Abstract
Acetone leaf extracts of Combretaceae species Combretum imberbe Wawra, Combretum nelsonii Duemmer, Combretum albopunctatum Suesseng, and Terminalia sericea Burch ex DC and a mixture of asiatic acid and arjunolic acid isolated from C. nelsonii were tested for antifungal activity against Candida albicans, Cryptococcus neoformans, Microsporum canis, and Sporothrix schenckii on wounds of immunocompromised Wistar rats. The therapeutic agents were selected based on low MIC values ranging 0.02–2.5 mg/mL and low toxicity (LC50) ranging 75.7–168.6 μg/mL. Seven circular, full-thickness wounds were made on the back skin of 24 Wistar rats, under general anesthetic and using an aseptic technique. Rats were infected with different fungal pathogens in groups of six. The treatments were administered topically using 20% concentrations of each extract in aqueous cream. Amphotericin B was used as positive control. Erythema, exudate, crust formation, swelling, and ulceration were used to determine the wound healing process. Throughout the experiment, body temperature, measured using a subcutaneous probe, and weight of the rats were found to be within normal ranges. Epithelial closure in all rats occurred by 17 days. There was no significant difference in contraction of the lesion areas treated with different extracts. The variability in erythema at each lesion in rats infected with different fungal pathogens differed with treatments; the lesion without treatment took a longer time to heal in all cases. Exudate formation was observed until day 12 in rats infected with C. albicans and day 8 in rats infected with C. neoformans. In lesions infected with M. canis and S. schenckii, exudate formation was observed until day 10. The treated group presented a rigid, dark, and thick crust formation after day 3 until day 15. During histopathological evaluations, scant fungi were noted in all the wounds, indicating that infection had occurred but had generally cleared. The antifungal potential of crude extracts of selected plants and a mixture of asiatic acid and arjunolic acid on the wounds of immunocompromised rats was confirmed. The extracts of these plants may possibly be further developed into drugs for topical treatment of fungally infected wounds.
Introduction
The skin provides a natural barrier against the environment and exerts a variety of essential protective functions. Upon disruption of the skin’s integrity, either by acute injuries or by chronic insults, a multi-step process is initiated, leading to at least partial reconstruction of the wounded tissue and reestablishment of the skin’s barrier function (CitationSchäfer & Werner, 2008).
Wound healing is a dynamic pathway that optimally leads to restoration of tissue integrity and function. Wound healing is the result of the accumulation of processes, including coagulation, inflammation, ground substance and matrix synthesis, angiogenesis, fibroplasia, epithelialization, wound contraction, and remodeling. These complex, overlapping processes are best organized into three phases of healing, which are the inflammatory phase, the proliferative phase, and the maturation phase (Citationde la Torre, 2006).
The inflammation stage begins immediately after injury, first with vasoconstriction that favors homeostasis and releases inflammation mediators. The proliferative phase is characterized by granulation tissue proliferation, formed mainly by fibroblasts and the angiogenesis process. The remodeling stage is characterized by reformulations and improvement in components of the collagen fiber that increases the tensile strength (CitationMandelbaum et al., 2003).
Wound infection is likely the most common reason for poor wound healing. All wounds are contaminated with bacteria or fungi. Whether a wound becomes infected is determined by the host’s immune competence and the size of the bacterial or fungal inoculum. With normal host defenses and adequate debridement, a wound may bear a level of 100,000 (105) microorganisms per gram of tissue and still heal successfully. Beyond this number, a wound may become infected.
Wound care can be traced back to early civilizations, and many of these treatments were based on the use of herbal remedies. Approximately one-third of all traditional medicines in use are for the treatment of wounds and skin disorders, compared to only 1–3% of modern drugs (CitationMantle et al., 2001). Hence, if a compound has antioxidant potential and antimicrobial activity additionally, it can be a good therapeutic agent for accelerating the wound healing process (CitationHoughton et al., 2005).
Some of the species of the Combretaceae family are used for wound healing. CitationHutchings et al. (1996) have reported that the Vhavenda people in South Africa use the leaves of Terminalia sericea Burch ex DC for the treatment of wounds and menorrhagia, while the bark is used for wounds and the roots for diarrhea, infertility, and venereal diseases (CitationMabogo, 1990). CitationMabogo (1990) further reported that the roots of Combretum molle are used by the Vhavenda for wound healing, and in Ghana the leaves are used for wound dressing. The leaves of Combretum kraussii are used for wound application (CitationHutchings et al., 1996).
