmanes in the Urticaceae family. The chemotaxonomic significance of the isolation of these megastigmanes and flavonoid glycosides from Urtica species is discussed.
Introduction
“The genus Urtica (Urticaceae) contains about 50 taxa distributed from temperate to subtropical regions, e. g. Europe, North Africa, Northwest China, Japan and North America. In China, there are 23 taxa (16 species, 6 subspecies, and 1 variety). In China there are 23 taxa (16 species, 6 subspecies, and 1 variety), including seven species that are native to the Xinjiang Uighur Autonomous Region (northwest China) (CitationWang et al., 2002) (Urtica cannabina L., U. angustfolia Fisch., U. laetevirens Maxi., U. dioica L., U. kunlunshanica Y. Yang., U. thunbergiana Sieb. et Zucc., and U. urens L.).
The leaves of Urtica are nutritious and rich in micronutrients; however, they need to be steamed or otherwise cooked before ingestion to destroy their stinging hairs, which contain histamine, formic acid, acetylcholine, acetic acid, butyric acid, leukotrienes, 5-hydroxytryptamine, and other irritants (CitationWagner et al., 1994; CitationEmmelin & Feldberg, 1949). Contact with the hairs leads to a mildly painful sting, development of an erythematous macule, and itching or numbness for a period lasting from minutes to days.
Plants of the genus Urtica are commonly used in the traditional medicine of Turkey (CitationBaser et al., 1986), various countries in Europe (CitationCai, 2001), and Japan (CitationNamba, 1980) for the treatment of rheumatism, internal disease, skin conditions such as rashes, bleeding due to wounds, and benign prostatic hyperplasia (BPH) (CitationIida et al., 1994).
The hydrophilic components of Urtica, including polysaccharides and lectins, appear to be important medicine, and particularly inhibit prostatic hyperplasia (CitationLichius et al., 1999). The importance of Urtica root lignans, such as (-)-3,4-divanillyltetrahydrofuran, to improve benign prostatic hyperplasia (BPH) and other androgen- and estrogen-sensitive conditions, may be due to interference with the binding of sex hormone binding globulin (SHBG) to testosterone, the testosterone receptor, and/or the SHBG receptor (CitationSchottner et al., 1997; CitationHryb et al., 1995). The steroidal compounds stigmasterol, stigmast-4-en-3-one, and campesterol inhibit the prostatic sodium/potassium pump, which may contribute to the effects of Urtica on BPH (CitationHirano et al., 1994). However, the small quantity of β-sitosterol contained in Urtica root (<0.01% of the total mass) is unlikely to have an effect on BPH, given that 60 mg β-sitosterol daily is the usual amount necessary to reduce symptoms (CitationBerges et al., 1995). U. dioica agglutinin (UDA) is a heat- and acid-resistant lectin found in stinging Urtica, primarily in the root (CitationGalelli & Truffa, 1993). UDA activates the cell-mediated immunity, not observed for any other known plant lectin (CitationGalelli & Truffa, 1993). UDA appears to prevent formation of a systemic lupus erythematosus-like condition in mice, and has diverse antiviral effects in vitro (CitationMusette et al., 1996; CitationBalzarini et al., 1992). It also antagonizes the epidermal growth factor receptor that may inhibit the formation of BPH (CitationWagner et al., 1995).
In Xinjiang Uighur Autonomous Region (northwest China), U. cannabina aerial portion and fruit are medicinal plants used in Uighur traditional medicine (CitationLiu et al., 1999; CitationYasen et al., 1999). The fruit as a component of a preparation has been used for chronic bronchitis (CitationYasen et al., 1999). However, there have been few studies of the constituent chemicals of the fruit. CitationZhang et al. (2005) isolated scutellarein-7-O-α-l-rhamnoside and vicenin-2 from the leaves, while CitationMa et al. (2004) isolated β-sitosterol, scopoletin, caprylic alcohol, daucosterol, p-coumaric acid methyl ester, and p-coumaric acid from the whole herb. In the present study, we attempted to identify useful components of the fruit of U. cannabina to support its development as a Uighur traditional medicinal resource.
