Abstract
Ziziphus mauritiana Lamk. (Rhamnaceae) is a fruit tree that has been used as folkloric medicine for many ailments and diseases. In the present study, the hypoglycemic effect of seed extract of Ziziphus mauritiana in alloxan-induced diabetic mice was assessed. Seed extract was administered orally at doses of 100, 400, and 800 mg/kg body weight (bw) and also in combination with glyburide (800 mg/kg seed extract and 10 mg/kg glyburide) to different groups of mice (normal and alloxan-treated diabetic mice). Their blood glucose level (in acute and sub-acute study), body weight, and mortality rate were monitored. Oral glucose tolerance test (OGTT) was also performed. Oral administration of extract alone or in combination with glyburide reduced the blood glucose level in all the diabetic mice after acute and sub-acute (28 days) administration. Administration of the extract reduced the weight loss and mortality rate during the sub-acute study. The results of blood glucose level, loss in body weight, and mortality rate were more pronounced in the group treated with combination (800 mg/kg seed extract and 10 mg/kg glyburide). The extract also augmented the glucose tolerance in both normal and diabetic mice. These results suggest that the extract possesses synergistic hypoglycemic activity.
Introduction
Diabetes mellitus is a metabolic disorder which affects carbohydrate, protein, and fat metabolism. The disease is characterized by hyperglycemia together with biochemical alterations of glucose and lipid metabolism (CitationDavis & Granner, 1996). The worldwide prevalence of diabetes mellitus is estimated to be 2.8% (CitationWild et al., 2004). It increases the risk of many disorders such as atherosclerotic arterial disease 2-6 fold (CitationSacks, 1997). Many synthetic oral hypoglycemic agents such as sulfonylureas, biguanides, thiazolidinediones, meglitinide derivatives, and α-glucosidase inhibitors are presently in use but they all have several side effects (CitationEdwin et al., 2006). This necessitates the use of herbal preparations, plant decoctions or infusions, which usually have fewer side effects, easy availability, and cost effectiveness.
From time immemorial in many under-developed and developing countries plants are used as an alternative source of treatment for various pathophysiological conditions including diabetes mellitus.
Ziziphus mauritiana Lamk. (Rhamnaceae) is a tropical or subtropical fruit tree, commonly known as Indian jujube or ber, native to Asia, mainly India and China, though also found in Africa. It has been used as a folk remedy for a long time for several ailments and diseases. A bark paste of the tree is applied on sores (CitationMorton, 1987; CitationVerheij & Calabura, 1991), the fruit (CitationNdhala et al., 2006) as well as leaf extracts (CitationDahiru et al., 2005; CitationDahiru & Obidoa, 2007) have been reported to exhibit antioxidant activity. CitationPisha et al. (1995) and CitationRamadoss et al. (2000) reported that the bark of Z. mauritiana possesses cytotoxic effect on tumor cell lines. The fruit has been reported as an immune stimulant in traditional Chinese medicine (CitationChevallier, 1996). The leaves of the plant have shown a hypoglycemic effect (CitationIgnacimuthu & Amalraj, 1998). CitationCisse et al. (2000) studied the effect of aqueous extract of Z. mauritiana leaves in diabetic mice. There are number of cyclopeptides, isoquinoline, alkaloids (CitationTschesche & Kaussmann, 1975), flavonoids, terpenoids (CitationLee et al., 2003), saponins, tannins (CitationGlombitza et al., 1994; CitationAdzu et al., 2001) reported to be present in various amounts in different parts of Ziziphus species, which may be responsible for its biological activities.
The importance of the present study lies in the fact that work was premeditated with seeds as the test material, which are usually thrown away as waste. A literature survey revealed that the seeds of Z. mauritiana have not been studied for various hypoglycemic and antihyperglycemic activities. The present investigation was conducted to study the effect of aqueous alcoholic seed extract of Z. mauritiana on blood glucose levels and on the oral glucose tolerance test in alloxan-induced diabetic mice.
