Abstract
The antioxidant, antiplatelet, and cytoxoxic effects of seven South African plant extracts, namely, Combretum vendae A.E. van Wyk (Combretaceae), Commiphora harveyi (Engl.) Engl. (Burseraceae), Khaya anthotheca (Welm.) C.DC (Meliaceae), Kirkia wilmsii Engl. (Kirkiaceae), Loxostylis alata A. Spreng. ex Rchb. (Anacardiaceae), Ochna natalitia (Meisn.) Walp. (Ochnaceae), and Protorhus longifolia (Bernh. Ex C. Krauss) Engl. (Anacardiaceae), were evaluated using established in vitro assays. All the extracts showed comparably low toxicity except for the extract of C. harveyi that showed high hemagluttination assay titer value, which indicates toxicity. The extracts of P. longifolia, K. wilmsii, O. natalitia, L. alata, C. harveyi, and C. vendae exhibited antioxidant properties in the qualitative assay using DPPH. In the quantification of antioxidation using ABTS, only the extracts of P. longifolia, L. alata, and C. vendae showed antioxidant activity with respective TEAC values of 1.39, 1.94, and 2.08. Similarly, in the quantitative DPPH assay, L. alata (EC50, 3.58 ± 0.23 µg/mL) and K. wilmsii (EC50, 3.57 ± 0.41 µg/mL) did not differ significantly (p ≤ 0.05) from the control. K. anthotheca showed a higher EC50 (176.40 ± 26.56 µg/mL) value, and differed significantly (p ≤ 0.05) from all the other extracts and control. In addition, the extracts of C. vendae and C. harveyi showed significant (p ≤ 0.05) antiplatelet activity and did not differ from the control (aspirin) with EC50 of 0.06 ± 0.01 µg/mL and 0.19 ± 0.00 µg/mL, respectively. Lower EC50 values in the antioxidant and antiplatelet studies are indicative of superior activity of the plant extract against oxidation and platelet aggregation.
Introduction
Bioactive compounds commonly found in plants have been shown to have possible health benefits partly due to their antioxidative properties (CitationCao & Cao, 1999). It is well known that reactive oxygen species (ROS) are involved in a diversity of important processes in medicine, including among others: inflammation, atherosclerosis, cancer, and reperfusion injury (CitationKehrer, 1993). One of the major fundamental tissue-destructive mechanisms is oxidative stress through an excessive release of reactive oxygen metabolites (ROM) (CitationMcCord, 2000). Although their generation by phagocytes (and to a lesser extent by eosinophils, lymphocytes, and fibroblasts) is essential for an effective host defense against bacterial infection, continuous overproduction during inflammatory processes may also cause extensive tissue destruction (CitationWeiss, 1989). One way by which a substance can interfere with these processes is by acting as an antioxidant or free radical scavenger. Antioxidants abate inflammation and protect tissues from oxidative damage caused by free radicals. Inflammation has been described as the release of chemicals from tissues and migrating cells. Agents such as prostaglandins, leukotrienes, histamine, bradykinin, platelet-activating factor (PAF) 1, and interleukin 1 (IL 1) play some part in the inflammatory processes. In addition, prostaglandins and other inflammatory mediators produce oxidant products that play an important role in tissue oxidation (CitationPekoe et al., 1982). Many anti-inflammatory drugs are able to react with oxidants in vitro, so considerable interest has been expressed in the possibility that oxidant scavenging contributes to the action of these drugs in vivo (CitationHalliwell et al., 1988). Despite the presence of wide arrays of anti-inflammatory drugs (steroidal and nonsteroidal), they still present a wide range of side effects for which the major reason is nonselective inhibition of cyclooxygenase I (COX I) and cyclooxygenase II (COX II) (CitationVane & Botling, 1995). Prostaglandins, thromboxanes, and platelet-activating factor (PAF) contribute to platelet aggregation, and, moreover, platelets have been shown to play an important role in acute inflammation by releasing arachidonic acid (AA) metabolites and PAF (CitationHolmsen et al., 1977; CitationVincent et al., 1977). Furthermore, thromboxane A2 (TXA2), an AA product formed via the COX pathway in platelets, has been reported to be a potent vasoconstrictor and pro-aggregatory agent (CitationPage et al., 1984). COX inhibitors, such as aspirin, are known to inhibit platelet aggregation (CitationSaeedu et al., 1997).
A large number of naturally occurring compounds, such as flavonoids, catechins, lignans, and phenolic acids contained in plants and herbal remedies, have been shown to have antioxidant properties (CitationDall’Acqua et al., 2008). These reasons have recently prompted research into natural antioxidants. The aim of this study was to test the antioxidant, antiplatelet, and cytotoxicity effects of acetone extracts of seven South African tree leaves that were previously screened and shown to have antifungal, antibacterial, and other pharmacological effects.