Herein, we report the isolation of the megastigmanes 1 and 2 from a methanol (MeOH) extract of U. cannabina fruit. To the best of our knowledge, this is the first report on the Urticaceae family. Compounds 3-7 were also isolated from this plant for the first time. In addition, the chemotaxonomic significance of these compounds was evaluated and compared among Urtica species.
Materials and methods
General experimental procedures
Melting points (mp) were determined using a Yanaco micro melting point apparatus, and were uncorrected. UV spectra were obtained using a Shimadzu UV 1600 spectrophotometer and optical rotation data were obtained using a Horiba SEPA-300 digital polarimeter. Electron Ionization Mass Spectrometry (EI-MS) and Fast Atom Bombardment Mass Spectrometry (FAB-MS) data were obtained using a JEOL JMS-700 mass spectrometer. Circular dichroism (CD) spectra were obtained in MeOH using a JASCO J-820 spectrometer. 1H- and 13C-NMR spectra were obtained using a JEOL JNM-AL 400 spectrometer with tetramethylsilane as an internal standard. TLC was performed using silica gel 60 F254 TLC plates (Merck) and RP-18 F254S TLC plates (Merck). Silica gel 60 N (particle size: 100-200 μm, Kanto Chemical) was used for silica gel open column chromatography. Separation was carried out using normal and reverse phase HPLC with an SPD-20A detector (Shimadzu) at 254 nm.
Plant materials
Dried fruits of U. cannabina were purchased from Xinjiang Hospital of Uighur Medicine (Urumqi, Xinjiang, China), for use as an herbal medicine in May 2006. A voucher specimen was identified by Kuang Hai Xue (Heilongjiang University of Traditional Chinese Medicine) and deposited at the Department of Natural Medicine and Phytochemistry, Meiji Pharmaceutical University (Tokyo) (Accession No. 06UC001).
Extraction and isolation
Dried fruits of U. cannabina (8.5 kg) were extracted with 13 L of MeOH under reflux for 4 h (3 times). The MeOH extract was concentrated under reduced pressure to yield a sample of 416.6 g, of which 410 g was suspended in water and partitioned with ethyl acetate (AcOEt). The AcOEt-soluble portion (10 g) was subjected to column chromatography on silica gel and eluted stepwise using a series of n-hexane/AcOEt mixtures (20:1, 10:1, 5:1, 2:1, 1:1, 1:2, and 1:5), AcOEt, and MeOH to yield 10 fractions (A1-A10). Fraction A7 (1034.6 mg) was further separated by chromatography on a Sephadex LH-20 column (φ 40 × 1200 mm, GE Healthcare) using MeOH into 7 subfractions, of which subfraction A7-2 (147.4 mg) was subjected to preparative reverse phase HPLC (COSMOSIL 5C18-ARII column of φ 10 × 250 mm, at 1 mL/min with UV detection at 254 nm) using MeOH/H2O (5:4) to yield compound 1 (31.2 mg). Fraction A6 (1072.6 mg) was purified by normal phase HPLC (COSMOSIL 5SL-II column of φ 20 × 250 mm, at 2 mL/min, with UV detection at 254 nm) using n-hexane/AcOEt (1:2) to give 4 subfractions, of which subfraction A6-2 (32.3 mg) was purified by reverse phase HPLC on the COSMOSIL 5C18-ARII column as above using MeOH/H2O (2:3) to yield compound 2 (5.8 mg). Fraction A10 (2.6 g) was further separated by chromatography on the Sephadex LH-20 column (φ 40 × 1200 mm, GE Healthcare) using MeOH to yield 8 subfractions, of which subfraction A10-7 (100.5 mg) was purified by reverse phase HPLC on the COSMOSIL 5C18-ARII column as above using MeOH/H2O (5:4) to yield compounds 3 (2.9 mg) and 4 (7.7 mg). Fraction A9 (2.2 g) was further separated by chromatography on the Sephadex LH-20 column (φ 40 × 1200 mm, GE Healthcare) using MeOH to yield 9 corresponding fractions, of which subfraction A9-7 (38 mg) was purified by reverse phase HPLC on the COSMOSIL 5C22-ARII column using MeOH/H2O (5:4) to yield compounds 5 (6.3 mg) and 6 (5.0 mg). Subfraction A9-8 (9.4 mg) was purified by reverse phase HPLC on the COSMOSIL 5C22-ARII column using MeOH/H2O (5:4) to yield compound 7 (2.7 mg).
