Abstract
Prasaplai is a Thai traditional medicine for relieving dysmenorrhea and adjusting the menstrual cycle. Three fatty acid esters, (E)-4-(3,4-dimethoxyphenyl)but-3-en-1-yl linoleate (1), (E)-4-(3,4-dimethoxyphenyl)but-3-en-1-yl oleate (2) and (E)-4-(3,4-dimethoxyphenyl)but-3-en-1-yl palmitate (3) are formed during storage from the reaction of chemical components in two herbs, i.e., fatty acids in Nigella sativa (L.) (Ranunculaceae) and (E)-4-(3,4-dimethoxyphenyl)but-3-en-1-ol (compound D) in Zingiber cassumunar (Roxb.) (Zingiberaceae). The formations of these artifacts were monitored for 1 year and their amounts were analyzed by HPLC at certain periods of time. The results showed that artifact formation was saturated after 73 days of storage. The amount of each artifact in the saturation period ranged from 3.93 ± 0.06 to 4.30 ± 0.18% w/w for compound 1, 1.69 ± 0.08 to 1.9 ± 0.13% w/w for compound 2 and 0.09 ± 0.003 to 0.1 ± 0.005% w/w for compound 3. Cytotoxicity of the artifacts was evaluated using NCI-H187, KB, and BC cancer cell lines and found that the IC50 of all artifacts in all tests were higher than 20 μg/mL. For acute toxicity in mice, the LD50 of each artifact was more than 300 mg/kg.
Introduction
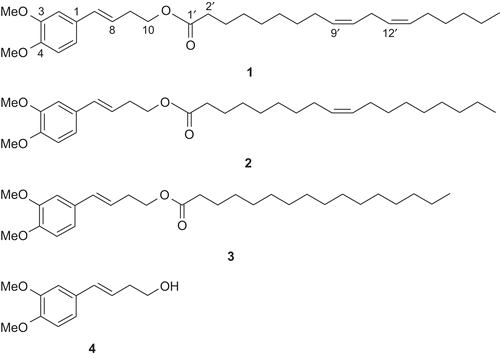
Prasaplai preparation is a traditional drug formulation which is listed in the Thai traditional common household drug list for relieving dysmenorrhea and adjusting the menstruation cycle (CitationNEDLC, 1999). Prasaplai is composed of ten crude plants and two pure compounds. The crude plants are the root of Acorus calamus (L.) (Araceae), the bulb of Allium sativum (L.) (Alliaceae), the pericarp of Citrus hystrix (DC.) (Rutaceae), the rhizome of Curcuma zedoaria (Roscoe.) (Zingiberaceae), the bulb of Eleutherine palmifolia (L.) Merr (Iridaceae), the seed of Nigella sativa (L.) (Ranunculaceae), the fruits of Piper retrofractum (Vahl.) (Piperacea), and P. nigrum (L.) (Piperaceae), the rhizomes of Zingiber cassumunar (Roxb.) (Zingiberaceae) and Z. officinale (Roscoe.) (Zingiberaceae). The two pure compounds are sodium chloride and camphor (CitationPoomchusri, 1973). Three artifacts (E)-4-(3,4-dimethoxyphe-nyl)but-3-en-1-yl linoleate (1), (E)-4-(3,4-dimethoxy-phenyl)but-3-en-1-yl oleate (2) and (E)-4-(3,4-dimethoxy-phenyl)but-3-en-1-yl palmitate (3) () are originated during storage of the preparation. They were found after mixing all the ingredients together on day 1. After investigation of the origin of the artifacts by a systematic preparation of two component mixtures and subsequent HPLC analysis, it was found that the artifacts were formed by the interaction of linoleic, oleic and palmitic acids from N. sativa seeds and (E)-4-(3,4-dimethoxyphenyl)but-3-en-1-ol (compound D) from Z. cassumunar rhizomes (CitationNualkaew et al., 2004). Compounds 1-3 all showed positive results against Mycobacterium tuberculosis H37Ra with minimum inhibition concentrations (MICs) of 200, 100, and 200 µg/mL, respectively while compound 2 showed anti-HSV-1 at IC50 of 42.6 µg/mL and compound 3 showed cytotoxicity against Vero cells with an IC50 of 38 µg/mL (CitationTangyuenyongwatana & Gritsanapan, 2007).
