Abstract
Ethyl acetate extract of the whole plant of Nervilia aragoana Gaud. (Orchidaceae) and ethanol extract of the leaves of Atlantia monophylla Linn. (Rutaceae) were evaluated for antifungal and antioxidant activities. At 5 mg/mL concentration of the extracts, the former exhibited more inhibitory activity than the latter against fungi. The order of MIC values for Nervilia aragoana were Saccharomyces cerevisiae (1.4 mg/mL) > Aspergillus niger (1.2 mg/mL) > Aspergillus fumigatus (0.95 mg/mL) > Cryptococcus neoformans (0.75 mg/mL). In the case of Atlantia monophylla values were Cryptococcus neoformans (1 mg/mL) > Candida albicans (0.95 mg/mL) > Aspergillus niger (0.65 mg/mL). TLC-DPPH method assay was carried out to evaluate the antioxidant potential. Further DPPH radical, superoxide, nitric oxide, H2O2 scavenging, and reducing power activities were carried out. N. aragoana (85%) extract exhibited more scavenging activity than that of A. monophylla (66%) by DPPH free radical scavenging method. A. monophylla extract exhibited more superoxide, nitric oxide, H2O2 scavenging activities than that of N. aragoana. The acute toxicity studies of both extracts have shown no mortality rate even up to 3 g/kg body weight in albino rats. Screening for secondary metabolites showed the presence of carbohydrates in both extracts. Flavonoids were found only in the ethyl acetate extract of N. aragoana. Tannins, alkaloids, triterpenoids and steroids were present in A. monophylla. Total phenols present in N. aragoana and A. monophylla were 340 and 560 mg/g extract of gallic acid equivalents, respectively.
Introduction
Fungi are the eukaryotic, opportunistic pathogens mostly seen in immuno-compromised patients. Disseminated fungal infections are on the increase (CitationDenning, 1991) and the therapeutic difficulties encountered in their management have prompted investigators and clinicians to be more creative in the development of antifungal drugs. Increased fungal infections are commonly observed in patients with immuno-suppressed conditions such as AIDS, many of whom are surviving longer with supportive therapy, and increased numbers of transplant patients as well as cancer patients undergoing aggressive chemotherapy (Citationde Marie et al., 1994). Medicinal plants have been the basis for the treatment of various infectious diseases in traditional medicine, and possess a variety of compounds of known therapeutic properties (CitationTempone et al., 2008). Plant-derived compounds are gaining much importance in pharmaceutical and therapeutically applications as there are usually fewer side effects and are not very toxic. Much attention has been paid to plant-derived antifungal compounds, (CitationGurgel et al., 2005) based on the knowledge that plants have their own defense systems against fungal pathogens (CitationFontenelle et al., 2007; CitationWojtaszek, 1997).
Oxidative stress, induced by oxygen radicals, is believed to be a primary factor in various degenerative diseases such as cancer (CitationMuramatsu et al., 1995), atherosclerosis (CitationSteinberg et al., 1989), gastric ulcer (CitationDas et al., 1997) and other conditions (CitationOliver et al., 1987). Antioxidants play an important role in inhibiting and scavenging radicals, thus providing protection to humans against infection and degenerative disorders. Antioxidants are compounds that help to inhibit many oxidation reactions caused by free radicals such as singlet oxygen, superoxide, peroxy radicals, hydroxyl radicals and peroxy nitrate, thereby preventing or delaying damage to the cells and tissues (CitationKarthikumar et al., 2007). Although there are some synthetic antioxidant compounds such as butylated hydroxyl anisole (BHA) and butylated hydroxyl toluene (BHT), which are commonly used in processed foods, it has been reported that these compounds may have side effects (CitationPourmorad et al., 2006; CitationWitschi, 1981; CitationKehrer & DiGiovanni, 1990; CitationYamamoto et al., 1980).
Nervilia aragoana Gaud. (Orchidaceae) is distributed in hilly wet areas of Western Ghats of Kerala, Karnataka, Tamil Nadu and in some rare places of Andhra Pradesh (India).
Atlantia monophylla Linn. (Rutaceae) has been earlier reported for insecticidal activity against pests of agricultural importance (CitationSukumar et al., 1991; CitationSivagnaname & Kalyanasundaram, 2004).