Compound 1
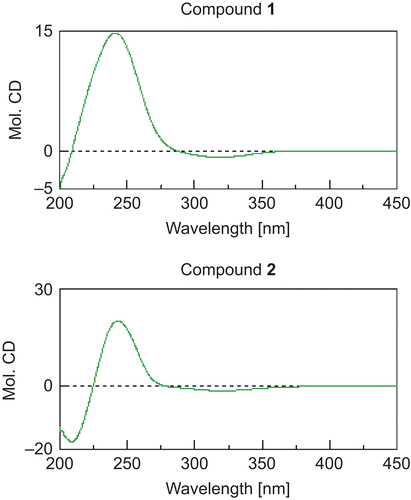
(+)-Blumenol A (1): white crystal, mp 108-109°C. [α]D26 + 245.2° (c = 0.69, MeOH). UV λmax (MeOH) nm (log ϵ) 237 (4.06). CD (c = 1.34 × 10−4 M, MeOH) Δϵ (nm): +14.89 (241.6), -0.85 (320.2). Positive-mode FAB-MS m/z 225 (M+H)+, 247 (M+Na)+. Negative-mode FAB-MS m/z 223 (M-H)−. NMR ( and ).
Table 1. 1H-NMR data of compounds 1 and 2.
Table 2. 13C-NMR data of compounds 1 and 2.
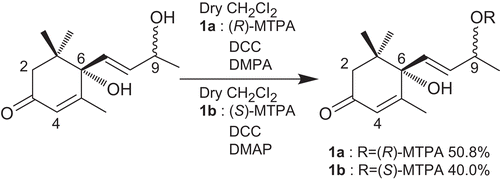
Preparation of (R)- and (S)-MTPA esters 1a and 1b from compound 1
A working solution of (R)-MTPA [(R)-MTPA (Sigma-Aldrich) 51.5 mg and DCC (Nacalai) 36 mg in 0.5 mL of dry CH2Cl2] was prepared. Compound 1 (2.9 mg, 13 μmol) and DMAP (Wako) (16.4 mg) were dissolved in dry CH2Cl2 (0.5 mL) and stirred at room temperature for 30 min. The (R)-MTPA working solution was then added and the mixture was stirred at room temperature for 1 h. After the addition of 5 mL each of water and CH2Cl2, the solution was washed successively with 5% HCl (5 mL), saturated NaHCO3 (5 mL) and brine (5 mL). The organic soluble part was dried with Na2SO4 and concentrated in vacuo to yield a residue (CitationKai et al., 2007). 1a (2.9 mg, 50.8%) was purified on a silica gel (2 mL) column using n-hexane/AcOEt. Using a similar procedure, 1b (2.3 mg, 40%) was prepared from compound 1 (2.9 mg, 13 μmol) with (S)-MTPA (49.7 mg), DCC (36 mg) and DMAP (17.3 mg) ().
9-O-(R)-MTPA ester (1a)
Colorless oil, [α]D29 + 189.3° (c = 0.097, CHCl3). UV λmax (CHCl3) nm (log ϵ) 239.5 (4.24). EI-MS m/z (rel. int. %) 440 (1) (M)+, 206 (26), 189 (77), 150 (100), 1H-NMR ().
Table 3. 1H-NMR spectral data of compound 1 and derivatives 1a and 1b.