Some plants are used in the management of fresh wounds, while others are used for the treatment of chronic wounds. The common ways of treatment are either direct application of crushed fresh/dried plant on the wound or repeated wash of the wound with a plant decoction. Despite the widespread use of local plants in wound healing, only a few of them have been investigated for their potential usefulness using excision and incision wound models in rats.
The present study was designed to test the in vivo wound healing and antifungal activity of the extracts of four selected Combretaceae species, namely: Combretum imberbe Wawra, Combretum nelsonii Duemmer, Combretum albopunctatum Suesseng, and Terminalia sericea Burch ex DC. These plants were selected based on low minimum inhibitory concentration (MIC) values ranging 0.02–2.5 mg/mL (CitationMasoko et al., 2005, Citation2006) and low LC50 values ranging 75.7–168.6 μg/mL (CitationMasoko, 2006). In addition, a mixture of isolated compounds of Combretum nelsonii was included in the experiments. Aspects evaluated included wound healing, erythema, exudate formation, crust formation, possible toxic effects of the extracts, and histopathology.
Materials and methods
Plant collection
Leaves were collected in the summer of 2003 from trees in the Lowveld National Botanical Gardens in Nelspruit, Mpumalanga Province, South Africa. Voucher specimens in the garden herbarium and tree labels verified the identity of the plants. Plants were confirmed by Professor J. N. Eloff of the University of Pretoria. Species were selected based against their high antifungal activity in previous in vitro studies (CitationMasoko et al., 2005, Citation2006). Leaves were collected from four species belonging to different sections of the genus (CitationCarr, 1988): C. imberbe Wawra (Hypocrateropsis Engl. & Diels), C. nelsonii Duemmer (Angustimarginata Engl. & Diels), C. albopunctatum Suesseng (Ciliatipetala Engl. & Diels), and T. sericea Burch ex DC (Psidiodes).
Plant storage
Leaves were separated from the stems, and dried at room temperature. The dried plants were milled to a fine powder in a Macsalab mill (model 200 LAB; Eriez®, Bramley, South Africa), and stored at room temperature in closed containers in the dark until used.
Extraction procedure
Dried plant leaves from each species were individually extracted by treating 500 g of finely ground plant material with 5 L of acetone (technical grade; Merck) in large glass containers. Containers were vigorously shaken for 3–5 h in a Labotec model 20.2 shaking machine at high speed. The sediment was allowed to settle, and the supernatant was filtered and evaporated using a rotavaporator (R-114; Büchi) and decanted into pre-weighed labeled beakers. The process was repeated three times to exhaustively extract the plant material, and the extracts were combined. The solvent was removed under a stream of air in a fume cupboard at room temperature. The extract was then weighed to quantify the extraction efficiency.
Minimum inhibitory concentration
The MIC values of the extracts were determined by a serial dilution microplate assay method using tetrazolium violet to indicate growth of the fungi, as described by CitationEloff (1998) and further modified by CitationMasoko et al. (2005). The MIC was recorded as the lowest concentration of extract that inhibited antifungal growth after 24 and 48 h.
In vitro cytotoxicity assay
Viable cell growth after incubation with test extracts was determined using the tetrazolium-based colorimetric (MTT) assay described by CitationMosmann (1983). Normal Vero kidney cells (monkey) of a subconfluent culture were used. The LC50 value was calculated as the concentration of test compound resulting in a 50% reduction of absorbance compared to untreated cells.
Isolation of compounds
Isolation of compounds was discussed in our previously published work (CitationMasoko et al., 2008). A mixture of arjunolic acid and asiatic acid was isolated.
Preparation of extracts and compounds
The dried extracts were ground with a mortar and pestle to a fine powder and mixed with aqueous cream (Reitzer Pharmaceuticals (Pty) Ltd.) consisting of distilled water, white petroleum jelly, mineral oil, emulsifying wax, and phenoxyethanol to a concentration of 20% (2 g/10 g), and isolated compound and amphotericin B to a concentration of 1%, and kept at 4°C until use.
Selection of rats
Twenty-four healthy male Wistar rats weighing 150–200 g were used. The test was conducted using a single gender as a way of reducing variability and to minimize the numbers required (CitationOECD, 2000). At the commencement of the study, each rat was 8–12 weeks old and the weight variation of animals used did not exceed ±20% of the mean weight of all previously dosed animals (CitationNIEHS, 2001).