Materials and methods
Chemicals
Alloxan was procured from Sigma (St. Louis, MO), glyburide from Ranbaxy Pharma (New Delhi), blood glucose estimation kit was purchased from Bayer Health Care, Ireland. All other chemicals were of analytical grade, available locally.
Plant material and extract preparation
Fruits of Ziziphus mauritiana var. Umran were collected from the Botanical Gardens of Punjabi University, Patiala, Punjab, India and authenticated by R.C. Gupta, Botany Department, Punjabi University, Patiala, Punjab, India (voucher specimen DOB (305) PUP).
The pulp of Ziziphus mauritiana was peeled off, seeds were shade dried at room temperature, and reduced to coarse powder. The dried and powdered seeds were percolated four times with ethanol:water (1:1) at room temperature. The combined extracts were filtered (Whatmann paper), centrifuged (3200 g, 4°C, 30 min) and concentrated under reduced pressure in a thin film evaporator at 50° ± 5°C. The golden colored paste so formed was dissolved in methanol and concentrated in a thin film evaporator. Finally, the extract was completely dried under vacuum in the desiccator. The whole procedure yielded 9-10% (w/w) of the extract in terms of dried starting material. The extract was standardized using High performance liquid chromatography (HPLC) to reduce the extract to extract variation.
Standardization of ZMS extract
Triterpenoid betulinic acid was used as a marker compound and was quantified in the extract as described by CitationMalik et al. (2007) and CitationZhao et al. (2007). Briefly, the Standardization of Ziziphus mauritiana aqueous:ethanol seed extract (ZMS) extract was standardized on the basis of triterpenoid, betulinic acid using HPLC. The extract was dissolved in methanol:water (1:1) and filtered through 0.45 µm Millipore filter before injection into HPLC system. Marker compound was quantified at 30°C employing a Shimadzu HPLC system comprising of LC-10ATVP pump, diode array detector (SPD-M10 AVP), Phenomenex C18 column (5 µm, 250 mm × 4 mm, ID), column oven (CTO 10ASVP) and class VP software 6.10 by UV detection at 254 nm. The mobile phase consisted of acetonitrile:water.
Animals
Swiss albino mice (25-30 g) of either sex were maintained on standard laboratory diet (Kisan Feeds, Mumbai) and having free access to tap water were employed in the present study. They were housed in the departmental animal house and were exposed to a 12 h cycle of light and dark. The experimental protocol was approved by the Institutional Animal Ethics Committee and care of the animals was carried out as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forest, Government of India (Reg. No. 107/1999/CPCSEA).
Acute toxicity
Acute toxicity of Ziziphus mauritiana seed extract was determined as described by CitationMalik et al. (2007). Mice were given a single oral dose each of 100 mg to 2000 mg/ kg, p.o., and 100 mg to 1000 mg/kg bw, i.p. Animals were observed for behavior and mortality rate, and blood was tested for various parameters Total leukocyte count (TLC), Differential leukocyte count (DLC), Hemoglobin content (Hb) over a period of one month. There was no mortality or abnormality detected as compared to the untreated control group (data not shown). The spleen was excised and weighed after one month.
Induction of experimental diabetes
Experimental diabetes in mice who had been subjected to overnight fasting was induced by intraperitoneal (i.p.) administration of aqueous alloxan monohydrate in acetate buffer (0.15 M, pH 4.5) as described by CitationOzbek et al. (2004). Total dose of alloxan (450 mg/kg bw) was administered in three injections at intervals of 48 h (50 mg/kg bw each time). After 48 h, animals showing blood glucose level above 200 mg/dL (diabetic) were selected for study.
Collection of blood and determination of blood glucose
Blood of control and experimental mice was collected from orbital sinus puncture using a heparinized capillary glass tube. The blood samples so collected were analyzed for blood glucose levels by glucose estimation kit Bayer health care (Bayer diagnostics Europe Ltd.), Chapel lane, Swords, Co. Dublin, Ireland.