The aims of the present study were to investigate: 1) antifungal activity; 2) in vitro antioxidant activity and 3) acute toxicity studies of ethyl acetate extract (whole plant) of N. aragoana and ethanol extract (leaves) of A. monophylla. This is probably the first scientific report on antifungal and antioxidant activities of both the plants.
Materials and methods
Plant collection
Nervilia aragoana was collected from Western Ghats of India during the month of September 2007 and authenticated by P. Sudhakaran, Curator at Raiyrath Nursery, Thrissur, Kerala. Leaves of Atlantia monophylla were collected from Tirumala hills of Eastern Ghats of India during the month of December 2007 and authenticated by N. Yasadomma, Department of Botany, S.V. University, Tirupati. The whole plant of N. aragoana and leaves of A. monophylla were shade dried, powdered and used in the present studies.
Solvent extraction
Whole plant powder (100 g) of N. aragoana was extracted by Soxhlet for 6-8 h with ethyl acetate, and the extract was distilled to remove the solvent. The yield of the ethyl acetate extract was 2.25 g and soluble in 10% DMSO.
Leaf powder (100g) of A. monophylla was placed in a Corning jar, 300 mL of ethanol was added and soaked for 48 h. After that the ethanol extract was collected and Rota vaporized at 40°C to drive off the ethanol. The yield was 9 g which was also soluble in 10% DMSO.
Fungal strains
Fungal cultures used in the present study were procured from IMTECH, Chandigarh, India. These include Candida albicans ATCC 10231 (MTCC 227), Aspergillus niger ATCC 16404 (MTCC 1344), Aspergillus fumigatus (MTCC 1811), Cryptococcus neoformans ATCC 32045 (MTCC 1347) and Saccharomyces cerevisiae ATCC 9763 (MTCC 171).
Antifungal activity
Antifungal activity of both the plant extracts was tested using the diffusion method according to CitationBauer et al. (1966) and CitationNCCLS (2000). The fungal strains were maintained on potato dextrose agar (PDA) medium (Hi-Media). Sterile discs of Whatman No.1 filter paper of about 6 mm diameter were impregnated on the surface of the media. Different concentrations of both the extracts were prepared and applied on the discs and incubated for 48–72 h at 28°C. The results were recorded by measuring the inhibitory zone around the discs.
Minimum inhibitory concentration
Different concentrations from 0.05 mg to 2 mg/ml were prepared. The cells were adjusted so that 0.1 mL of culture contains 1x108 CFU/mL. Inoculum (0.1 mL) was added to each test tube and incubated at 28°C for 48–72 h. The lowest concentration (highest dilution) of the extract that produced no visible signs of fungal growth (no turbidity) was regarded as the minimum inhibitory concentration (MIC).
Antioxidant activity
TLC-DPPH antioxidant screening
Each extract (10 µL) was loaded as a 1 cm band on the origin of TLC (Merck, Silica gel 60 F254 plates). Plates were developed using methanol and ethyl acetate (5:5) for Nervilia aragoana and 100% methanol for Atlantia monophylla. To detect antioxidant activity, chromatograms were sprayed with 0.2% DPPH in methanol as an indicator (CitationDeby & Margotteaux, 1970) until just wet, and dried in a fume hood. The presence of antioxidant compounds were detected as yellow spots against a purple background on TLC plates.
Free radical scavenging activity
Free radical scavenging activity was determined by using the 2,2′-diphenly-1-picrylhydrazyl (DPPH) method followed by CitationBurits and Bucar (2000). One mL of various concentrations of the extracts in methanol was added to 4 mL of 0.004% methanol solution of DPPH. After a 30-min incubation period at room temperature, the absorbance was read against blank at 517 nm. The inhibition of free radicals by DPPH in percentage terms (I%) was calculated by using the following equation.
I % = [(A control – A sample)/A blank] × 100
where A control is the absorbance of the control reaction (containing all reagents except the test compound), and A sample is the absorbance of the test compound.
