Abstract
An activity-directed fractionation and purification process was used to isolate 1,1-diphenyl-2-picrylhydrazyl radical (DPPH•) scavenging components from fruits of Capparis spinosa L. (Capparaeae). Ethyl acetate and aqueous fractions showed greater DPPH• scavenging activities compared to the petroleum ether fractions. The ethyl acetate fraction was subjected to purification using column chromatography. A new antioxidant cappariside (4-hydroxy-5-methylfuran-3-carboxylic acid, 1), together with seven known organic acids (2–8) for the first time from plants of genus Capparis and four known organic acids (9–12) were isolated from C. spinosa. The structures were elucidated by extensive analysis of 1D- and 2D-NMR spectroscopic. In addition, compounds 1, 2, 4, 5, 9, 10 and 12 indicated strong scavenging capacity for 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals with a SC50 value of 0.204 ± 0.002, 0.007 ± 0.0, 0.011 ± 0.0, 0.044 ± 0.0016, 0.032 ± 0.0, 0.090 ± 0.001, and 0.350 ± 0.017 mM, respectively.
Introduction
Capparis spinosa L., Caper, (Capparaeae) is a plant originating from dry regions in west or central Asia and spread particularly across the Mediterranean basin (CitationTrombetta et al., 2005). The plant is widely distributed in the Xinjiang Uighur Autonomous Region of China. C. spinosa is used not only as food, but also as medicine. Fruits of perennial shrubs of the genus Capparis have medicinal and aromatic properties (CitationEddouks et al., 2004). Fruits with small, soft seeds are preferred for the production of pickles (CitationÖzcan & Aydin, 2004; CitationMatthäus & Ozcan, 2005). C. spinosa is used in phytomedicine around the world as an antihyperglycemic (CitationEddouks et al., 2004), antihepatotoxic (CitationEddouks et al., 2005; CitationGadgoli & Mishra, 1999), antioxidant (CitationGermano et al., 2002; CitationSteenkamp et al., 2004), antifungal (CitationAli-Shtayeh & Abu Ghdeib, 1999), anti-inflammatory (CitationAl-Said et al., 1988) and antidiabetic (CitationEddouks et al., 2004; CitationYaniv et al., 1987). In China, C. spinosa has been used since ancient times in traditional medicine especially for the treatment of rheumatism and gout (CitationFu et al., 2008). With regard to the constituents of the C. spinosa, a number of flavonoids, alkaloids, terpenoids, volatile oils and fatty acids have been reported (CitationMatthäus & Ozcan, 2002; CitationSharaf et al., 1997; CitationKhanfar et al., 2003; CitationYang et al., 2008).
Free radicals have been found to be related to illnesses, cell damage, cell death, and gene mutation (CitationGutteridge & Halliwell, 1990), and the relationship between the intake of foods containing antioxidant components and the illnesses caused by oxidative damage has become an important research topic in the food and nutrition area. The methanol extract of C. spinosa buds showed strong antioxidant activities in vitro tests, and the antioxidant efficiency of the methanol extract may be attributed to its phenolic acid content (CitationGermano et al., 2002). But up to now there is little information available regarding the ingredients on antioxidant activities of C. spinosa. There were no detailed studies of the chemical composition of C. spinosa growing wild in China. In order to clarify its bioactive compounds with antioxidant activity, we studied the chemical constituents of the C. spinosa fruits systematically. Our detailed investigation has led to the discovery of a new organic acid (named cappariside), together with eleven known compounds: protocatechuic aldehyde, E-butenedioic acid, ethyl 3, 4-dihydroxybenzoate, syringic acid, 5-hydroxymethylfurfural, 5-hydroxymethyl furoic acid, 2-furoic acid, protocatechuic acid, vanillic acid, succinic acid, and 4-hydroxybenzoic acid were isolated from the fruits of C. spinosa. Among the total eleven known compounds, protocatechuic aldehyde, E-butenedioic acid, ethyl 3, 4-dihydroxybenzoate, syringic acid, 5-hydroxymethylfurfural, 5-hydroxymethyl furoic acid and 2-furoic acid were isolated for the first time from plants of genus Capparis.