Effect of seed extract on blood glucose level in alloxan-induced diabetic mice
Effect of seed extract on blood glucose levels was estimated according to the method of CitationDunn and McLetchie (1943). Diabetic Swiss albino mice of either sex were divided into six groups (n = 6), i.e.: Group I, vehicle treated (distilled water 10 ml/kg); Group II, glyburide treated (10 mg/kg bw); Group III, extract treated (100 mg/ kg bw); Group IV, extract treated (400 mg/kg bw); Group V, extract treated (800 mg/kg bw); Group VI, glyburide (10 mg/kg bw) and extract treated (800 mg/kg bw). All drugs were given orally.
The acute study involved the estimation of blood glucose levels at 0, 2, 4, 6, and 24 h after administration of extract and glyburide, with all the doses given at one time. The sub-acute study involved the repeated administration of drug for 28 days at a prefixed time and blood glucose levels were estimated on days 7, 14, 21, and 28. The data were represented as mean blood glucose level and standard error mean (SEM). Body weights of the mice were also noted during the study period of 28 days and represented as mean change in body weights. The death of the mice was monitored and percentage mortality was calculated.
Effect of seed extract of Ziziphus mauritiana on oral glucose tolerance test (OGTT) in normal and diabetic mice
Non-diabetic and diabetic mice were divided into six groups (n = 6), i.e.: Group I, vehicle, (distilled water 10 mL/kg); Group II, glyburide treated (10 mg/kg bw); Group III, extract treated (100 mg/kg bw); Group IV, extract treated (400 mg/kg bw), Group V, extract treated (800 mg/kg bw); Group VI, glyburide (10 mg/kg bw) and extract treated (100 mg/kg bw). Prior to commencement of experiment, the animals were fasted overnight. Extract, glyburide and combination of both were administered orally and blood glucose levels were monitored. After 30 min of drug and extract administration, animals were loaded with d-glucose solution (2.5 mg/kg) and blood glucose levels were monitored at 0, 30, 60, and 120 min after glucose loading.
Statistical analysis
All the results were expressed as mean ± SEM. Data of tests were statistically analyzed using one-way ANOVA followed by Tukey’s multiple range test, applied for post hoc analysis. A value of p <0.001 was considered to be statistically significant.
Results
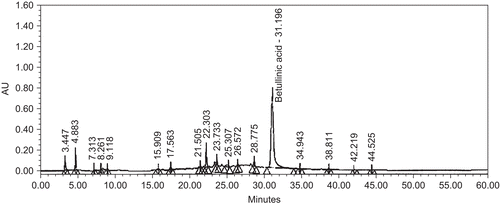
To evade extract to extract variation and global diversity, the standardization of plant extract is mandatory. For this, we generated the chemoprofiling data of ZMS extract based on naturally occurring triterpenoid betulinic acid (). Seed extract of Ziziphus mauritiana was found to possess 2.62% betulinic acid.
Figure 1. Standardized chromatogram of seed extract of Ziziphus mauritiana. Chromatogram of seed extract of Ziziphus mauritiana employing HPLC profile of chemical marker. HPLC was performed using acetonitrile:water gradient over a period of 60 min: acetonitril 10%, 0.01–5 min; 10%–60%, 5–30 min; 60%–98%, 30–40 min; 98%, 40–45 min; 98%–10%, 45–55 min; 10%, 55–60 min. Other conditions were same as described in materials and methods.
Acute toxicity study revealed that the extract was safe up to a dose level of 1000 mg/kg bw. Although at higher doses no lethality was observed, the animals treated with dose ranges above 1000 mg/kg bw showed lethargic behavior.