Housing and feeding conditions
Rats were kept at the Onderstepoort Biomedical Research Centre, University of Pretoria, and housed in separate cages at a temperature of 22°C (±2°C) and relative humidity of 50–60% in a light/dark cycle of 12 h. The rats were fed conventional rodent diets (Epol (Pty) Ltd.) with an unlimited supply of drinking water (CitationNIEHS, 2001). Environmental enrichment, e.g., bedding (wood, wool) was provided to keep rats busy. Previous work suggests that the provision of enrichment items, which give laboratory rats the opportunity to perform exploratory and gnawing activities, can be used to improve their well-being and to distract them from tampering with dressings (CitationZhu et al., 1996).
Preparation of animals
The cages of the rats were labeled, to permit identification. Rats were kept in their cages for at least 5 days prior to treatment to allow acclimatization to the laboratory conditions (CitationSpielmann et al., 1999). They were also handled daily during this period. The rats were immunosuppressed by subcutaneous injection of 500 µg of estradiol valerate.
Wound creation
The hair on the back area was removed with electrical clippers. The area was disinfected using 0.5% chlorhexidine in 70% alcohol and allowed to dry. Rats were anesthetized with isoflurane (0.01–0.05 μg/kg). Seven evenly spaced wounds were made on each rat using 6 mm diameter punch biopsies (CitationSimonsen et al., 2002). The whole process was carried out in a biosafety class II cabinet to limit infection. A temperature probe (Identipet microchip) was injected into each rat subcutaneously in the rump area so that the body temperature could be routinely monitored without unduly disturbing the rat.
Administration of treatments
The treatments were applied three times a week as follows: A, no treatment; B, isolated compound; C, C. nelsonii crude extract; D, C. imberbe crude extract; E, C. albopunctatum crude extract; F, T. sericea crude extract; 20% of crude extract; and G, amphotericin B, on various sites on the rats. These sites were randomly allocated when the treatment was repeated. Six rats were used for each fungal infection.
Observations
Each animal served as its own control with six test sites for the crude and isolated compounds, one as a positive control with amphotericin B, and one site as a negative control. A blinded study was done, and all observations were recorded for each rat. Every Monday, Wednesday, and Friday at the same time, each rat was taken out of the cage and given anesthetic with isoflurane, the dressing was removed, and the different parameters were measured. The rats were weighed and the body temperature read electronically (electronic reader information). The lesions were scored using the following parameters: presence and type of exudate, erythema, swelling, ulceration, and crust formation. The scoring criteria are shown in . The lesion diameters were also measured in millimeters (mm) in the vertical and horizontal planes using the same electronic digital calipers. The wounds were then cleaned using clean cotton wool and the treatments were applied as previously indicated. The wounds were then re-dressed and the rats were placed in the cage to allow them to recover.
Table 1. Evaluation of erythema and exudate.
The rats were observed individually at least once during the first 30 min and periodically during the first 24 h (with special attention given during the first 4 h) after treatment. All rats were observed daily for any indication of illness and interference with the wound dressing. Clinical signs of disease included changes in the skin and fur, diarrhea, lethargy, sleep, weight loss, and coma. Provision was made for the early termination of rats that were very ill or that interfered excessively with the wound dressings. They were euthanized by carbon dioxide inhalation when the wound had fully contracted and there was evidence of scar formation. At necropsy, a pathologist examined the liver, heart, lungs, intestines, lymph nodes, and kidneys of the rats for gross abnormalities.
Pathological and histopathological studies
Histopathological studies were done with the help of a pathologist at the end of the experiment. Wound tissue specimens from treated and non-treated rats were collected in 10% buffered formalin, and, after processing, 6 μm thick sections were cut and stained with hematoxylin and eosin (CitationMcManus & Mowry, 1960). Sections were qualitatively assessed under a light microscope and graded with respect to congestion, edema, infiltration of polymorphonuclear leukocytes (PMNLs) and monocytes, necrosis, fibroblast proliferation, collagen formation, angiogenesis, and epithelialization (CitationShukla et al., 1999). Necropsies were performed, and the presence of fungi was determined using the periodic acid–Schiff (PAS) stain.
The research was approved by the Research and Animal Use and Care Committee of the University of Pretoria (VI 010/05 approval number).