Materials and methods
Plant materials
The mature fruits of C. spinosa were collected in July 2005 from Turpan, Xinjiang Uighur Autonomous Region, China, and identified by Chang-hong Wang. A voucher specimen was deposited at the herbarium of the Shanghai R&D Center for Standardization of Chinese Medicines, Shanghai, China. Before chemical analysis, the fruits of C. spinosa were milled into a coarse powder.
General procedures and reagent
Melting points were measured with a Büchi Melting Point B-540 apparatus (Flawil, Switzerland). MS spectra were obtained on an LCQ DECA XP (Thermo Finnigan, San Jose, CA) instrument. The HREI-MS spectra were obtained on a Micromass CGT (Waters Co., Milford, MA, USA) instrument. The 1H-NMR and 13C-NMR spectra were carried out on a Brucker AM 500 MHz and Brucker AM 400 MHz using TMS as internal standard. The chemical shift values (δ) are reported in ppm units and the coupling constants (J) are in Hz. Thin layer plate was precoated with silica gel F254 (10 × 20 cm, 0.3 mm thick; Yantai, China) were used for TLC, and visualization of the TLC plates was carried out under UV at 254, as well as by spraying the plates with FeCl3. Optical density measurements were made with a SPECTRA MAX190 microplate spectrophotometer (Molecular Devices, Sunnyvale, CA). 1, 1-Diphenyl- 2-picrylhydrazyl (DPPH•) and α-tocopherol were purchased from Sigma-Aldrich (St. Louis, MO). All other chemicals were of analytical reagent grade and used without any further purification.
Extraction and isolation of bioactive compounds
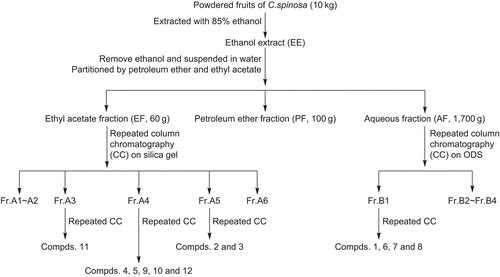
C. spinosa antioxidants were extracted and fractionated according to their polarity as shown in . Briefly, the powdered fruits (10 kg) of C. spinosa were extracted successively three times with 80 L of 85% ethanol under reflux. The 85% ethanol extract was then filtered through absorbent gauze, and the filtrate was concentrated under reduced pressure to remove ethanol and then dried by lyophilization to afford a residue (2.1 kg) which was named EE. The residue was suspended in water (4 L), and subsequently successively partitioned three times with the same volumes of petroleum ether (60~90°C) and ethyl acetate. The petroleum ether and ethyl acetate extracts were separately combined and evaporated to dryness under reduced pressure, while the aqueous layer was lyophilized to dryness. These three fractions were designated as PF (100 g), EF (60 g) and AF (1700 g), respectively. The DPPH• scavenging activities of the four fractions including EE, PF, EF and AF were determined using a spectrophotometric method. Greater antioxidant activities were found in the EF and AF fractions compared with PF and EE.
The DPPH• -active EF fraction (60 g) was fractionated on a silica gel column with a gradient mixture of ethyl acetate and petroleum ether, and finally with ethyl acetate to give six fractions (Fr.A1–Fr.A6). Fraction A3 was subjected to further chromatography on a silica gel column and eluted using a stepwise gradient of petroleum ether and acetone (6:1) to afford a further three sub-fractions (Fr.A3.1–Fr.A3.3), and compound 11 (35 mg) was obtained by crystallization from Fr.A3.2. Fraction Fr.A4 was subjected to a silica gel column and eluted with petroleum ether-acetone (4:1), afforded three sub-fractions (Fr.A4.1–Fr.A4.7). Compound 9 (25 mg), compound 10 (33 mg) and compound 12 (7 mg) were obtained from sub-fraction Fr.A4.3 Compound 4 (7 mg) and compound 5 (8 mg) were obtained from sub-fraction Fr.A4.6 by crystallization with a methanol solvent system. Fraction Fr.A5 was subjected to a silica gel column and eluted with petroleum ether and acetone (1:1) to afforded three sub-fractions (Fr.A5.1–20). Sub-fraction Fr.A5.6 was subjected to further chromatography on a silica gel column, using petroleum ether-acetone (1:1) as eluent, yielded compound 2 (10 mg) and compound 3 (70 mg).