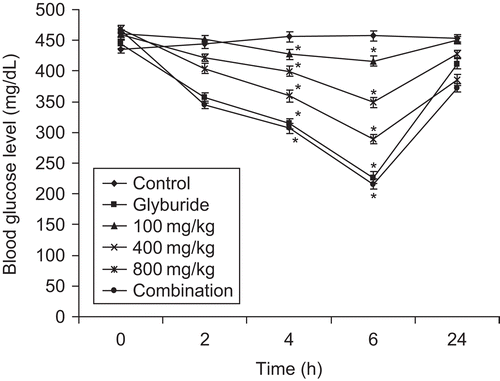
A single administration of seed extract (100, 400, 800 mg/kg bw), glyburide (10 mg/kg bw) and the combination of both significantly reduced (p < 0.001) the blood glucose levels at 2, 4, and 6 h. The onset of anti-hyperglycemic effect of glyburide was 2 h and that of the extract at 800 mg/kg was 4 h (). The peak of the effect was attained at 6 h but the effect diminished at 24 h. The combination of glyburide and seed extract exerted an antihyperglycemic effect at 2 h and showed better reduction in glucose levels (26% ± 2.5%) than glyburide alone (19% ± 1.5%).
Figure 2. Effect of acute treatment of seed extract of Ziziphus mauritiana, glyburide and combination (seed extract and glyburide) on blood glucose level in alloxan-induced diabetes in mice. Blood glucose levels were assessed at regular interval of 0, 2, 4, 6 and 24 h after administration of extract at different concentration, glyburide and combination. The results are presented as mean ± SEM (n = 6). *p<0.001 compared with untreated control group.
Sub-acute administration of the extract, glyburide and combination caused a significant (p < 0.001) reduction in blood glucose levels as compared to control (). Maximum activity of the extract was observed at 800 mg/kg on day 28. A similar pattern was observed in the glyburide-treated group. However, the combination of seed extract and glyburide resulted in a better and significant (p <0.001) response in terms of reduction in blood glucose levels. The blood glucose level on day 28 shown by the combination group was 169.97 ± 2.1 mg/dL, whereas that of the glyburide-treated group was 183.5 ± 2 mg/dL. Administration of vehicle (distilled water) in diabetic mice resulted in decrease in body weight during the period of 28 days (), while the treatment of diabetic animals with different concentrations of seed extract (100, 400, and 800 mg/kg bw) prevented the decrease in body weight showing the advantageous outcome of extract administration. Treatment of diabetic mice with vehicle only resulted in the death of 50% of the total animals during the sub-acute study. However, the administration of the extract at 100, 400, and 800 mg/kg bw reduced the mortality rate to 33.3%, 33.3% and 0%, respectively. The extract in combination also resulted in 0% mortality. In OGTT, administration of glucose (2.5 g/kg) increased the blood glucose levels significantly (p < 0.001) after 30 min of glucose loading in diabetic mice () and normal mice (). Extract, glyburide, and combination of extract and glyburide produced significant (p < 0.001) increase in glucose threshold within 30 min of glucose loading.
Figure 3. Effect of sub-acute treatment of seed extract of Ziziphus mauritiana, glyburide and combination (seed extract and glyburide) on blood glucose level in alloxan-induced diabetes in mice. Blood glucose levels were assessed on day 0, 7, 14, 21 and 28 after simultaneous administration of extract at different concentration, glyburide and combination. The results are presented as mean ± SEM (n = 6). *p <0.001 compared with untreated control group.
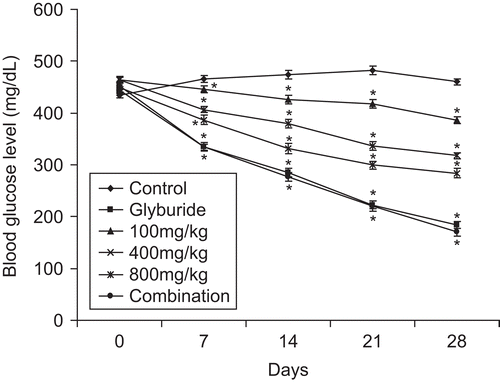
Figure 4. Effect of treatment of seed extract of Ziziphus mauritiana, glyburide and combination (seed extract and glyburide) on blood glucose level in OGTT in diabetic mice. Blood was collected and assessed for blood glucose levels at 0. 30, 60, and 120 min after loading of glucose in diabetic mice. The results are presented as mean ± SEM (n = 6). *p <0.001 compared with untreated control group.