Superoxide anion scavenging activity
Measurement of superoxide anion scavenging activity of both plant extracts was carried out by the modified CitationLiu et al. (1991) method (CitationGulcin et al., 2004). 1.5 mL of Tris-HCl buffer (pH 8.0, 32 mM), 0.5 mL of Nitroblue tetrazolium (NBT) (300 µM), 0.5 mL of nicotinamide adenine dinucleotide (NADH) solution of 468 µM and different concentrations of plant extracts (10-100 µg/mL) were taken. The reaction was initiated by the addition of 1 mL phenozine methosulphate (PMS) (30 µM) to the mixture. The reaction mixture was incubated at 25°C for 5 min and the absorbance was measured at 560 nm. Decreased absorbance of the reaction mixture indicates the increased superoxide anion scavenging activity. Ascorbic acid was used as a standard. The % of inhibition was calculated.
Nitric oxide scavenging activity
Nitric oxide scavenging activity was measured by the method of CitationMarcocci et al. (1994). Nitric oxide radicals (NO) were generated from sodium nitroprusside. 1 mL of sodium nitroprusside (10 mM), 1.5 mL of phosphate buffer saline (0.2 M, pH 7.4) was added to the different concentrations of the both plant extracts and incubated for 150 min at 25°C. After incubation 1 mL of the reaction mixture was treated with 1 mL of Griess reagent (1% sulfanilamide, 2% H3PO4, and 0.1% naphthylethylenediamine dihydrochloride). The absorbance of the chromatophore was measured at 546 nm. Butylated hydroxyl toluene was used as a standard. The % of inhibition was calculated.
H2O2 scavenging activity
The H2O2 scavenging activity of both plant extracts was determined according to the method of CitationRuch et al. (1989). A solution of H2O2 (40 mM) was prepared in phosphate buffer (pH 7.4). Different concentrations of both plant extracts in 3.4 mL phosphate buffer were added to a H2O2 solution (0.6 mL, 40 mM). The absorbance value of the reaction mixture was recorded at 230 nm. The % of inhibition was calculated.
ABTS radical scavenging activity
The antioxidant activity of both the extracts was determined with stable ABTS+ cation radical method of CitationRe et al. (1999). ABTS (2 mM) was prepared by dissolving in 50 mL of phosphate buffered saline (pH 7.4). ABTS+ was produced by reacting 50 mL of stock solution with 200 µL of 70 mM potassium per sulfate (K2S2O8) water solution. The mixture was left to stand in the dark at room temperature for 15-16 h before use. For the evaluation of antioxidant activity, the ABTS+ solution was diluted with PBS to obtain the absorbency of 0.8 ± 0.03 at 734 nm. Different concentrations of both the extracts were prepared and mixed with 3 mL of ABTS solution. The absorbance was read at room temperature after 10 min at 734 nm. PBS solution was used as a blank sample. The percentage of inhibition was calculated.
Reducing power
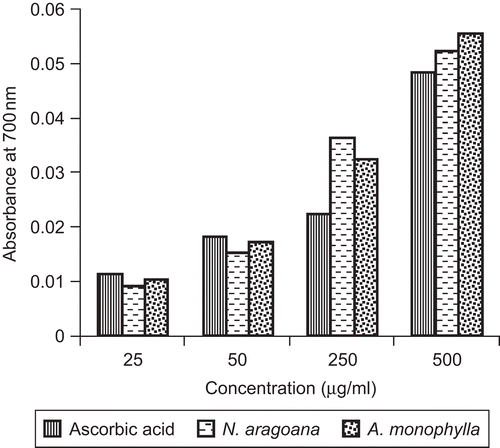
The reducing power was determined according to the CitationOyaizu (1986) method. Different concentrations of both extracts were prepared in methanol mixed with phosphate buffer (2.5 mL, 0.2 M, pH 6.6) and potassium ferricyanide [K3Fe (CN) 6] (2.5 mL, 1%). The mixture was incubated at 50°C for 20 min and 2.5 mL of trichloroacetic acid (10%) was added to the mixture, which was then centrifuged at 3000 rpm for 10 min. The upper layer of the solution (2.5 mL) was mixed with distilled water (2.5 mL) and FeCl3 (0.5 mL, 0.1%). The absorbance was measured at 700 nm. Increased absorbance of the reaction mixture indicated increased reducing power. Ascorbic acid was used as a standard.
Screening for secondary metabolites
The qualitative analyses for screening of secondary metabolites performed according CitationHarborne and Baxter (1995). Various tests were carried out to confirm the presence of phenols, carbohydrates, tannins, alkaloids, triterpenoids, and steroids.