9-O-(S)-MTPA ester (1b)
Colorless oil, [α]D28 + 159.1° (c = 0.067, CHCl3). UV λmax (CHCl3) nm (log ϵ) 239.5 (4.32). EI-MS m/z (rel. int. %) 440 (1) (M)+, 206 (25), 189 (77), 150 (100), 1H-NMR ().
Compound 2
(+)-Dehydrovomifoliol (2): yellow oil, [α]D26 + 119.9° (c = 0.48, MeOH). UV λmax (MeOH) nm (log ϵ) 239 (4.04). CD (c = 1.26 × 10−4 M, MeOH) Δϵ (nm) +20.44 (243). EI-MS m/z (rel. int. %) 222 (1) (M)+, 166 (32), 149 (10), 124 (100), NMR ( and ).
Compound 3
Isovitexin (3): yellow amorphous solid, mp 210°-215°C. [α]D27 + 40.8° (c = 0.21, MeOH). UV λmax (MeOH) nm (log ϵ) 207 (3.06), 271.0 (2.73), 333.5 (2.74). FAB-MS m/z (neg.) 431 (M-H)−. 1H-NMR (400 MHz, DMSO-d6) δ 6.77 (1H, s, H-3), 6.5 (1H, s, H-8), 7.93 (2H, d, J = 8.8, H-2′, 6′), 6.93 (2H, d, J = 8.8, H-3′, 5′), 4.59 (1H, d, J = 9.8, H-1″ of Glc), 13.55 (1H, brs, OH-5), 13C-NMR ().
Table 4. 13C-NMR data of compounds 3-7.
Compound 4
Isoquercitrin (4): yellow powder, mp 175°-177°C. [α]D23 -5.2° (c = 0.10, MeOH). UV λmax (MeOH) nm (log ϵ) 202.5 (4.59), 257 (4.29), 356.5 (4.16). FAB-MS m/z (neg.) 463 (M-H)−. 1H-NMR (400 MHz, CD3OD) δ 6.2 (1H, d, J = 2.2, H-6), 6.39 (1H, d, J = 2, H-8), 7.71 (1H, brs, H-2′), 6.87 (1H, d, J = 8.2, H-5′), 7.58 (1H, dd, J = 8.2, H-6′), 5.25 (1H, d, J = 7.6, H-1″ of Glc), 13C-NMR ().
Compound 5
Astragalin (5): yellow powder, mp 174°-178°C. [α]D27 -11.3° (c = 0.32, MeOH). UV λmax (MeOH) nm (log ϵ) 266 (4.26), 350 (4.22). FAB-MS m/z (neg.) 447 (M-H)−. 1H-NMR (400 MHz, CD3OD) δ 6.22 (1H, d, J = 2, H-6), 6.41 (1H, d, J = 2, H-8), 8.05 (2H, d, J = 8.9, H-2′, 6′), 6.89 (2H, d, J = 8.9, H-3′, 5′), 5.22 (1H, d, J = 7.6, H-1″ of Glc), 13C-NMR ().
Compound 6
Afzelin (6): yellow powder, mp 173°-175°C. [α]D26 -104° (c = 0.1, MeOH). UV λmax (MeOH) nm (log ϵ) 265 (3.98), 342 (3.81). FAB-MS m/z (neg.) 431 (M-H)−. 1H-NMR (400 MHz, DMSO-d6) δ 6.21 (1H, d, J = 2, H-6), 6.40 (1H, d, J = 2, H-8), 7.75 (2H, d, J = 8.8, H-2′, 6′), 6.91 (2H, d, J = 8.8, H-3′, 5′), 5.3 (1H, d, J = 1.5, H-1″ of Rha), 12.6 (1H, brs, OH-5), 13C-NMR ().