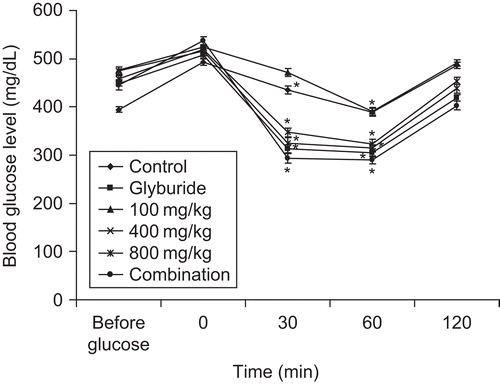
Figure 5. Effect of treatment of seed extract of Ziziphus mauritiana, glyburide and combination (seed extract and glyburide) on blood glucose level in OGTT in normal mice. Blood was collected and assessed for blood glucose levels at 0, 30, 60, and 120 min after loading of glucose in normal mice. The results are presented as mean ± SEM (n = 6). *p<0.001 compared with untreated control group.
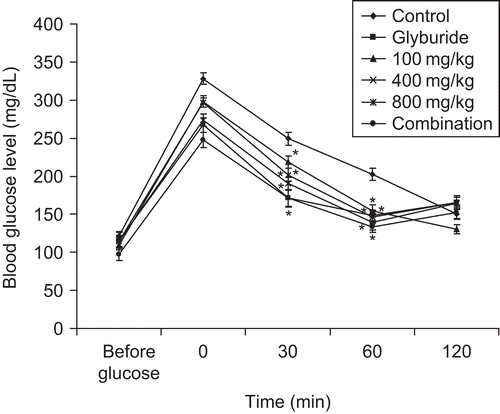
Table 1. Effect of aqueous-ethanol seed extract of Ziziphus mauritiana on body weight (g) in alloxan-induced diabetic mice.
Discussion
Numerous plants have been used traditionally to treat diabetes and some of them have been proven scientifically and reported to be hypoglycemic agents. The hypoglycemic activity in these plants is assumed to be due to the active principles present. Compounds such as polysaccharides (CitationTomoda et al., 1985), flavonoids, terpenoids, tannins (CitationRehar et al., 1991) and alkaloids (CitationKarawya & Wahab, 1984) have been reported to be responsible for hypoglycemic effect.
Ziziphus mauritiana leaves have been reported to possess antidiabetic potential (CitationIgnacimuthu & Amalraj, 1998). The antihyperglycemic activity of aqueous leaf extract of Ziziphus mauritiana has also been reported (CitationCisse et al., 2000) and was found to be comparable to the known antihyperglycemic agent, glibenclamide. The root of Ziziphus has also been studied for antihyperglycemic activity and was found to possess a pronounced effect even on oxidative stress caused by diabetes (CitationHussein et al., 2006). In an earlier finding it had been postulated that the seed extract of Zizyphus jujuba is helpful in reducing blood glucose level (CitationKim, 2002), but the studies lacked detailed effect of Ziziphus mauritiana seed extract on acute and sub-acute administration. Our study is an attempt to know more about the effect of Ziziphus mauritiana on acute, sub-acute and OGTT pattern in normal and diabetic mice. As mentioned earlier, terpenoids also possess hypoglycemic activity. Hence, for standardization purposes, triterpene betulinic acid was identified and quantified in the extract as a marker compound. The acute toxicity showed that animals treated with higher doses exhibited lethargic behavior, but there was no mortality. This may be due to the sedative action of Ziziphus seeds, as the seeds are used traditionally for insomnia and have been reported to possess sedative action (CitationWatanabe et al., 1973; CitationMorishita et al., 1987). Moreover, the body weight and spleen weight did not show any abnormal rise. The weight of the lymphoid organ is a good indicator of toxicity. Thus, the extract apparently is safe for use. In the current study, the hypoglycemic activity of aqueous-ethanol seed extract of Ziziphus mauritiana was evaluated in alloxan-induced diabetic mice. Significant (p < 0.001) reduction in blood glucose level was experiential at 4 h and maximum reduction occurred at 6 h of extract treatment in acute study at the dose range of 800 mg/kg. The combination of glyburide (10 mg/kg bw) and extract (800 mg/ kg bw) resulted in significant reduction (p < 0.001) at 2 h which was higher than that of glyburide treatment alone. The more pronounced effect of the extract may be due to limited or compromised action of insulin in diabetic conditions and on the contrary, more due to the anti-hyperglycemic principles present in the extract. There are many anti-hyperglycemic principles which have been reported earlier from the plant origin (CitationHikino et al., 1985; CitationTakahashi et al., 1985).