Determination of total phenols
Total phenols in both plant extracts were determined by Folin-Ciocalteau reagent using gallic acid as standard (CitationSingleton & Rossi, 1965). The absorbance was measured at 650 nm. The concentration of total phenols was expressed in terms of grams of gallic acid equivalents.
Acute toxicity studies
The Nervilia aragoana ethyl acetate extract and Atlantia monophylla ethanol extract were dissolved in 10% DMSO. The extract was orally administered to different group of male albino rats in doses ranging from 500 mg to 3 g/kg body weight for the LD50 study using the method of CitationGhosh (1984). No mortality was observed in the groups even after 7 days treatment.
Results and discussion
Screening for antifungal and antioxidant activities of both plants was carried out with different solvents (complete data not presented). We have observed that ethyl acetate extract of Nervilia aragoana and ethanol extract of Atlantia monophylla were good in terms of antifungal and antioxidant activities.
Antifungal activity
Medicinal plants possess a variety of compounds of known therapeutic properties (CitationChopra et al., 1992; CitationHarborne & Baxter, 1995; CitationAhmad & Beg, 2001). Hence, much attention has been paid to plant-derived antifungal compounds based on the knowledge that plants have their own defense system (CitationFontenelle et al., 2007). The therapeutic use of such plant products is an alternative strategy to prevent the spread of diseases (CitationElkovich, 1988). Both the plant extracts showed good antifungal activity. The ethyl acetate of Nervilia aragoana showed a wider range of antifungal activity on fungi used in the present study compared to the ethanol extract of Atlantia monophylla. At 5 mg/mL both the plant extracts exhibited antifungal activity. The results of antifungal activity of both plants are presented in . The following is the order of susceptibility of the test fungi, namely: C. neoformans (26 mm) > A. fumigatus (24 mm) > S. cerevisiae (16 mm) > A. niger (12 mm) > C. albicans (8 mm) at 5 mg/mL concentration in the case of N. aragoana. A. monophylla exhibited the order of susceptibility of the test fungi namely, A. niger (18 mm) > C. albicans (16 mm) > C. neoformans (14 mm). But A. fumigatus and S. cerevisiae have not shown susceptibility.
Table 1. Antifungal activity and MIC values of ethyl acetate extract of N. aragoana and ethanolic extract of A. monophylla.
MIC values of both the extracts were also represented in . In the case of N. aragoana, the order of MIC values were S. cerevisiae (1.4 mg/mL) > A. niger (1.2 mg/mL) > A. fumigatus (0.95 mg/mL) > C. neoformans (0.75 mg/mL). In the case of A. monophylla, the order of MIC values were, C. neoformans (1 mg/mL) > C. albicans (0.95 mg/mL) > A. niger (0.65 mg/mL).
Antioxidant activity
Results of TLC-DPPH chromatograms (forming yellow spots) indicated the presence of antioxidant compounds in both the plant extracts (). Free-radical reactions have been implicated in the pathology of many human diseases/disease conditions like atherosclerosis, ischemic heart disease, aging process, inflammation, diabetes, immune-suppression, neurodegenerative diseases, etc. (CitationMaxwell, 1995; CitationDroge, 2002; CitationBeckman & Ames, 1998). The disturbance in “redox homeostasis” occurring when antioxidant defenses are inadequate can damage lipids, proteins, carbohydrates, and DNA. Drugs with multiple protective mechanisms, including antioxidant activity, may be one way of minimizing tissue injury (CitationHalliwell, 1991).
Figure 1. Screening of antioxidant activity by TLC-DPPH method (A) Ethyl acetate extract of N. aragoana and (B) Ethanolic extract of A. monophylla).