In this study, the observation of the artifacts formation was extended for one year and the duration promoting the highest artifact formation was evaluated. The cytotoxicity of all artifacts against human cancer cell lines which has not been reported before was evaluated on NCI-H187 (Human small cell lung carcinoma), KB (Human epidermoid carcinoma of cavity) and BC (breast cancer cell line). The acute toxicity of each artifact was also evaluated in mice. These pieces of information are essential for the safety of Prasaplai administration.
Materials and methods
General
Reagents were of reagent grade and purchased from Aldrich (USA). HPLC grade mobile phase was obtained from Merck (Germany). All artifacts and compound D were synthesized in our laboratory according to the procedures (CitationTangyuenyongwatana & Gritsanapan, 2007, Citation2008) with the purity more than 95%.
Plant materials
The rhizomes of Z. cassumunar cultivated in Thailand and the seeds of N. sativa imported from India were used in this study. The crude drugs were identified by Wandee Gritsanapan at the Department of Pharmacognosy, Faculty of Pharmacy, Mahidol University (Bangkok, Thailand). All plant materials were also compared with specimens at the Forest Herbarium, Department of National Park, Wildlife and Plant Conservation, Ministry of Natural Resources and Environments, Bangkok. The voucher specimens of Z. cassumunar (SMU 029) and N. sativa (SMU 026) were deposited at the Department of Pharmacognosy, Faculty of Pharmacy, Mahidol University, Bangkok, Thailand.
Animals
Twenty-five male and female ICR mice weighing 22-25 g were obtained from The National Laboratory Animal Center, Mahidol University, (Salaya, Nakon Pathom, Thailand) for acute toxicity study. Five mice of the same sex were housed in a cage with free access to food and water. They were housed in a quiet room under a 12 h light: 12 h dark cycle at 25° ± 2°C for 14 days. The test was approved for ethical clearance by Institutional Animal Care and Use Committee, Faculty of Pharmacy, Mahidol University (Proof no. 009/2550).
HPLC analysis method
A Knauer Wellchrom HPLC pump K1001 was used with Knauer Wellchrom fast-scanning photometer K-2600 (Germany). The separation was performed on Kromasil 5 µ 100A C18 (250x4mm column, Phenomenex, California, USA), flow rate was 0.8 mL/min and the solvent system was a gradient elution of 1% acetic acid in water and CH3CN starting from 85:15, 70:30, 55:45, 50:50, 30:70, 15:85, 0:100 and 0:100 at 0, 8, 25, 30, 55, 65, 80 and 100 min, respectively. The method was validated by analysis of five different concentrations of standard solutions. Calibration was performed by a least-square linear regression of the peak area versus the respective standard concentration. The limits of quantitation (LOQs) were at the lowest concentration level among the linear ranges. The intraday precision of injection was determined by using the results of three replicate injections of the standard solutions containing compound D and three artifacts. The interday precision was studied by comparing the assays on different days (3 days). The recovery studies were carried out by adding three individual concentrations of standard compounds (low, medium and high spike) to the dried powder sample. The extraction and analysis of the spiked material was done under the same procedure as the sample.
Artifacts formation versus time
The dried rhizomes of Z. cassumunar (250 g) and the dried seeds of N. sativa (250 g) were mixed in a mortar and ground for 15 min. The mixture was kept in an ambient temperature (25°C) and light protected. The average relative humidity ranged from 62% to 74% during this experiment. The mixture was analyzed and the data including humidity was collected in the certain period of time on day 4(29 September 2007, 72%), 17(12 October 2007, 70%), 30(26 October 2007, 70%), 51(15 November 2007, 62%), 73(7 December 2007, 63%), 123(26 January 2008, 64%), 175(19 March 2008, 63%), 264(17 June 2008, 70%), and 347(11 September 2008, 71%). The analysis was carried out by weighing 200 mg of the mixture and transferring it to a 25 mL volumetric flask. Ethanol 70% v/v (20 mL) was added and the mixture was sonicated for 30 min. After final extraction, the volume was adjusted with 70% ethanol and the mixture was mixed well. The mixture was then filtered through a membrane filter (0.45 µ) and 20 µL of the filtrate was injected into the HPLC system.