Materials and methods
Plant collection
The leaves of Commiphora harveyi (Engl.) Engl. (Burseraceae), Combretum vendae A.E. van Wyk (Combretaceae), Khaya anthotheca (Welm.) C.DC (Meliaceae), Loxostylis alata A. Spreng. ex Rchb. (Anacardiaceae), and Protorhus longifolia (Bernh. Ex C. Krauss) Engl. (Anacardiaceae) were collected at the University of Pretoria Botanical Garden, South Africa. Kirkia wilmsii Engl. (Kirkiaceae) and Ochna natalitia (Meisn.) Walp. (Ochnaceae) were collected at the Lowveld National Botanical Garden in Nelspruit, South Africa. All plants were collected in November 2006 and were identified and authenticated by Lorraine Middleton, the herbarium curator, and Magda Nel at the Botanical Garden of the University of Pretoria. Voucher specimens of the plants were deposited at the herbarium of the Department of Plant Sciences, University of Pretoria, South Africa.
Plant storage
Immediately after collection and transportation to our laboratory, leaves were separated from stems and dried at room temperature with good ventilation. The dried plants were milled to a fine powder in a Macsalab mill (model 200 LAB; Eriez®, Bramley, South Africa) and stored at room temperature in closed containers in the dark until used.
Plant extraction
Plant samples from each species were individually extracted by weighing four aliquots of 1 g of finely ground plant material and extracting with 10 mL of acetone, hexane, dichloromethane (DCM), or methanol (technical grade; Merck) in polyester centrifuge tubes. Tubes were vigorously shaken for 1 h using a Labotec model 20.2 shaking machine at a moderate speed. Extracting at lower speed for a longer period allows the solvent to penetrate more into the plant tissues, allowing the extraction of more of the compounds contained in the plant species (CitationSilva et al., 1998). After centrifuging at 3500 × g for 10 min, the supernatant was decanted into pre-weighed, labeled containers. The whole process was repeated three times to exhaustively extract the plant material and the extracts were combined. The solvent was removed under a stream of air in a fume cupboard at room temperature to quantify the extraction.
Evaluation of antioxidant activity
Qualitative antioxidant screening was employed using 2,2-diphenyl-1-picryl hydrazyl (DPPH) (CitationTakao et al., 1994). Thin layer chromatography (TLC) plates loaded with 100 µg of each extract were developed in a chloroform/ethyl acetate/formic acid (5:4:2; CEF) solvent system and sprayed with 0.2% DPPH in methanol. Compounds with antioxidant activity were visualized as yellow bands against a purple background (Bors et al., Citation1992). Similarly, quantification of antioxidant activity was done by spectrophotometric means using two radicals, ABTS [2,2’-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)] and DPPH.
In the ABTS method, the Trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid) equivalent antioxidant capacity (TEAC) assay Citation(Re et al., 1999) was determined. This was based on scavenging of the ABTS radical into a colorless product by antioxidant substances. The blue/green chromophore ABTS+ was produced through the reaction between ABTS and potassium persulfate. The absorbance was read at 734 nm using a Versamax microplate reader (Molecular Devices). Trolox is a vitamin E analog and was used as a standard in this assay. The percentage change in absorbency was calculated as shown below:
Initial absorbency of ABTS+ – New absorbency of ABTS+/Initial absorbency of ABTS+ × 100
Curves were plotted with the dependent variable being the percentage change in absorbency and the independent variable being the different concentrations at which test substances were analyzed. Mathematical comparison of the antioxidant activities of different plant extracts was done by dividing the slope obtained for extract by that of Trolox, to get the Trolox equivalent antioxidant capacity (TEAC). An extract with a TEAC value of 1 indicates an antioxidant value equivalent to that of Trolox. A decrease or increase of antioxidant activity is depicted by a lower or higher value of TEAC, respectively.
The DPPH free radical assay was conducted as described by CitationMensor et al. (2001). Briefly, 10 µL of 0.3 mM DPPH in ethanol was added to 25 µL of each concentration of extract tested and allowed to react at room temperature in the dark for 30 min. Appropriate blank and negative control solutions were prepared for each test. L-ascorbic acid (vitamin C) was used as positive control. The decrease in absorbance was measured at 518 nm. Values obtained were converted to percentage antioxidant activity (AA%) using the formula:
AA% = 100 – {[(Abssample – Absblank) × 100]/Abscontrol}
where Abssample is the absorbance of the sample, Absblank is the absorbance of the blank, and Abscontrol is the absorbance of the control. The EC50 value, defined as the concentration of sample leading to a 50% reduction of the initial DPPH concentration, was calculated from the linear regression of plots of concentration of the test extracts (µg/mL) against the mean percentage of antioxidant activity obtained from three replicate assays, using Microsoft Office Excel. EC50 values obtained from the regression lines showed a coefficient of determination r2 ≥ 95%. A lower EC50 value indicates high antioxidant activity.