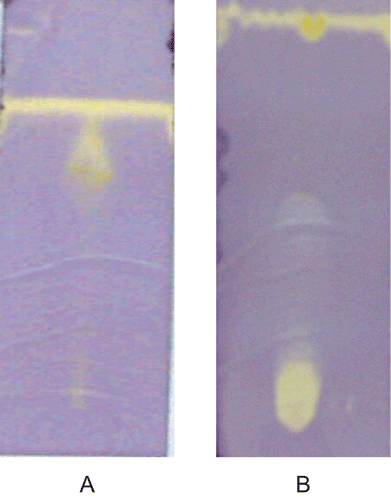
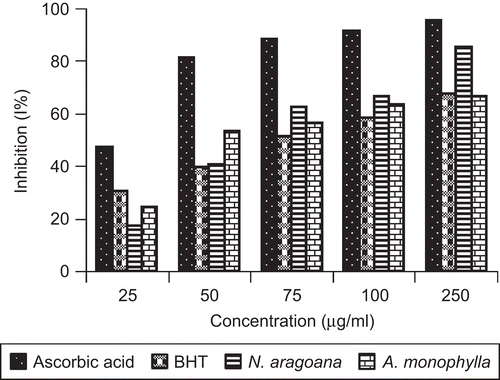
The hydrogen atom or electron donation abilities of the corresponding extracts and some pure compounds were measured from the bleaching of the purple-colored methanol solution of 2,2′-diphenly-1-picrylhydrazyl (DPPH). At 250 µg/mL concentration, the ethyl acetate extract of N. aragoana exhibited 85% of DPPH radical scavenging activity than ethanol extract of A. monophylla that exhibited 66% (). CitationSanchez-Moreno et al. (1998) classified the kinetic behavior of antioxidant as follows: <5 min (rapid) 5–30 min (intermediate) and >30 min (slow). According to this classification both the extracts decolorizes rapidly within 5 min.
Figure 2. DPPH free radical scavenging activity of ethyl acetate extract of N. aragoana and ethanolic extract of A. monophylla.
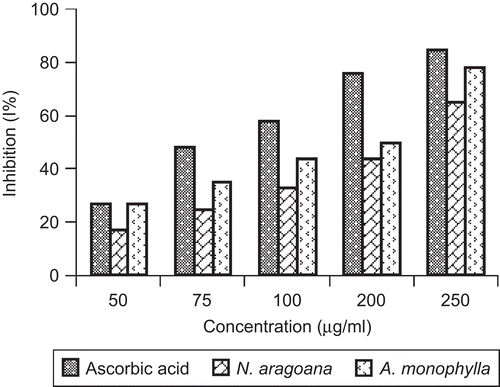
It is well known that superoxide anions damage biomolecules directly or indirectly by forming H2O2, OH, peroxy nitrite or singlet oxygen during aging and pathological events such as ischemic reperfusion injury. Superoxide has also been observed to directly initiate lipid peroxidation (CitationYen & Duh, 1994). The superoxide scavenging activity of both extracts was increased markedly with the increase in concentrations. At 250 µg/mL, the inhibition of SOD activity was 65% in case of N. aragoana and 78% in case of A. monophylla ().
Figure 3. Superoxide scavenging activity of ethyl acetate extract of N. aragoana and ethanolic extract of A. monophylla.
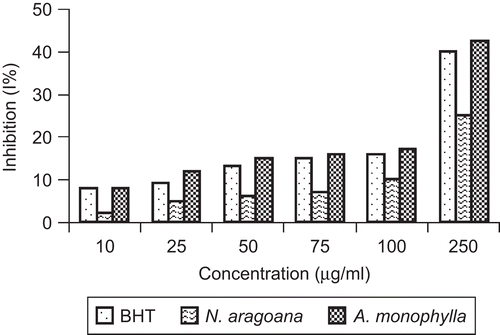
Nitric oxide is a diffusible free radical which plays many roles as an effector molecule in diverse biological systems including neuronal messenger, vasodilation and antimicrobial and antitumor activities (CitationMiller et al., 1993). Nitric oxide inhibitors have been shown to have beneficial effects on some aspect of inflammation and tissue damage seen in inflammatory diseases. At 250 µg/mL, the inhibition of both extracts on nitric oxide activity was 25% (N. aragoana) and 42.5% (A. monophylla) ().
Figure 4. Nitric oxide scavenging activity of ethyl acetate extract of N. aragoana and ethanolic extract of A. monophylla.
Hydrogen peroxide is a weak oxidizing agent and can inactivate a few enzymes directly, usually by oxidation of essential thiol (-SH) groups. Hydrogen peroxide can cross cell membranes rapidly, once inside the cell, H2O2 can probably react with Fe2+ and possibly Cu2+ ions to form hydroxyl radical and this may be the origin of many of its toxic effects (CitationHalliwell & Gutteridge, 1993). It is therefore biologically advantageous for cells to control the amount of hydrogen peroxide that is allowed to accumulate. At 250 µg/mL the inhibition of H2O2 scavenging activity of A. monophylla was 75% and N. aragoana was 65% ().