Results
All the rats infected with different fungal pathogens, i.e., C. albicans, C. neoformans, M. canis, and S. schenckii gradually lost weight (results not shown), which was not the case in the pilot study (CitationMasoko et al., 2006), and this was attributed to an immunosuppressive reaction and the additional treatment (isolated compound). On day 15 all the rats gained weight except rat 3. We decided to leave the rats for 6 additional days without handling them, and an increase in weight was observed. Rat 22 lost more weight after day 5 but gradually recovered the weight lost after day 8. The temperatures of the rats were within the expected range (35–37°C) at the end of the experiment (day 15).
Lesion size
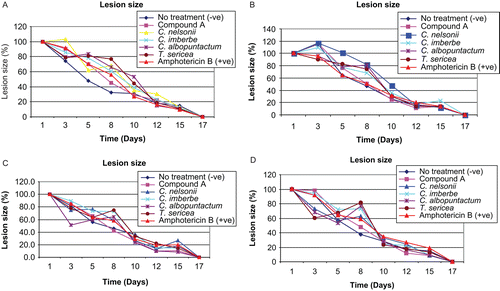
Circular, full-thickness lesions were created on the backs of rats. Lesion sizes were measured: C. albicans (), C. neoformans (), M. canis (), and S. schenckii (). The open lesion healed by the process of wound contraction. Epithelial closure occurred by 17 days in all rats. The transient formation of granulation tissue was vigorous on day 12 after wounding. There was no significant difference in the contraction of lesion areas treated with different extracts. In all the experiments, lesions treated with isolated compound healed faster than with the crude extracts and amphotericin B.
Erythema
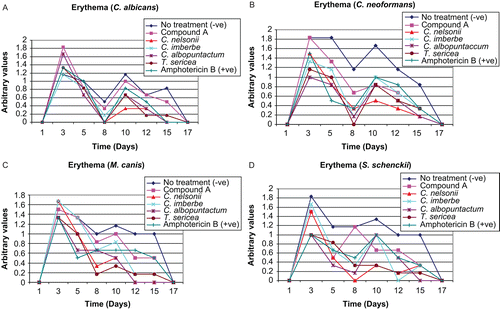
A fundamental property of the skin is its ability to respond to treatment. In rat populations, these responses are clearly adaptive, whereby the first response, erythema (redness), is a sign that the immune system is active and the healing process has begun. The resulting healing was quantified on the basis of erythema: C. albicans (), C. neoformans (), M. canis (), and S. schenckii (). A scoring system was used to determine the degree of erythema. Consequently, a scale of 1–5 was used, 1 being the lowest and 5 being the highest value. Averages from all six rats were used in all groups infected with different pathogens. The variability in the results for erythema at each lesion in rats infected with different fungal pathogens differed between treatments; lesions without treatment took a longer time to heal in all cases. Although the differences were not statistically significant, the plant extracts tended to decrease erythema in practically all cases.
Figure 2. The influence of the crude extracts, isolated compound, and amphotericin B (positive control) on wound erythema in rats infected with C. albicans (A), C. neoformans (B), M. canis (C), and S. schenckii (D).
Exudate
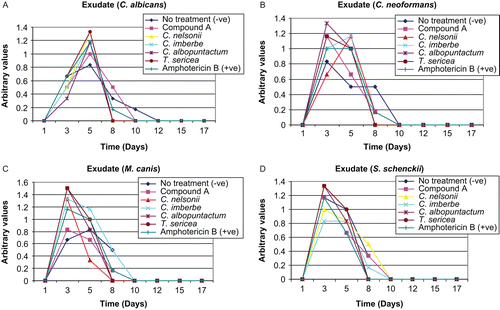
Exudate formation was one of the parameters used to quantify the healing process: C. albicans (), C. neoformans (), M. canis (), and S. schenckii (). Exudate formation was observed until day 12 in rats infected with C. albicans and day 8 in rats infected with C. neoformans, excepting the lesions that were not treated. In lesions infected with M. canis and S. schenckii, exudate formation was observed until day 10. There was less exudate formation in the untreated lesions.
Crust formation
The wound healing process was also quantified by crust formation (results not shown). The treated group presented a rigid, dark, and thick crust. This was probably due to proteins and wound exudates interconnected with the extract constituents favoring local homeostasis, and protecting the new tissue by forming an external cover that furnished mechanical protection. Crust formation in all infected rats followed the same pattern, i.e., crust started forming after day 3 until day 15. There was no marked difference in crust formation with all the treatments.