Cytotoxicity determination of NCI-H187, KB and breast cancer cells
For NCI-H187 (Human small cell lung carcinoma, ATCC CRL-5804) cytotoxicity determination, the metabolic activity assay was performed by using MTT microtiter plates assay (CitationVistica et al., 1991). Ellipticine and doxorubicin were used as positive controls. For KB (human epidermoid carcinoma of cavity, ATCC CCL-17) and BC (breast cancer cell line) assays, the tests were determined by colorimetric cytotoxicity assay that measures cell growth from cellular protein content (CitationSkehan et al., 1990). Ellipticine and doxorubicin were used as positive controls while DMSO was used as a negative control.
Acute toxicity study
Acute toxicity of artifacts was determined for an oral lethal dose in mice (CitationLorke, 1983). LD50 value of not less than 300 mg/kg body weight was required for a non-toxic result. The animals were randomized into three experimental groups. Each tested group of mice consisting of 5 males and 5 females was orally administered at a dose of 300 mg/kg. Mice in groups 1 and 2 were administered with 40% propylene glycol or distilled water, respectively, while mice in group 3 were administered with the artifacts. The animals were observed for mortality or any signs of abnormalities periodically during the first 24 h and twice daily for 7 days thereafter. Clinical signs, morbidity and mortality of tested groups were observed for 14 days and compared to the respective control group. The positions, shapes, sizes and colors of visceral organs, namely, heart, kidneys, lungs, stomach, intestine, liver, pancreas and sex organs were visually observed for any sign of gross lesions.
Results and discussion
The assay linearity was determined by the analysis of five different concentrations of standard solutions. The linear ranges of compounds 1, 2, 3, and compound D were 54-272 µg/mL (Y = 105593X+1772), 37-188 µg/mL (Y = 102038X+700), 1.8-32 µg/mL (Y = 536401X+1332), and 20-100 µg/ml (Y = 775997X+4257), respectively. The correlation coefficients (r2) ranged from 0.9992-0.9997 while limits of quantitation (LOQs) ranged from 0.9-1.6 µg/mL. The intraday precision of injection results demonstrated the relative standard deviation (RSD) of precision less than 3% and interday precision less than 4%. The recovery studies were preceded by spiking the standard artifact compounds in three different concentrations to a control mixture of Z. cassumunar and N. sativa. After HPLC analysis, the mean recovery values with RSD of compound 1, compound 2, compound 3, and compound D were 101.2% ± 2.39%, 98.27±1.00, 101.3% ± 2.3% and 101.5% ± 2.57%, respectively.