Otherwise, the DPPH• -active AF fraction was submitted to octadecyl silane (ODS) column chromatography, eluted with mixture of methanol and water (from 5:95, 30:70, 60:40, to 90:10; 5000 mL for each step), to yield four fractions (Fr.B1–Fr.B4). Fraction B1 was subjected to further chromatography on an ODS column and eluted with a mixture of methanol and water (5:95) to yield a further three sub-fractions (Fr.B1.1–3). Compound 1 (5 mg), Compound 6 (45 mg), compound 7 (70 mg) and compound 8 (20 mg) were obtained from Fr.B1.1, Fr.B1.2 and Fr.B1.3, respectively.
DPPH• scavenging capacity
The antioxidant capacity of these compounds was carried out by determination photometrically through their scavenging activity against the stable artificial free radical, 1,1-diphenyl-2-picrylhydrazyl (DPPH•), using the method as described previously (CitationEl-Desouky et al., 2007) with slight modification. In brief, 200 μL of 0.208 mM DPPH solution (in ethanol) was added to an Eppendorf tube containing a 200 μL aliquot of different concentrations sample (in water). The mixture was vortexed for 1 min and kept at room temperature for 30 min in the dark. The assay was carried out using 96-well plates and the bleaching of DPPH• was monitored at an absorbance of 515 nm. The scavenging potential was compared with a solvent (negative control) and α-tocopherol (positive control). The percentage of DPPH bleaching was utilized to calculate the SC50 (half maximal scavenging concentration).
The percentage DPPH• scavenged by each sample extract was calculated using the following equation:
% Scavenging = [1–(As–Ab) / (Ac–Ab)] × 100% (1)
where As, Ab, and Ac represent the absorbance of sample, the blank, and the control reaction at 30 min.
SC50 values were calculated using non-linear regression and expressed in mg dried material equivalents/mL for sample extracts or in mM for pure compounds. SC50 values express the concentration of antioxidant samples necessary to scavenge 50% radicals in the reaction mixture. The non-linear regression analysis was performed using GraphPad Prism 5 (GraphPad Software, San Diego, CA). α-Tocopherol was used as a positive control. All the antioxidant measurements were performed in duplicate, and the data were expressed as average ± standard deviations (SD).
Results and discussion
Isolation of antioxidant compounds
Fruits of C. spinosa were re-extracted with 80 L of 80% ethanol. The ethanol extract was further fractionated by being partitioned with petroleum ether (60~90°C) and ethyl acetate. All the fractions including EE, PF, EF, and AF were evaluated for their DPPH• scavenging capacities using a conventional spectrophotometric assay. Among the four fractions, EF and AF showed the greatest DPPH• scavenging activities with SC50 values of 0.321 and 0.421 mg dried raw material equivalents/mL, respectively (). In the present study, further experiments were carried out on the EF and AF fraction to separate its antioxidant components. A new compound (1), named cappariside, together with eleven known organic acids (2–12), protocatechuic aldehyde, E-butenedioic acid, ethyl 3,4-dihydroxybenzoate, syringic acid, 5-hydroxymethylfurfural, 5-hydroxymethyl furoic acid, 2-furoic acid, protocatechuic acid, vanillic acid, succinic acid, and 4-hydroxybenzoic acid were isolated from the fruits of C. spinosa. Among the total eleven known compounds, protocatechuic aldehyde, E-butenedioic acid, ethyl 3,4-dihydroxybenzoate, syringic acid, 5-hydroxymethylfurfural, 5-hydroxymethyl furoic acid and 2-furoic acid were isolated for the first time from plants of genus Capparis.
Table 1. The DPPH• scavenging activities of the extracts and the phenolic acids isolated from C. spinosaa.
Structure elucidation for isolated compounds
The structures of the isolated compounds were identified on the basis of spectroscopic analyses including 1D-and 2D-NMR spectroscopy and electrospray ionization mass spectrometry (ESIMS).