Histopathological evaluations
Quantitative histopathological findings were determined in four rats, each rat representing the group, i.e., the first six rats were infected with C. albicans (), rat 7 to rat 12 with C. neoformans (), rat 13 to rat 18 with M. canis (), and rat 19 to rat 24 with S. schenckii (). Clumps of fungi were observed with all treatments and in controls for all rats infected with different fungal pathogens. Epithelialization was observed in the dermis except for rats infected with C. neoformans, where it was observed on the epidermis in the wound treated with C. nelsonii and the untreated wound. Clumps of degenerating neutrophils, and necrotic changes in the upper dermis with loss of epidermis, were also observed up to day 17. Scant fungi were noted in all the wounds, indicating that infection had occurred but had generally cleared. Exceptions were treatments with the isolated compound and T. sericea on the S. schenckii infected wounds where there were high numbers of fungi.
Table 2. Quantitative histopathological findings of wounds of rats infected with C. albicans after topical application of different creams.
Table 3. Quantitative histopathological findings of wounds of rats infected with C. neoformans after topical application of different creams.
Table 4. Quantitative histopathological findings of wounds of rats infected with M. canis after topical application of different creams.
Table 5. Quantitative histopathological findings of wounds of rats infected with S. schenckii after topical application of different creams.
Antifungal activity
MIC values ranged 0.02–2.5 mg/mL. C. nelsonii and T. sericea extracts were the most active against all tested pathogens, with an average MIC value of 0.16 mg/mL. C. neoformans and S. schenckii were most susceptible, with average MIC values of 0.11 and 0.03 mg/mL, respectively ().
Table 6. Minimum inhibitory concentration (MIC) values of selected extracts after 24 h incubation at 37°C.
Discussion
The clinical treatment of skin infected with fungi has become a major problem, especially in immunocompromised patients. Therapeutic agents selected for the treatment of infected wounds had ideally shown antifungal activity in in vitro studies. We also evaluated whether these agents would improve phases of wound healing without producing deleterious side effects. We further reported that the selected extracts were not toxic on normal Vero kidney cells (monkey) () (CitationMasoko, 2006).
Table 7. LC50 values from MTT cytotoxicity assay of the optimal extracts.
This study describes some unique features with respect to the therapeutic effect of leaf extracts of selected plants on dermal wounds of rats infected with fungal pathogens. Plant products are potential agents for wound healing and the treatment of fungal infections (CitationMasoko et al., 2005, Citation2006), and are largely preferred because of their widespread availability, low toxicity, and effectiveness as crude preparations. We have reported that Combretum and Terminalia species have antifungal activity () (CitationMasoko et al., 2005, Citation2006; CitationMasoko & Eloff, 2005, Citation2006). These findings prompted us to further investigate the in vivo activity of the four most active extracts.
These experiments were designed to afford a simple in vivo method for comparing the relative effectiveness of various plant extracts against fungal pathogen wound infections. The duration of therapy and the dosage employed determined the end point of the experiment, and gave an index of their relative effectiveness. A preliminary survey of the therapy revealed that a considerable number of plant extracts were effective locally in the prevention of fungal infections. The choice depended upon consideration of toxicity that was determined in other studies (CitationMasoko, 2006).
CitationInngjerdingen et al. (2004) reported that some Combretaceae species had wound-healing activities when the plant powder was applied directly on the wound. The treatments were usually repeated every day until the wounds were healed. In this study, we treated rats with the selected leaf extracts every second day. Wound healing is a multifactorial process whereby microbial infections and the formation of free radicals may contribute to retard or inhibit its resolution. Free radicals can oxidize the endogenous inhibitors or proteases; this reduces their ability to inhibit elastase and the proteases responsible for deterioration of the extracellular matrix (CitationKudi et al., 1999). The possibility of wound healing due to free radicals was eliminated in previous studies (CitationMasoko, 2006), where the antioxidant activity of the selected plants was studied. The selected plant extracts and compound(s) did not have antioxidant activity based on the DPPH (1,1-diphenyl-2-picrylhydrazyl) assay.
There was no evidence of systemic infection caused by the irritant effects. The rats were divided into four groups based on fungal pathogens. In this study, rats were immunocompromised by subcutaneous injection of 500 µg of estradiol valerate. Estradiol pretreatment is known to inhibit innate and acquired immune defenses (CitationCarlson et al., 1991).
Acetone extracts of leaves and a mixture of asiatic acid and arjunolic acid demonstrated wound healing properties comparable to that of an antibiotic powder (amphotericin B). Even the untreated wounds healed, but not at the same rate as the treated wounds. It is important to note that throughout the period of wound treatment, the extracts did not cause irritation or pain to the animals, as the rats showed neither signs of restlessness nor scratching/biting of wound sites when the extracts were applied.