Glyburide is a second generation oral sulfonylurea antidiabetic agent which is used as a diet adjunct to lower blood glucose levels in diabetic patients. Glyburide acts by stimulating the pancreatic islet cells, which result in secretion of insulin. Sulfonylurea acts by binding to and blocking the ATP-sensitive K+ channel. The drug thus is similar to the physiological secretagogues like glucose and leucine which lower the conductance of this channel. Reduced K+ conductance causes membrane depolarization and an influx of Ca2+ through the voltage sensitivity Ca2+ channel. Prolonged administration of glyburide produces extra pancreatic effect and thus contributes to hypoglycemic activity (CitationShah et al., 2006). The results of both acute and sub-acute study hypothesized that the late-onset and prolonged duration of the action of the extract may be due to the improved pancreatic cytoarchitecture. Moreover, the extract alone or in combination with glyburide protected the weight loss induced by alloxan, which are in corroboration with the earlier studies from other plant extracts (CitationBadole et al., 2006). Administration of the extract also reduced the mortality rate from 50% to 33.3% when the dose was given at 400 mg/kg bw. Moreover, there was no mortality when the dose of the given extract was given at 800 mg/kg. Thus it was apparent that when the drug was not administered, progression of diabetes resulted in mortality of mice, whereas the extract treatment resulted in reduction of mortality or even no mortality.
The protective effect against diabetes induced weight loss is supported by earlier studies (CitationSwanston-Flatt, 1989). Sub-acute treatment of extract for 28 days also resulted in reduction of blood glucose level. In the oral glucose tolerance test (OGTT), the doses increased the tolerance of glucose suggesting increased peripheral utilization of glucose in both diabetic as well as non-diabetic mice.
The antihyperglycemic effects observed in alloxan-induced diabetes and OGTT can be attributed to several mechanisms. The antihyperglycemic activity exhibited by the extract could be through glucose/insulin metabolism and/or by enhancing the peripheral insulin sensitivity (CitationTalpur et al., 2002) or by enhancing insulin release by the islets of Langerhans (CitationGray & Flatt, 1998). However, the exact mechanism for the hypoglycemic activity shown by the seed extract of Ziziphus mauritiana is not known.
In conclusion, our results imply that seed extract of Ziziphus mauritiana contains principles that possibly exert multiple actions involving different mechanisms in exerting hypoglycemic and antihyperglycemic effects. Further investigations are in progress to identify the active constituents responsible for hypoglycemic activity of Ziziphus mauritiana.
Acknowledgements
The authors are grateful to the Department of Biotechnology, Punjabi University, Patiala for providing necessary laboratory facilities to execute this work
Declaration of interest
The Authors would like to thank the DST (Department of Science and Technology), Governmentt of India for the financial aid provided by them in the form of a FIST (Fund for Improvement of S&T Infrastructure in Universities and Higher Educational Institutions) FIST grant for the purchase of instruments.