Compound 7
Quercitrin (7): yellow powder, mp 178°-180°C. [α]D27 -96° (c = 0.1, MeOH). UV λmax (MeOH) nm (log ϵ) 206 (4.64), 256.5 (4.19), 351.5 (4.06). FAB-MS m/z (neg.) 447 (M-H)−. 1H-NMR (400 MHz, DMSO-d6) δ 6.18 (1H, d, J = 2.2, H-6), 6.37 (1H, d, J = 2.2, H-8), 7.26 (1H, d, J = 2.2, H-2′), 6.85 (1H, d, J = 8.3, H-5′), 7.22 (1H, d, J = 8.3, 2.2, H-6′), 5.2 (1H, d, J = 1.2, H-1″ of Rha), 12.58 (1H, brs, OH-5), 13C-NMR ().
Results and discussion
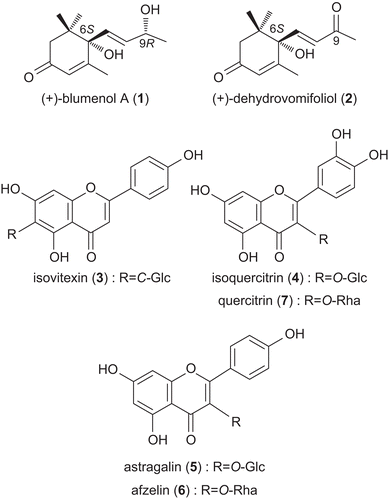
Here we report the first isolation of megastigmanes 1 and 2 and flavonoid glycosides 3-7 () from U. cannabina as well as the first isolation of megastigmanes from plants of the Urticaceae family.
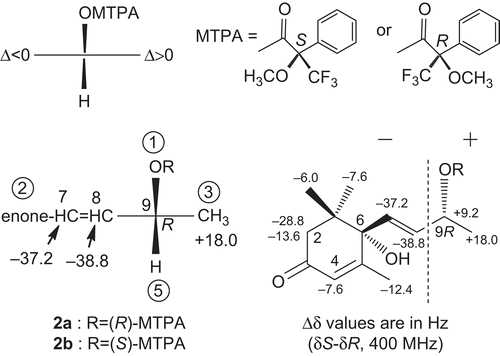
The absolute configuration of 1 at C-6 was determined by application of the circular dichroism (CD) helicity rule (CitationWeiss et al., 1973) based on the known compound (+)-dehydrovomifoliol (2). The CD spectrum of 2 showed a positive Cotton effect (Δϵ +20.44) at 243 nm (), so the 6-position was determined to have the S configuration. On the basis of this evidence, because the CD spectrum of 1 exhibited a positive Cotton effect (Δϵ +14.89) at 241.6 nm (), we also concluded that the 6-position of 1 has the S configuration. In order to clarify the absolute stereostructure at the 9-position of compound 1, a modified Mosher’s method was used. Compound 1 was treated with either (R)- or (S)-α-methoxy-α-(trifluoromethyl)phenylacetic acid (MTPA), N,N-dicyclohexylcarbodiimide (DCC), and 4-(dimethylamino)pyridine in dry dichloromethane at room temperature to yield the 9-O-(R)-MTPA ester 1a (50.8%), and the 9-O-(S)-MTPA ester 1b (40.0%), respectively (). As shown in , the NMR signals due to protons attached at the 2a, 2b, 4, 7, 8, 11, 12, and 13-positions in 1a were observed at a lower field than in 1b (negative Δδ), while the signal of the protons at the 10-position in 1a were observed at a higher field than in 1b (positive Δδ). Consequently, the absolute configuration at the 9-position was elucidated to be R. On the basis of this evidence, the structure of 1 was determined to be (+)-blumenol A.
The other five compounds were identified as isovitexin (3), isoquercitrin (4), astragalin (5), afzelin (6), and quercitrin (7) by comparison of their spectroscopic data (NMR, UV, MS, melting points and optical rotation) with reported values.