All the rats lost weight in this study until day 12, and started increasing in weight from day 15. It was assumed that this was due to immunocompromisation of the rats. After 3 weeks of the experiment, bandages were removed and all rats were kept for another week. Thereafter it was found that all the rats had gained weight, except rat 3, whose mass remained constant. The temperatures were also within the normal range.
After 3 weeks all 24 rats were euthanized with CO2 and necropsies performed. From rats infected with C. albicans (rats 1–6), organs were taken from rat 1 for histopathological studies. Also, the lungs of rat 3 had block specks and the intestines were blue; the lungs were taken to the bacteriology laboratory for culturing. The organs of rat 7 together with the lungs of rat 10 were taken for histopathological studies. The organs in group 2 (rats infected with C. neoformans) (rats 7–12) were normal. Again, the organs of rat 13 in group 3 (rats 13–18, infected with S. schenckii) and the liver of rat 16 were taken for pathology section, together with the organs of rat 19 and the left lymph node of rat 23 in group 4 (rats 19–24, infected with M. canis).
Unfortunately, some of the results of histopathological studies are not reported here due a delay in evaluation of the samples by the pathologists at Onderstepoort. Only the results from four rats are discussed. The following comparisons were made from histopathology results. There was generally more fibrosis with the crude extracts, but an exception was amphotericin B treatment of S. schenckii. The other parameters of healing, i.e. angiogenesis and epithelialization, were present or complete, with the exception of S. schenckii infections where the extracts performed better; this was possibly a synergistic effect. No noticeable differences in wound necrosis were observed. Neutrophils were evident and at deeper levels in the untreated group. Plasma cells, lymphocytes, and macrophages were the most predominant cell types. These are the most predominant cells in fungal infections, and are also more common in chronic infections. Macrophages are also the most active cells in wound healing, acting as potent wound debriders.
A close examination of tissue sections revealed that there was marked infiltration of lymphocytes, eosinophils, neutrophils, mast cells, and macrophages and enhanced proliferation of fibroblasts as a result of treatments. Increased cellular infiltration observed from hematoxylin and eosin (H&E) staining in treated rats may be a result of a chemotactic effect enhanced by the extract, which might have attracted inflammatory cells toward the wound site. Increased cellular proliferation may be a result of the mitogenic activity of the plant extract, which might have significantly contributed to the healing process. Early dermal and epidermal regeneration in the treated rats also confirmed that the extract had a positive effect toward cellular proliferation, granular tissue formation, and epithelialization.
Histopathological studies of the wounds revealed prominent aspects, i.e., both the antibiotic and the plant extracts individually were capable of healing the wounds. Further study is required regarding the relative quantities of plant extracts that are necessary for optimal effect, and the maximum period for which the extract can be kept stable. Whether such preparations should be and could be sterilized is also an aspect that may be pertinent. Finally, the most important question that arises from the study is: which constituent of the extracts evokes the wound healing effect? Bioactivity may also be associated with other components such as prostaglandin precursors or some other molecule; identification and isolation of such a molecule may also be desirable. Until such a possibility is brought to reality, the plant extracts in their natural form may be our only choice. The isolated mixture of asiatic acid and arjunolic acid from C. nelsonii, which showed high activity in in vitro studies, did not have the same effect in the in vivo studies. The rat infected with M. canis and treated with the mixture demonstrated the formation of fibroblasts and infiltration of the cells in deeper tissues. In a similar manner, in the rat infected with S. schenckii, in the wound treated with the mixture, the formation of fibroblasts occurred in extensive deeper tissues and there was a delay of epithelialization.
In some instances there were more traces of fungal hyphae in wounds treated with amphotericin B compared to the extracts, i.e., in the rat infected with S. schenckii. Maybe this antibiotic was not the right choice for control, and the presumption is that any other antibiotic could have behaved in a manner similar to amphotericin B, but certainly that needs to be experimentally confirmed. Amphotericin B was selected as it is the most potent broad-spectrum antifungal available. However, it is not usually used as a topical agent: the inidazoles are better choices. These aspects, if considered important at all, will have to be studied separately.