References
- Adzu B, Amos S, Wambebe C, Gamaniel K (2001): Antinociceptive activity of Zizyphus spina christi root bark extract. Fitotherapia 72: 344–350.
- Badole SL, Shah SN, Patel NM, Thakurdesai PA, Bodhankar L (2006): Hypoglycemic activity of aqueous extract of Pleurotus pulmonarius in alloxan-induced diabetic mice. Pharm Biol 44: 421–425.
- Chevallier A (1996): The Encyclopedia of Medicinal Plants. London, Dorling Kindersley, p. 203.
- Cisse A, Nadiaye A, Lopez-Sall P, Seck F, Faye B (2000): Antidiabetic activity of Zizyphus mauritiana Lam. Dakar Med 45: 105–107.
- Dahiru D, Obidoa O (2007): Pretreatment of albino rats with aqueous leaf extract of Zizyphus mauritiana protects against alcohol induced liver damage. Trop J Pharm Res 6: 705–710.
- Dahiru D, William ET, Nadru MS (2005): Protective effect of Zizyphus mauritiana leaf extract on carbon tetrachloride-induced liver injury. Afr J Biotechnol 4: 1177–1179.
- Davis SN, Granner DK (1996): Insulin, oral hypoglycemic agents and the pharmacology of endocrine pancreas, in: Goodman LS, Gilman AG, eds, The Pharmacological Basis of Therapeutics, ninth edition. New York, Mc-Graw-Hill, pp. 1487–1517.
- Dunn JS, McLetchie NGB (1943): Experimental alloxan diabetes in rats. Lancet 2: 384–387.
- Edwin E, Sheeja E, Chaturvedi M, Sharma S, Gupta VB (2006): A comparative study on antihyperglycemic activity of fruits and barks of Ficus bengalesis (L.). Adv Pharmacol Toxicol 7: 69–71.
- Glombitza KW, Mahran GH, Mirhom YW, Michel CG, Motawi TK (1994): Hypoglycemic and antihyperglycemic effect of Zizyphus spina christi. Planta Med 60: 244–247.
- Gray AM, Flatt PR (1998): Insulin releasing and insulin like activity of Agaricus campestris. J Endocrinol 157: 259–266.
- Hikino H, Takahashi M, Konno C, Ishimoro A, Kawamura T, Namiki T (1985): Effect of glycans of Saccharum officinarum on carbohydrate and lipid metabolism of rats. J Ethanopharmacol 14: 261–268.
- Hussein HM, El-Sayed EM, Said AA (2006): Antihyperglycemic, antihyperlipidemic and antioxidant effects of Zizyphus spina christi and Zizyphus jujube in alloxan diabetic rats. Int J Pharmacol 2: 563–570.
- Ignacimuthu S, Amalraj T (1998): Effect of leaf extract of Ziziphus jujube on diabetic rats. Indian J Pharmacol 30: 107–108.
- Karawya MS, Wahab SA (1984): Diphenylamine, an antihyperglycemic agent from onion and tea. J Nat Prod 47: 775–780.
- Kim H S (2002): Effects of the Zizyphus jujube seeds extract on the lipid components in hyperlipidemic rats. J Food Sci Nutr 7: 72–77.
- Lee HW, Park YS, Choi JW, Yui SY, Shin WS (2003): Antidiabetic effects of chitosan oligosaccharides in neonatal streptozotocin induced non-insulin dependent diabetes mellitus in rats. Biol Pharm Bull 26: 1100–1103.
- Malik F, Singh J, Khajuria A, Suri KA, Satti NK, Singh S, Kaul MK, Kumar A, Bhatia A, Qazi GN (2007): A standardized root extract of Withania somnifera and its major constituent withanolide-A elicit humoral and cell-mediated immune responses by up regulation of Th1-dominant polarization in BALB/c mice. Life Sci 80: 1525–1538.