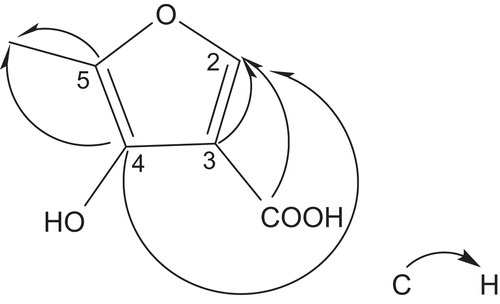
Compound 1 was obtained as colorless needles from methanol, and exhibited a molecular formula of C6H6O4 as determined by HREI-MS m/z: 142.0266 (calculate for 142.0264). In the 1H-NMR, the spectrum showed the following significant proton signals at the low-field region of the spectrum (δ 7.93, s, H-2), and the methyl signal (δ 2.31 s, CH3). Resonance at δC 140.7 was assigned to C-2 by HSQC spectrum. HMBC () correlation of the methyl proton at δH 2.31 with C-5 at δC 143.2 and C-4 at δC 152.2 suggested this methyl was attached to C-5. Olefinic protons at δH 7.93 (s, 1H) to furan carbons at δC 146.1 (C-3) and carbonyl-C at 170.6 (COOH) suggested carbonyl-C was attached to C-3. Major HMBC correlations were shown in . All of the above arguments determined the structure of 1 as 4-hydroxy-5-methylfuran-3-carboxylic acid and named as cappariside. The 1H-NMR (500 MHz, in CD3OD) and 13C-NMR (125 MHz, in CD3OD) see .
Table 2. 1H- and 13C-NMR data for compound 1 (1H = 500 MHz, 13C = 125 MHz, CD3OD, δ in ppm and J in Hz)a
Compound 2 was obtained as colorless needles from acetone, and exhibited a molecular formula of C7H6O3 as determined by ESIMS m/z: 137 [M-1]. Mp: 153 155°C. 1H-NMR (CD3COCD3, 500 MHz) δ (ppm): 9.67 (s, 1H), 8.64 (2H, br.s, OH), 7.24 (1H, d, J = 1.84 Hz, H-2), 7.22 (1H, dd, J = 8.03, 1.86 Hz, H-6), 6.87 (1H, d, J = 8.01 Hz, H-5). The 1H- and 13C-NMR data agreed well with the reported compound, protocatechuic aldehyde (CitationGao et al., 2005).
Compound 3 was obtained as colorless needles from pyridine, and exhibited a molecular formula of C4H4O4 as determined by ESIMS m/z: 115[M-1]. Mp: 287~288°C. 1H-NMR (CD3OD, 500 MHz) δ (ppm): 6.81 (1H, d, J = 15.8 Hz, H-2), 6.48 (1H, d, J = 15.8 Hz, H-3). 13C-NMR (CD3OD, 125 MHz) δ (ppm): 134.8 (C-2, C-3), 166.1 (C-1, C-4). The 1H- and 13C-NMR data agreed well with the reported compound, E-butenedioic acid (CitationZhao et al., 2004).
Compound 4 was obtained as colorless needles from ethyl acetate, and exhibited a molecular formula of C9H10O4 as determined by ESIMS m/z: 182 [M-1]. Mp: 131~133°C. 1H-NMR (MeOD, 500 MHz) δ (ppm): 7.37 (1H, d, J = 2 Hz, H-2), 7.31 (1H, dd, J = 2, 8.3 Hz, H-6), 6.76 (1H, d, J = 8.3 Hz, H-5), 4.13 (2H, q, OCH2), 1.19 (3H, t, -CH3); 13C-NMR (CD3OD, 125 MHz) δ (ppm): 166.6 (C = O), 150.7 (C-4), 145.6 (C-3), 123.3 (C-1, 6), 122.9 (C-2, 1), 117.2 (C-5), 115.7 (C-2), 60.8 (OCH2), 14.6 (CH3). The 1H- and 13C-NMR data agreed well with the reported compound, ethyl 3,4-dihydroxybenzoate (CitationYao & Min, 2005).
Compound 5 was obtained as colorless needles from ethyl acetate, and exhibited a molecular formula of C9H10O5 as determined by ESIMS m/z: 197[M-1]. Mp: 179 180°C. 1H-NMR (CD3OD, 500 MHz) δ (ppm): 7.2 (2H, s, H-2,6), 3.76 (6H, s, OCH3). 13C-NMR (CD3OD, 125 MHz) δ (ppm): 167.5 (COOH), 148.4 (C-3, 5), 141.6 (C-4), 121.5 (C-1), 108.2 (C-2, 6), 56.7 (OCH3). The 1H- and 13C-NMR data agreed well with the reported compound, syringic acid (CitationChen et al., 2007).