(+)-Blumenol A (1) has been previously isolated from the leaves of Podocarpus blumei Endl. (Podocarpaceae) (CitationGalbraith & Horn, 1972), while (+)-dehydrovomifoliol (2) has been isolated from the aerial parts of Beta vulgaris L. var. cicla L. (Chenopodiaceae) (CitationKim et al., 2004). However, this is the first report of the isolation of megastigmanes from plants of the Urticaceae family. Afzelin (6) and quercitrin (7) have been isolated from the fruits of Ocotea velloziana Meisn. (Lauraceae) (CitationGarcez et al., 1995) and Juniperus communis L. (Cupressaceae) (CitationHiermann et al., 1996). Isovitexin (3) has been isolated from other Urticaceae species, including the leaves of Phenax angustifolius Wedd. (CitationPiccinelli et al., 2005). Isoquercitrin (4) and astragalin (5) have been isolated from flowers of U. dioica (CitationWang et al., 2006), and roots of U. fissa (CitationChaurasia & Wichtl, 1987).
The presence of megastigmanes 1 and 2, afzelin (6) and quercitrin (7) has chemotaxonomic significance for Urticaceae family. Isovitexin (3) does not appear to have a significant chemotaxonomic presence among Urtica species, although it has been reported in Phenax, another member of the Urticaceae family. As isovitexin has not previously been reported in Urtica species, its presence in U. cannabina is also of chemotaxonomic significance.
Naturally occurring flavonoids have been isolated from more than 6000 species, and several have been reported to exhibit anti-allergic, anti-inflammatory, anticancer and antiviral activities. Megastigmanes have been reported to exhibit radical scavenging activity (CitationMatsunami et al., 2006). In future studies, the compounds identified in this plant may be investigated for activity against chronic bronchitis, which this plant used for in Uighur traditional medicine.
Acknowledgement
Authors are grateful to Professor Dr. Uper Hamurat of Xinjiang Medical University in China, and Dr. Hisahiro KAI of Kyushu University of Health and Welfare in Japan. A part of this work was supported by Japan China Medical Association in 2007.
Declaration of interest
The authors report no conflict of interest. The authors are responsible for the content and writing of the paper.
References
- Balzarini J, Neyts J, Schols D, Hosoya M, Van DE, Peumans W, De CE (1992): The mannose-specific plant lectins from Cymbidium hybrid and Epipactis helleborine and the (N-acetylglucosamine)n-specific plant lectin from Urtica dioica are potent and selective inhibitors of human immunodeficiency virus and cytomegalovirus replication in vitro. Antiviral Res 18: 191–207.
- Baser KHC, Honda G, Miki W (1986): Herb Drugs and Herbalists in Turkey. Tokyo, Toyo Publishing, pp. 146, 161, 188, 193, 222.
- Berges RR, Windeler J, Trampisch HJ, Senge T (1995): Randomised, placebo-controlled, double-blind clinical trial of beta-sitosterol in patients with benign prostatic hyperplasia. Beta-sitosterol study group. Lancet 345: 1529–1532.
- Cai Q (2001): Rheuma-Hek capsule. World Phytomedicines 16: 282.
- Chaurasia N, Wichtl M (1987): Flavonol glycosides from Urtica dioica. Planta Med 53: 432–444.
- Emmelin N, Feldberg W (1949): Distribution of acetylcholine and histamine in nettle plants. New Phytol 48: 143–148.
- Galbraith MN, Horn DHS (1972): Structures of the natural products blumenols A, B, and C. J Chem Soc Chem Comm 3: 113–114.
- Galelli A, Truffa BP (1993): Urtica dioica agglutinin. A superantigenic lectin from stinging nettle rhizome. J Immunol 151: 1821–1831.
- Garcez WS, Yoshida M, Gottlieb OR (1995): Benzylisoquinoline alkaloids and flavonols from Ocotea vellosiana. Phytochemistry 39: 815–816.
- Hiermann A, Kompek A, Reiner J, Auer H, Schubert-Zsilavecz M (1996): Investigation of flavonoid pattern in fruits of Juniperus communis. Sci Pharm 63: 437–444.
- Hirano T, Homma M, Oka K (1994): Effects of stinging nettle root extracts and their steroidal components on the Na+, K (+)-ATPase of the benign prostatic hyperplasia. Planta Med 60: 30–33.