Some organ samples were also studied. In most instances the lungs showed diffuse, sub-acute mono-morphonuclear (mainly lympho-plasmacytic, with lesser numbers of macrophages) interstitial pneumonia with moderate to severe, diffuse hemorrhage. The spleen showed mild, red-pulp hyperplasia with many hemosiderin-laden macrophages. The small intestine showed mild to moderately increased numbers of eosinophils within the lamina proprium of the small intestine wall. Some fungal spores were seen within the lumen, but no signs of any reaction were visible. Pre-scopular lymph nodes of rat 23 infected with S. schenckii showed moderate cortical hyperplasia. A handful (four or five) of fungal spores was seen in two foci just below the capsule, two of them within a macrophage phagosome. This is a common mode of spread of this fungus and, as exhibited, rodents are particularly susceptible to S. schenckii infections.
Pulmonary lesions are commonly seen in experimental rodents, and are possibly a result of various environmental factors/stressors. The moderate amounts of hemosiderin within splenic macrophages may be a result of pulmonary hemorrhage or the wounds created for the experiment. The few fungal spores within the lymph nodes of rat 23 were most likely a result of lymphatic drainage from the experimental wounds, as opposed to direct infection, as there were no signs of inflammation in the tissues surrounding the lymph nodes. The occasional fungal spores seen on all of the epidermal surfaces of the skin samples can be regarded as incidental. These spores were always in association with normal skin; hardly ever were they seen over the lesion itself.
Healing is a physiological process and does not normally require much help, but still wounds cause discomfort and are prone to infection and other complications. Therefore, the use of agents to expedite healing is required. Further, some diseases such as diabetes, immunocompromised conditions, and ischemia, and conditions including malnourishment, aging, local infection, and local tissue damage due to burns or gunshot wounds, lead to a delay in healing. Such conditions often require the use of agents that can facilitate the healing process (CitationMensah et al., 2001).
The rat model described in this study was used for the first time by our group to test for fungal pathogens. We have observed that rats must be immunocompromised to ensure a localized fungal infection. We have demonstrated that all the extracts used possess antifungal activity. Although amphotericin B gave better results, the isolated mixture of asiatic acid and arjunolic acid gave promising results, and thus can be considered for future treatment due to the toxicity of amphotericin B. Exudate formation, erythema, and lesion size are good parameters to consider for wound healing. Ulcerations did not occur, indicating that wound healing progressed normally. Generally the technique works, and it can be used as a model for future studies. The main objective was to test the activity of the plant extracts on infected animals. There was no systemic infection except in one rat, and the infection was not from the fungal pathogens used.
Conclusions
The results of this study have confirmed the antifungal potential of crude extracts and the wound healing properties of selected plants and a mixture of asiatic acid and arjunolic acid in a rat model. The extracts of these plants may possibly further be developed into phytomedicines for the management of septic wounds, because they did not show any signs of irritancy to the rats. The model used was successful, as there were no systemic infections in all rats and the wounds healed within 3 weeks.
Infected rat models have often been used for determination of the wound healing properties of various dressings and topical formulations; it is generally acknowledged that these models may not reflect accurately the biological processes occurring in humans during wound healing, likely due to significant inter-species skin differences in morphology and function (CitationDorsett-Martin, 2004). However, there is still a potential to consider using the rat model for animal and human infections.
In conclusion, treatment using the leaves of selected plants exhibited significant pro-healing activity in infected wounds when topically applied on rats, by affecting various stages of the healing process.
Acknowledgements
Rudi Kotze and Johan Hurter gave permission to collect plant material from the Lowveld National Botanical Garden. Mr N. P. Selahle helped with the care of rats and the experiment. Histopathological studies were done with the help of a pathologist (Dr Joshua Dabwroski).
Declaration of interest
The National Research Foundation (NRF) provided funding. There is no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Carlson D, Haurie A, Leizarowitz A (1991): Infinite Horizon Optimal Control: Deterministic and Stochastic Systems, 2nd ed. Berlin, Springer, pp. 90–108.
- Carr JD (1988): Combretaceae in Southern Africa. Johannesburg, The Tree Society of Southern Africa, pp. 1–100.
- de la Torre JI (2006): Wound Healing, Chronic Wounds [Online]. Available at: http://www.emedicine.com/plastic/TOPIC457.htm.
- Dorsett-Martin WA (2004): Rat models of skin wound healing: A review. Wound Repair Regen 12: 591–599.
- Eloff JN (1998): A sensitive and quick method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med 64: 711–713.