Compound 6 was obtained as colorless needles from ethyl acetate, and exhibited a molecular formula of C6H6O3 as determined by ESIMS m/z: 125[M-1]. 1H-NMR (CD3OD, 500 MHz) δ (ppm): 9.46 (1H, s, CHO), 7.24 (1H, d, J = 3.51 Hz, H-4), 6.5 (1H, d, J = 3.51 Hz, H-3), 4.51 (3H, s, CH2). The 1H-NMR data agreed well with the reported compound, 5-hydroxymethylfurfural (CitationZhang & Wang, 2001).
Compound 7 was obtained as colorless needles from methanol, and exhibited a molecular formula of C6H6O4 as determined by ESIMS m/z: 141[M-1]. 1H-NMR (CD3OD, 500M Hz) δ (ppm): 7.06 (1H, d, J = 3.42 Hz, H-3), 6.36 (1H, d, J = 3.43 Hz, H-4), 4.47 (3H, s, CH2).13C-NMR(CD3OD, 125 MHz) δ (ppm): 162.08 (C-2′), 160.99 (C-2), 145.99 (C-5), 120.28 (C-3), 110.51 (C-4), 57.8 (C-5′). The 1H- and 13C-NMR data agreed well with the reported compound, 5-hydroxymethyl furoic acid (CitationChen et al., 2005).
Compound 8 was obtained as colorless needles from methanol, and exhibited a molecular formula of C5H4O3 as determined by ESIMS m/z:111[M-1]. Mp: 133~134°C. 1H-NMR (MeOD, 400 MHz) δ (ppm): 11.16 (1H, s, COOH), 7.78 (1H, d, J = 0.82 Hz, H-3), 7.22 (1H, dd, J = 3.32, 0.36 Hz, H-5), 6.62 (1H, dd, J = 3.33, 1.73 Hz, H-4); 13C-NMR (MeOD, 125 MHz) δ (ppm): 159.4 (C-1′), 147.6 (C-5), 145.9 (C-2), 118.6 (C-3), 112.7 (C-4). The 1H and 13C-NMR data agreed well with the reported compound, 2-furoic acid (CitationWang & Pei, 2000).
Compound 9 was obtained as colorless needles from acetone, and exhibited a molecular formula of C7H6O4 as determined by ESIMS m/z: 153[M-1]. Mp: 200 202°C. 1H-NMR (CD3OD, 500 MHz) δ (ppm): 7.31 (1H, d, J = 1.7 Hz, H-2), 7.2 (1H, dd, J = 8.1, 1.7 Hz, H-6), 6.9 (1H, d, J = 8.1 Hz, H-5). 13C-NMR (CD3OD, 125 MHz) δ (ppm): 170.7(COOH), 151.8(C-4), 146.3(C-3), 124.2(C-6), 123.5(C-1), ll8.1(C-2), ll6.1(C-5). The 1H- and 13C- NMR data agreed well with the reported compound, protocatechuic acid (CitationZhang & Wang, 2001).
Compound 10 was obtained as colorless needles from acetone, and exhibited a molecular formula of C8H8O4 as determined by ESIMS m/z: 167[M-1]. Mp: 210 213°C. 1H-NMR (CD3COCD3, 500 MHz) δ (ppm): 12.44 (1H, br.s, COOH), 9.81 (1H, br. s, OH), 7.46 (1H, dd, J = 8.2, 1.7 Hz, H-6), 7.43 (1H, d, J = 1.7 Hz, H-2), 6.77 (1H, d, J = 8.2 Hz, H-5), 3.79 (3H, s, OCH3); 13C-NMR (CD3COCD3, 125 MHz) δ (ppm): 167.5 (C-1), 152.1 (C-4), 148.1 (C-5), 124.9 (C-7), 122.9 (C-2), 115.5 (C-3), 113.5 (C-6), 56.4 (OCH3). The 1H- and 13C-NMR data agreed well with the reported compound, vanillic acid (CitationShen et al., 2002).