- Hryb DJ, Khan MS, Romas NA, Rosner W (1995): The effect of extracts of the roots of the stinging nettle (Urtica dioica) on the interaction of SHBG with its receptor on human prostatic membranes. Planta Med 61: 31–32.
- Iida M, Asami Y, Tanaka T, Honda G, Tabata M, Sezik E, Yesilada E (1994): Studies on genus Urtica in Turkey (1). Identification of Urtica in Turkey by internals of axis and petiole. Nat Med 48: 237–243.
- Kai H, Baba M, Okuyama T (2007): Two new megastigmanes from the leaves of Cucumis sativus. Chem Pharm Bull 55: 133–136.
- Kim I, Chin YW, Lim SW, Kim YC, Kim J (2004): Norisoprenoids and hepatoprotective flavone glycosides from the aerial parts of Beta vulgaris var. cicla. Arch Pharm Res 27: 600–603.
- Lichius JJ, Renneberg H, Blaschek W, Aumüller G, Muth C (1999): The inhibiting effects of components of stinging nettle roots on experimentally induced prostatic hyperplasia in mice. Planta Med 65: 666–668.
- Liu YM, Liu WX, Shawuti YKM, Zou Y (1999): Pharmacography of Uighur (Part Two). Urumuqi, Xinjiang Science Technology Hygiene Publishing House, pp. 510.
- Matsunami K, Takamori I, Shinzato T, Aramoto M, Kondo K, Otsuka H, Takeda Y (2006): Radical-scavenging activities of new megastigmane glucosides from Macaranga tanarius (L.) Mull.-Arg. Chem Pharm Bull 54: 1403–1407.
- Ma XM, Guo YJ, Wang LS (2004): Study on chemical contents of Urtica cannabina L. Zhongguo Zhongyao Zazhi 29: 472.
- Musette P, Galelli A, Chabre H (1996): Urtica dioica agglutinin, a V beta 8.3-specific superantigen, prevents the development of the systemic lupus erythematosus-like pathology of MRL lpr/lpr mice. Eur J Immunol 26: 1707–1711.
- Namba T (1980): Coloured Illustrations of Wakan-Yaku, Vol. I. Osaka, Hoikusha Publishing, pp. 106.
- Piccinelli AL, Mahmood N, Mora G, Poveda L, De Simone F, Rastrelli L (2005): Anti-HIV activity of dibenzylbutyrolactone-type lignans from Phenax species endemic in Costa Rica. J Pharm Pharmacol 57: 1109–1115.
- Schottner M, Gansser D, Spiteller G (1997): Lignans from the roots of Urtica dioica and their metabolites bind to human sex hormone binding globulin (SHBG). Planta Med 63: 529–532.
- Wagner H, Willer F, Samtleben R, Boos G (1994): Search for the antiprostatic principle of stinging nettle (Urtica dioica) roots. Phytomedicine 1: 213–224.
- Wagner H, Geiger WN, Boos G, Samtleben R (1995): Studies on the binding of Urtica dioica agglutinin (UDA) and other lectins in an in vitro epidermal growth factor receptor test. Phytomedicine 2: 287–290.
- Wang MY, Wei YF, Huang XY (2002): Researches in medicinal plant of Urtica L. J Chin Mater Med 25: 58–60.
- Wang MY, Wei YF, Li XB (2006): Chemical constituents of anti-rheumatism fraction from Urtica fissa. Zhongcaoyao 37: 1300–1303.
- Weiss G, Koreeda M, Nakanishi K (1973): Stereochemistry of theaspirone and the blumenols. J Chem Soc Chem Comm 16: 565–566.
- Yasen TRS, Tian RW, Cheng LY, Hamulati WPR, Liu YM (1999): People’s Republic of China Medical Department Drugs Standard Uighur Medicine Fascicle. Urumuqi, Xinjiang Science Technology Hygiene Publishing, pp. 198.
- Zhang ML, Li ZP, Jia XM (2005): Study on chemical constituents of Urtica cannabina L. Nat Prod Res Dev 17: 175–176.