- Morishita S, Mishima Y, Hirai Y, Saito T, Shoji M (1987): Pharmacological studies of water extract of Zizyphus seed and the Zizyphus seed containing drug. Gen- Pharmacol 18: 637–641.
- Morton J, (1987): Indian jujube, in: Fruits of Warm Climates, Mortan JF, ed. Center for New Crops & Plant Products, Purdue University. Available at http://www.hort.purdue.edu/newcrop/morton/indian_jujube.html (Accessed on 21/10/2006).
- Ndhala AR, Mupure CH, Chitindingue K, Benhura MAN, Muchuweti M (2006): Antioxidant potentials and degree of polymerization of six wild fruits. Sci Res Assay 1: 87–92.
- Ozbek H, Ceylan E, Kara M, Ozgokee F, Koyuncu M (2004): Hypoglycemic effect of Rheum ribes root in alloxan induced diabetic and normal mice. Scand J Lab Anim Sci 31: 113–115.
- Pisha E, Chai H, Lee IS, Chavedera TE, Farnsworth NR, Cordell GA, Beecher CW, Fong HH, Kinghorn AD, Brown DM (1995): Discovery of betulinic acid as a selective inhibitor of human myeloma that functions by induction of apoptosis. Nat Med 1: 1046–1051.
- Ramadoss S, Jaggi M, Siddiqui MJA (2000): Use of betulinic acid and its derivatives for inhibiting cancer growth and a method of monitoring this. Assignee: Dabur Research Foundation. US patent No. 6048847.
- Rehar G, Slijepcevic M, Krans L (1991): Hypoglycemic activity of triterpenes and tannins from Sarcopoterium spinosum and two Sanguisorba species. Planta Med 57: A57-A58.
- Sacks DB (1997): Implications for revised criteria for diagnosis and classification of diabetes mellitus. Clin Chem 43: 2230–2233.
- Shah SN, Bodhankar SL, Badole SL, Kamble HV, Mohan VJ (2006): Effect of trigonelline: An active compound from Trigonella foenum graecum Linn. in alloxan induced diabetes in mice. J Cell Tissue Res 6: 585–590.
- Swanston-Flatt SK, Day C, Flatt PR, Gould BJ, Bailey CJ (1989): Glycaemic effects of traditional European plant treatments for diabetes, studies in normal and streptozotocin diabetic mice. Diabetes Res 10: 69–73.
- Takahashi M, Konno C, Hikino H (1985): Isolation and hypoglycemic activity of Anemorans A, B, C and D glycans of Anemarrhema asphodeloides rhizomes. Planta Med 51: 100–102.
- Talpur N, Echard B, Dadgar A, Aggarwal S, Zhuang C, Bagehi D (2002): Effect of Maitake mushroom fractions on blood pressure of Zucker fatty rats. Mol Pathol Pharmacol 112: 68–82.
- Tomoda M, Shimada K, Konno C, Hikino H (1985): Structure of panaxan B, a hypoglycemic glycan of Panax ginseng roots. Phytochemistry 24: 2431–2433.
- Tschesche R, Kaussmann EU (1975): The cyclopeptide alkaloids in: The Alkaloids. Manske, RHF ed.New York, Academic Press, p. 165–205.
- Verheij EWM, Calabura M (1991): Plant resources of south-east Asia 2, in: Edible Fruits and Nuts, Verheij EWM, Coronel RE eds, Wageningen, PROSEA/Pudoc, pp. 222–223.
- Watanabe I, Saito H, Takagi K (1973): Pharmacological studies of Zizyphus seeds. Jap J Pharmacol 23: 563–571.
- Wild S, Roglic G, Green A, Sicree R, King H (2004): Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 27: 1047–1053.
- Zhao G, Yan W, Cao D (2007): Simultaneous determination of betulin and betulinic acid in white birch bark using RP-HPLC. J Pharma Biomed Analysis 43: 959–962.