Compound 11 was obtained as colorless needles from methanol, and exhibited a molecular formula of C4H6O4 as determined by ESIMS m/z: 117[M-1]. Mp: 185~187°C. 1H-NMR (CD3OD, 500 MHz) δ (ppm): 2.5 (4H, d, J = 15.8 Hz, H-2, 3); 13C-NMR (CD3OD, 125 MHz) δ (ppm): 176.5 (C-1, C-4), 30.1 (C-2, C-3). The 1H- and 13C- NMR data agreed well with the reported compound, succinic acid Citation(Ma & Liu, 2005).
Compound 12 was obtained as colorless needles from methanol, and exhibited a molecular formula of C7H6O3 as determined by ESIMS m/z: 137[M-1]. Mp: 213~214°C. 1H-NMR (CD3OD, 500 MHz) δ (ppm): 7.78 (2H, d, J = 8.8 Hz, H-3, 5), 6.72 (2H, d, J = 8.8 Hz, H-2, 6); 13C-NMR (CD3OD, 125 MHz) δ (ppm): 170.4 (C-7), 163.6 (C-4), 133.3 (C-2, C-6), 123 (C-1), 116.3 (C-3, C-5). The 1H- and 13C-NMR data agreed well with the reported compound, 4-hydroxybenzoic acid (CitationHu et al., 2006).
DPPH• scavenging capacities
The DPPH• scavenging capacity of all fractions and isolated pure phenolic compounds were determined and expressed as SC50 values. Among the fractions, the EF and AF fractions were found to be the most potent DPPH radical scavengers with SC50 values of 0.321 and 0.421 mg dried raw material equivalents/mL, respectively, while the PEF and EE was the less active scavenger (). The twelve compounds isolated from the DPPH• -active EF and AF fraction as well as the positive control, α-tocopherol, were also evaluated and compared for their DPPH• scavenging capacities, as shown in .
The scavenging percentage of the ethanol exact and petroleum ether (60°~90°C) were respectively 40.9% and 30.8% at the concentration of 1 mg·mL−1. Otherwise, the scavenging capacity of ethyl acetate fraction and aqueous fraction for 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals with a SC50 value of 0.321 ± 0.001 mg·mL−1 and 0.421 ± 0.001 mg·mL−1, respectively. The results showed the ethyl acetate fraction and aqueous fraction were the main antioxidant part of the ethanol exact. The antioxidant activity of phenolic acid compounds from the ethyl acetate fraction and the aqueous fraction on the DPPH• radical scavenging capacity was described in . So, the study clarified the antioxidant activity of compounds were cappariside (1), protocatechuic aldehyde (2), ethyl 3,4-dihydroxybenzoate (4), syringic acid (5), protocatechuic acid (9), vanillic acid (10), 4-hydroxybenzoic acid (12) mainly from the fruits of C. spinosa.
Acknowledgement
This work was supported by a research grant from foundation of Shanghai Municipal Education Commission (No. 05CZ31 awarded to Professor Chang-hong Wang).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ali-Shtayeh MS, Abu Ghdeib SL (1999): Antifungal activity of plant extracts against dermatophytes. Mycoses 42: 665–672.
- Al-Said MS, Abdelsattar EA, Khalifa SI, El-Feraly FS (1988): Isolation and identification of an anti- inflammatory principle from Capparis spinosa. Pharmazie 43: 640–641.
- Chen R, Liang JY, Yang Y, Liu R (2007): Chemical constituents from Physalis alkekengi and structural revision of Physalin G. Chin J Nat Med 5:186–189.
- Chen Y, Tian JK, Cheng YY (2005): Studies on chemical constituents of Ranunculus ternatus. Chin Pharm J 40: 1373–1375.
- Eddouks M, Lemhadri A, Michel J (2004): Caraway and caper: Potential anti-hyperglycaemic plants in diabetic rats. J Ethnopharmacol 94: 143–148.
- Eddouks M, Lemhadri A, Michel J (2005): Hypolipidemic activity of aqueous extract of Capparis spinosa L. in normal and diabetic rats. J Ethnopharmacol 98: 345–350.
- El-Desouky SK, Ki HK, Shi YR, Ahmad FE, Gamal-Eldeen AM, Young-Kyoon K (2007): A new pyrrole alkaloid isolated from Arum palaestinum Boiss. and its biological activities. Arch Pharm Res 30: 927–931.