- Houghton PJ, Hylands PJ, Mensah AY, Hensel A Deters AM (2005): In vitro tests and ethnopharmacological investigations: Wound healing as an example. J Ethnopharmacol 100: 100–107.
- Hutchings A, Scott AH, Lewis G, Cunninghan A (1996): Zulu Medicinal Plants, An Inventory. Pietermarizburg, South Africa University of Natal Press, pp. 128–163.
- Inngjerdingen K, Nergard CS, Diallo D, Mounkoro PP, Paulsen BS (2004): An ethnopharmacological survey of plants used for wound healing in Dogonland, Mali, West Africa. J Ethnopharmacol 92: 233–244.
- Kudi AC, Umoh JU, Eduvie LO, Gefu J (1999): Screening of some Nigerian plants for antibacterial activity. J Ethnopharmacol 67: 225–228.
- Mabogo DEN (1990): The Ethnobotany of the Vhavenda. MSc Thesis, University of Pretoria, pp. 1–100.
- Mandelbaum SH, Di Santis EP, Mandelbaum MHSA (2003): Cicatrization: current concepts and auxiliary resources – Part I. An Bras Dermatol 72: 393–410.
- Mantle D, Gok MA, Lennard TWJ (2001): Adverse and beneficial effects of plant extracts on skin and skin disorders. Adv Drug React Toxicol Rev 20: 89–103.
- Masoko P (2006): Characterisation of antifungal compounds isolated from Terminalia and Combretum species (Combretaceae). PhD Thesis, University of Pretoria, pp. 1–326.
- Masoko P, Eloff JN (2005): The diversity of antifungal compounds of six South African Terminalia species (Combretaceae) determined by bioautography. Afr J Biotechnol 4: 1425–1431.
- Masoko P, Eloff JN (2006): Bioautography indicates the multiplicity of antifungal compounds from twenty-four South African Combretum species (Combretaceae). Afr J Biotechnol 5: 1625–1647.
- Masoko P, Picard J, Eloff JN (2005): Antifungal activities of six South African Terminalia species (Combretaceae). J Ethnopharmacol 99: 301–308.
- Masoko P, Picard J, Eloff JN (2006): The antifungal activity of twenty-four South African Combretum species (Combretaceae). S Afr J Bot 73: 173–183.
- Masoko P, Mdee LK, Mampuru LJ, Eloff JN (2008): Biological activity of two related triterpenes isolated from Combretum nelsonii (Combretaceae) leaves. Nat Prod Res 22: 1074–1084.
- McManus JFA, Mowry RW (1960): Staining Methods: Histologic and Histochemical. New York, Harper & Row, pp. 81–91.
- Mensah AY, Sampson J, Houghton PJ, Hylands PJ, Westbrook J, Dunn M, Hughes MA, Cherry GW (2001): Effects of Buddleja globosa leaf and its constituents relevant to wound healing. J Ethnopharmacol 77: 219–226.
- Mosmann T (1983): Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55–63.
- NIEHS (2001): Guidance Document on Using in vitro Data to Estimate in vivo Starting Doses for acute toxicity. NIH number 01-4500. Research Triangle Park, NC, National Institute of Environmental Health Sciences.
- OECD (2000): Revised draft guidance document on the recognition, assessment and use of clinical signs as humane endpoints for experimental animals used in safety evaluation. Report of the international workshop on in vitro methods for assessing acute systematic toxicity. NIH number 01-4499. Research Triangle Park, NC, National Institute of Environmental Health Sciences.
- Schäfer M, Werner S (2008): Oxidative stress in normal and impaired wound repair. Pharmacol Res 58: 165–171.
- Shukla A, Rasik AM, Jain GK, Shankar R, Kulshrestha DK, Dhawan BN (1999): In vitro and in vivo wound healing activity of asiaticoside isolated from Centella asiatica. J Ethnopharmacol 65: 1–11.
- Simonsen L, Petersen MB, Groth L (2002): In vivo skin penetration of salicylic compounds in hairless rats. Eur J Pharm Sci 17: 95–104.
- Spielmann HE, Genschow M, Leibsch M, Halle W (1999): Determination of the starting dose for acute oral toxicity (LD50) testing in the up and down procedure (UDP) from cytotoxicity data. ATLA 27: 957–966.
- Zhu Shun-Wei, Yee BK, Nyffeler M, Winblad B, Feldon J, Mohammed AH (1996): Influence of differential housing on emotional behaviour and neurotrophin levels in mice. Behav Brain Res 169: 10–20.