Figure 5. Hydrogen peroxide scavenging activity of ethyl acetate extract of N. aragoana and ethanolic extract of A. monophylla.
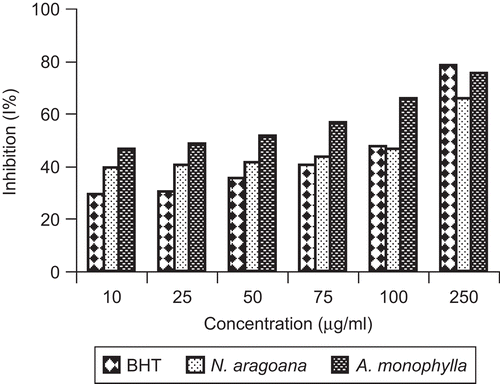
ABTS is a relatively stable free radical which involves in the direct generation of ABTS radical monocation without any involvement of intermediary cation. Here, the radical cation is formed prior to addition of the antioxidant test system, rather than the generation of the radical taking place continually in the presence of antioxidant. This method, used for the screening of antioxidant activity, is applicable to both lipophilic and hydrophilic antioxidants (CitationLong et al., 2000). The free radicals were scavenged by concentration-dependent manner. Maximum scavenging activity observed at 250 µg/mL and minimum activity was found to be 10 µg/mL for both the extracts. At 250 µg/mL, the inhibition of ABTS scavenging activity was found to be 91% in Nervilia aragoana and 93% in Atlantia monophylla (). Increased absorbance with the increased concentrations of the reaction mixture indicated the increased reducing power (). The reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity (CitationMeir et al., 1995).
Figure 6. ABTS scavenging activity of ethyl acetate extract of N. aragoana and ethanolic extract of A. monophylla.
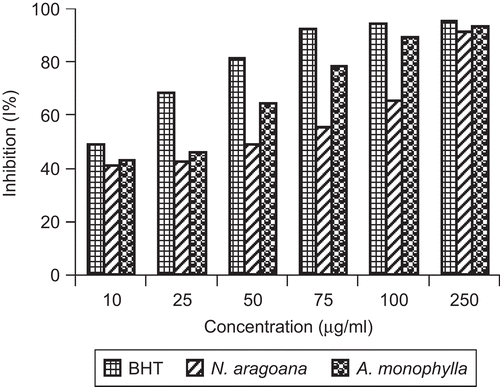
Figure 7. Reducing power of ethyl acetate extract of N. aragoana and ethanolic extract of A. monophylla.
Acute toxicity test gives clues on the range of doses that could be toxic to the animal. It could also be used to estimate the therapeutic index (LD50/ED50) of drugs and xenobiotics (CitationRang et al., 1999). The acute toxicity studies have showed no mortality rate even after 7 days treatment with both the extracts up to 3 g/kg body weight.
Phytochemical analysis
Screening for secondary metabolites revealed the presence of carbohydrates and flavonoids in the ethyl acetate extract of Nervilia aragoana. Steroids, triterpenoids, alkaloids and tannins are present in the ethanol extract of Atlantia monophylla (data not presented). Flavonoids are known to be synthesized in plants in response to microbial infections; they have been found in vitro to be effective antimicrobial substances against a wide range of microorganisms. Their activity is probably due to their ability to complex with extracellular and soluble proteins. Tannins, which are present as secondary metabolites in plants, may exhibit their mode of antimicrobial action by inactivating microbial adhesions, enzymes, cell envelope transport protein and complex with cell wall (CitationCowan, 1999). CitationScalbert (1991) reviewed the antimicrobial properties of tannins. The mechanism of action of terpenes is not fully understood but it is speculated to involve membrane disruption by the lipophilic compounds. Heterocyclic nitrogen compounds are called alkaloids. Recent in vitro studies have shown activity against bacteria, specifically enteric pathogens, and some activity against Candida spp. (CitationSawer et al., 1995). The concentration of total phenols present in the N. aragoana and A. monophylla are 340 mg/g and 560 mg/g extract of gallic acid equivalents.
Conclusions
Nervilia aragoana and Atlantia monophylla possess distinct antifungal and antioxidant properties which have been attributed to the presence of polyphenolic and other phytochemical constituents. As the plants are being used in traditional medicine there is ample opportunity to examine how they cure diseases on a more scientific basis. Thus there is a need to isolate and identify the compounds and use them in modern medicine. Investigations are in progress towards this goal.