- Fu XP, Wu T, Abdurahim M, Su Z, Hou XL, Aisa HA, Wu H (2008): New spermidine alkaloids from Capparis spinosa roots. Phytochemistry L 1: 59–62.
- Gadgoli C, Mishra SH (1999): Antihepatotoxic activity of p-methoxybenzoic acid from Capparis spinosa. J Ethnopharmacol 66: 187–192.
- Gao XZ, Zhou CX, Zhang SL, Yao W, Zhao Y (2005): Studies on the chemical constituents in herb of Ranunculus sceleratus. Chin J Chin Mater Med 30: 124–126.
- Germano MP, De Pasquale R, D’Angelo V, Catania S, Silvari V, Costa C (2002): Evaluation of extracts and isolated fraction from Capparis spinosa L. buds as an antioxidant source. J Agric Food Chem 27: 1168–1171.
- Gutteridge JMC, Halliwell B (1990): The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem Sci 15: 129–135.
- Hu J, Zhang WD, Liu RH, Zhang C, Shen YH, Xu XK, Liang MJ, Li HL (2006): Chemical constituents in root of Zanthoxylum nitidum. Chin J Chin Mater Med 31: 1689–1691.
- Khanfar MA, Sabri SS, Zarga MH, Zeller K (2003): The chemical constituents of Capparis spinosa of Jordanian origin. Nat Prod Res 17: 9–14.
- Ma BJ, Liu JK (2005): Chemical study on Russula densifolia. Nat Prod Res 17: 29–32.
- Matthäus B, Ozcan M (2002): Glucosinolate composition of young shoots and flower buds of capers (Capparis species) growing wild in Turkey. J Agric Food Chem 50: 7323–7325.
- Matthäus B, Ozcan M (2005): Glucosinolates and fatty acid, sterol, and tocopherol composition of seed oils from Capparis spinosa var. spinosa and Capparis ovata Desf. var. canescens (Coss.) Heywood. J Agric Food Chem 53: 7136–7141.
- Özcan M, Aydin C (2004): Physico-mechanical properties and chemical analysis of raw and brined caperberries. Biosyst Engin 89: 521–524.
- Sharaf M, El-Ansari MA, Saleh NA (1997): Flavonoids of four Cleome and three Capparis species. Biochem Syst Ecol 25: 161–166.
- Shen XL, Zeng HF, Chen Z, Zhong GH (2002): Study on chemical constituents isolated from Glycosmis citrifolia. Chin Pharm J 37: 14–17.
- Steenkamp V, Mathivha E, Gouws MC, Rensburg CEJ (2004): Studies on antibacterial, antioxidant and fibroblast growth stimulation of wound healing remedies from South Africa. J Ethnopharmacol 95: 353–357
- Trombetta D, Occhiuto F, Perri D, Puglia C, Santagati NA, Pasquale AD, Saija A, Bonina F (2005): Antiallergic and antihistaminic effect of two extracts of Capparis spinosa L. flowering buds. Phytother Res 19: 29–33.
- Wang SJ, Pei YH (2000): Studies on the chemical constituents of the leaves of Betula platyphylla Suk. J Shenyang Pharm Univ 17: 256–257.
- Yang T, Liu YQ, Wang CH, Wang ZT (2008): Advances on investigation of chemical constituents, pharmacological activities and clinical applications of Capparis spinosa. Chin J Chin Mater Med 33: 2453–2458.
- Yaniv Z, Dafni A, Friedman J, Palevitch D (1987): Plants used for the treatment of diabetes in Israel. J Ethnopharmacol 19: 145–151.
- Yao S, Min ZD (2005): Two new chalcones from the leaves of Artocarpus heterophyllus. Chin J Nat Med 3: 220–223.
- Zhang CL, Wang ZX (2001): Studies on the constituents of Cibotium barometz (L.) J. Sm. rhizome. Chin J Med Chem 11: 279–280.
- Zhao AH, Zhao QS, Peng LY, Zhang JX, Lin ZW, Sun HD (2004): A new chalcone glycoside from Bidens pilosa. Acta Botan Yunnan 26: 121–126.