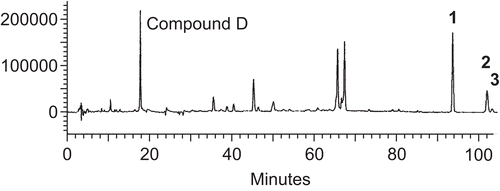
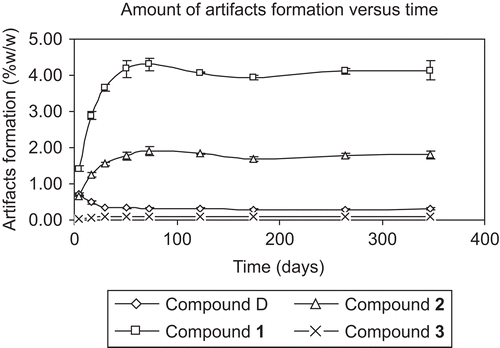
After mixing Z. cassumunar and N. sativa and keeping the mixture light proof at 25°C, amounts of compound D and artifacts formed in the mixture were analyzed by HPLC and the data are shown in . The HPLC pattern of each analysis was the same except for the height of the peaks (). The results showed the development of each artifact which correlated with storage time. From the graph (), the amounts of artifacts (compounds 1-3) extensively increased in the early period of mixing and continued to amplify in the first 30 days. Compound 1 had the greatest amount of development while compound 2 had a moderate growth and compound 3 showed slight formation during the time period. After 73 days of mixing, the amounts of artifacts formed slowly increased and had a tendency toward saturation. The amount of each artifact formed within the saturation period was ranged from 3.93% ± 0.06% to 4.3% ± 0.18% w/w for compound 1, 1.79% ± 0.06% to 1.9% ± 0.12% w/w for compound 2 and 0.09% ± 0.01% to 0.1% ± 0.01% w/w for compound 3. We reported a possible explanation for the formation of artifacts that might occur as a chemical reaction between alcohol and carboxylic acid with special condition (CitationTangyuenyongwatana & Gritsanapan, 2008). The reaction occurred in the environment of plant material in the dry form which acted as a matrix and capable of absorbing water from the reaction. The capability to hold the amount of water of the plant matrix accelerated the reaction to form the products. When the matrix was saturated with water, the formation of artifacts by esterification reaction no longer occurred.
Table 1. Amounts of artifacts and compound D during the period of 1 year storage.
On the other hand, the amount of compound D or (E)-4-(3,4-dimethoxyphenyl)but-3-en-1-ol, which is the precursor of all artifacts, dramatically decreased during the first 30 days after mixing (). After that, the amount of compound D was slightly decreased which was correlated with the increasing amounts of artifact formation.
The cytotoxicity tests of artifacts against human cancer cell lines NCI-H187, KB, and BC demonstrated IC50 higher than 20 μg/mL for all tests while the positive controls using ellipticine and doxorubicin showed IC50 values at 0.332 and 0.037 μg/mL against NCI-H187, 0.303 and 0.173 μg/mL against KB and 0.052 and 0.083 μg/mL against BC cell lines.
For acute toxicity test of each artifact, the dose of 300 mg/kg did not cause mortality or any sign of abnormality in tested mice. LD50 values of all artifacts were higher than 300 mg/kg. These pieces of information support the safety of the artifacts originated in the Prasaplai preparation which has been used as a traditional medicine for a long period of time. The artifacts formations may also occur in other traditional formulations and may have interesting biological activities which are waiting for exploration.
Acknowledgements
The authors thank the bioassay laboratory at BIOTEC of NSTDA, Thailand for helping us in the cytotoxicity study.
Declaration of interest
The authors report no conflict of interest. The authors are responsible for the content and writing of the paper.
References
- Lorke D (1983): A new approach to practical acute toxicity testing. Arch Toxicol 54: 275–287.
- NEDLC (1999). National List of Essential Drugs, Bangkok, National Essential Drug List Committee: p. 2.
- Nualkaew S, Gritsanapan W, Petereit F, Nahrstedt A (2004): New fatty acid esters originate during storage by the interaction of components in Prasaplai, a Thai traditional medicine. Planta Med 70: 1243–1246.
- Poomchusri NT (1973). Ayurvedic Study, second edition. Bangkok, Promjakkanpimp, p. 194.
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR (1990): New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82: 1107–1112.
- Tangyuenyongwatana P, Gritsanapan W (2007): Biological evaluations of fatty acid esters originated during storage of Prasaplai, a Thai traditional medicine. Nat Prod Res 21: 990–997.
- Tangyuenyongwatana P, Gritsanapan W (2008): A study on artifacts formation in the Thai traditional medicine Prasaplai. Planta Med 74: 1403–1405.
- Vistica DT, Skehan P, Scudiero D, Monks A, Pittman A, Boyd MR (1991): Tetrazolium-based assays for cellular viability: A critical examination of selected parameters affecting formazan production. Cancer Res 51: 2515–2520.