Acknowledgements
We also thank Dr. S.C. Basappa, former Deputy Director and Scientist, Central Food Technological Research Institute (CFTRI), Mysore (India), for his suggestions, encouragement and critical comments on the manuscript.
Declaration of interest
We thank the University Grants Commission, New Delhi for financial assistance.
References
- Ahmad I, Beg AZ (2001): Antimicrobial and phytochemical studies of 45 Indian medicinal plants against multi-drug resistant human pathogens. J Ethnopharmacol 74: 113–123.
- Bauer AW, Kirby WM, Sherris JC, Turck M (1966): Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Pathol 45: 493–496.
- Beckman KB, Ames BN (1998): The free radical theory of aging matures. Physiol Rev 78: 547–581.
- Burits M, Bucar F (2000): Antioxidant activity of Nigella sativa essential oil. Phytother Res 14: 323–328.
- Chopra RN, Nayer SL, Chopra IC (1992): Glossary of Indian Medicinal Plants, third edition. New Delhi, Council of Scientific and Industrial Research: 7–246.
- Cowan, MM (1999): Plant products as antimicrobial agents. Clin Microbio Rev 12: 564–582.
- Das D, Bandyopadhyay D, Bhattacharjee M, Banerjee RK (1997): Hydroxyl radical is the major causative factor in stress-induced gastric ulceration. Free Radic Biol Med 23: 8–18.
- Deby C, Margotteaux G (1970): Relationship between essential fatty acids and tissue antioxidant levels in mice. C R Soc Biol Fil 165: 2675–2681.
- de Marie S, Janknegt R, Bakker-Woudenberg IA (1994): Clinical use of liposomal and lipid-complexed amphotericin B. J Antimicrob Chemother 33: 907–916.
- Denning DW (1991): Epidemiology and pathogenesis of systemic fungal infections in the immunocompromised host. J Antimicrob Chemother 28: 1–16.
- Droge W (2002): Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95.
- Elkovich SD (1988): Terpenoids from the genus Artemisia as potential pesticides, in: Cutler HG, ed., Natural Products and their Potential Role in Agriculture, ACS Symposium series 380. Washington DC, American Chemical Society, pp. 250–261.
- Fontenelle RO, Morais SM, Brito EH, Kerntopf MR, Brilhante RS, Cordeiro RA, Tomé AR, Queiroz MG, Nascimento NR, Sidrim JJ, Rocha MF (2007): Chemical composition, toxicological aspects and antifungal activity of essential oil from Lippiasidoides Cham. J Antimicrob Chemother 59: 934–940.
- Ghosh MN (1984): Toxicity studies, in: Fundamentals of Experimental Pharmacology. Calcutta, Scientific Book Agency, pp. 153–158.
- Gulcin I, Uguz MT, Oktay M, Beydemir S, Kufrevioglu OI (2004): Evaluation of the antioxidant and antimicrobial activities of clary sage (Salvia sclarea L.). Turk J Agric For 28: 25–33.
- Gurgel LA, Sidrim JJC, Martins DT (2005): In vitro antifungal activity of dragon’s blood from Croton urucurana against dermatophytes. J Ethnopharmacol 97: 409–412.
- Halliwell B, Gutteridge JMC (1993): Free Radicals in Biology and Medicine. Oxford, Clarendon Press, p. 419.
- Halliwell B (1991): Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. Am J Med 91: 14–22.
- Harborne SB, Baxter H (1995): Phytochemical Dictionary: A Handbook of Bio-Active Compounds from Plants. London, Taylor and Francis, pp. 40–130.
- Karthikumar S, Vigneswari K, Jegatheesan K (2007): Screening of antibacterial and antioxidant activities of leaves of Eclipta prostrata (L). Scientific Res Essay 2: 101–104.
- Kehrer JP, DiGiovanni J (1990): Comparison of lung injury induced in four strains of mice by butylated hydroxytoluene. Toxicol Lett 52: 55–61.
- Liu Q, Zhu G, Huang P (1991): Antiinflammatory, analgesic and sedative effects of Leontice kiangnanensis. Zhongguo zhong Yao Za Zhi: 161: 50–65.
- Long LH, Kwee DC, Halliwell B (2000): The antioxidant activities of seasonings used in Asian cooking. Powerful antioxidant activity of dark soy sauce revealed using the ABTS assay. Free Radic Res 32: 181–186.
- Marcocci L, Maguire JJ, Droy-Lefaix MT, Packer L (1994): The nitric oxide scavenging properties of Ginkgo biloba extract EGb 761. Biochem Biophys Res Comm 201: 748–755.
- Maxwell SJ (1995): Prospects for the use of antioxidant therapies. Drugs 49: 345–361.
- Meir S, Kanner J, Akiri B, Hadas SP (1995): Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J Agric Food Chem 43: 1813–1815.
- Miller MJ, Sadowska-krowicka H, Chotinaruemol S, Kakkis JL, Clark DA (1993): Amelioration of chronic ileitis by nitric oxide synthase inhibition. J Pharmacol Exp Ther 264: 11–16.
- Muramatsu H, Kogawa K, Tanaka M, Okumura K, Koike K, Kuga T (1995): Superoxide dismutase in SAS human tongue carcinoma cell line is a factor defining invasiveness and cell motility. Cancer Res 55: 6210–6214.
- NCCLS (2000): Approved standard M7-A5. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, fifth edition. Wayne, PA, National Committee for Clinical Laboratory Standards, p. 20.
- Oliver CN, Ahn B, Moerman EJ, Goldstein S, Stadtmaan ER (1987): Age-related changes in oxidized protein. J Bio Chem 262: 5488–5491.
- Oyaizu M (1986): Studies on product of browning reaction prepared from glucose amine. Jap J Nutr 44: 307–315.
- Pourmorad F, Hosseinimehr SJ, Shahabimajd N (2006): Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr J Biotechnol 5: 1142–1145.
- Rang HP, Dale M, Ritter J (1999): Pharmacology, fourth edition. Churchill Livingstone, New York, pp. 351–369.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999): Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad Biol Med 26: 1231–1237.
- Ruch RJ, Cheng SJ, Klaunig JE (1989): Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 10: 1003–1008.
- Sanchez-Moreno C, Larrauri JA, Saura-calixto FA (1998): A procedure to measure the antiradical efficiency of polyphenols. J Sci Food Agric 76: 270–276.
- Sawer I, Berry M, Brown M, Ford J (1995): The effect of cryptolepine on the morphology and survival of Escherichia coli, Candida albicans and Saccharomyces cerevisiae. J Appl Bacteriol 79: 314–321.
- Scalbert A (1991): Antimicrobial properties of tannins. Phytochemistry 30: 3875–3883.
- Silva O, Duarte A, Cabrita J, Pimentel M, Diniz A, Gomes E (1996): Antimicrobial activity of Guinea-Bissau traditional remedies. J Ethnopharmacol 50: 55–59.
- Singleton VL, Rosi JA (1965): Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagent. Am J Enol Vitic 16: 144–158.
- Sivagnaname N, Kalyanasundaram M (2004): Laboratory evaluation of methanolic extract of Atlantia monophylla (Family: Rutaceae) against immature stages of mosquitoes and non-target organisms. Mem Inst Oswaldo Cruz 99: 115–118.
- Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum, JL (1989): Beyond cholesterol: Modification of low-density lipoprotein that increases its atherogenicity. N Engl J Med 320: 915–924.
- Sukumar K, Perich MJ, Boobar LR (1991): Botanical derivatives in mosquito control. A review. J Am Mosq Control Assoc 7: 210–217.
- Tempone AG, Sartorelli P, Teixeira D, Prado FO, Calixto IA, Lorenzi H, Melhem MS (2008): Brazilian flora extracts as source of novel antileishmanial and antifungal compounds. Mem Inst Oswaldo Cruz 103: 443–449.
- Witschi HP (1981): Enhancement of tumor formation in mouse lung by dietary butylated hydroxytoluene, Toxicology 21: 95–104.
- Wojtaszek P (1997): Oxidative burst an early plant response to pathogen infection. Biochem J 322: 681–692.
- Yamamoto K, Tajima K, Mizutani T (1980): The acute toxicity of butylated hydroxytoluene and its metabolites in mice. Toxicol Lett 6: 173–175.
- Yen GC, Duh PD (1994): Scavenging effect of methanolic extracts of peanut hulls on free- radical and active oxygen species. J Agric Food Chem 42: 629–632.