In vitro platelet aggregation assay
The modified method of CitationFratantoni and Poindexter (1990) was used to determine the inhibitory effect of the plant extracts on platelet aggregation. Briefly, fresh equine (Equus caballus) blood was collected from healthy representative breeds in the Equine Research Centre, Faculty of Veterinary Sciences, University of Pretoria, by Mrs, Stellest de Villiers into sterile 5 mL glass tubes containing 3.8% trisodium citrate solution as anticoagulant at a ratio of 1:9 volume of anticoagulant to blood.
The platelet rich plasma (PRP) and platelet poor plasma (PPP) were separated by centrifugation at 160 × g for 10 min and 1600 × g for 15–30 min at 20–25°C, respectively, using a Beckman® GS-15R centrifuge. PRP was later centrifuged at 1000 × g for 15 min at 20–25°C to sediment the platelets. The platelet count of the PRP was adjusted to 300,000/pL by adding PPP. Both PRP and PPP were then stored at room temperature. The cell suspension was adjusted to approximately 3.0 × 108 platelets per mL using phosphate buffered saline (PBS). Different concentrations of the extracts and aspirin as the reference drug were added and incubated at 37°C for 3 min. After incubation, platelet aggregation was induced by the addition of 50 µL of adrenaline.
The degree of platelet aggregation was determined spectrophotometrically at 600 nm after 30 min. Percentage platelet aggregation inhibition was calculated using the following equation:
X (%) = A – B/A × 100
where A = maximal aggregation of the control and B = maximal aggregation of sample-treated PRP.
The EC50 values were calculated by linear regression of plots using Microsoft Office Excel. The abscissa represented the concentration of tested plant extracts and the ordinate the average percent of antioxidant activity from three separate tests.
Cytotoxicity assay
MTT assay
The plant extracts were tested for cytotoxicity against the Vero monkey kidney cell line. The cells were maintained in minimal essential medium (MEM; Highveld Biological, Johannesburg, South Africa) supplemented with 0.1% gentamicin (Virbac) and 5% fetal calf serum (Adcock-Ingram). To prepare the cells for the assay, cell suspensions were prepared from confluent monolayer cultures and plated at a density of 0.5 × 103 cells into each well of a 96-well microtiter plate. After overnight incubation at 37°C in a 5% CO2 incubator, the subconfluent cells in the microtiter plate were used in the cytotoxicity assay. Stock solutions of the plant extracts were prepared by reconstitution to a concentration of 100 mg/mL in dimethylsulfoxide (DMSO). Serial 10-fold dilutions of each extract were prepared in growth medium (1–1000 µg/mL). The method described by CitationMosmann (1983) was used to determine the viability of cell growth after 120 h incubation with plant extracts. 3-(4,5-Dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) was used as an indicator of cell growth. The absorbance was measured at 570 nm. Berberine chloride (Sigma) was used as a positive control. Tests were carried out in quadruplicate and each experiment was done in triplicate.
Hemagglutination assay
Fresh equine (Equus caballus) blood was collected from a representative breed in the Equine Research Centre, Faculty of Veterinary Sciences, University of Pretoria into sterile 5 mL glass tubes containing 3.8% trisodium citrate solution. Erythrocytes were fixed with formalin and prepared according to the method of CitationSadique et al. (1989) as modified by CitationIwalewa et al. (2005). Briefly, 20 mL of the mixed blood was centrifuged at 4000 rpm for 10 min, using a Beckman® GS-15R centrifuge. The packed red blood cells (RBCs) were washed with 10 mM PBS, pH 7.2, until a clear supernatant was obtained. The washed, packed RBCs were suspended in 5% (v/v) formaldehyde–PBS (1:12.3, v/v) solution. The mixture was left at room temperature for 24 h. The final, fixed RBCs were washed and centrifuged with PBS three times, and preserved with 1 mL (50 mg/mL) gentamicin containing 0.1% methyl paraben to prevent microbial growth, and stored at 4°C.
PBS (100 µL) was added to wells of 96-well microtiter plates. The first row was used as a control without extracts. The extracts (100 μL) were added into the first well of the second row, and a two-fold serial dilution was made until the last well (well 12). Then, 50 μL of equine RBCs was added to all the wells. They were incubated at room temperature for 1 h. The presence of buttons in the center of the well indicated no agglutination, and the hemagglutination titer values of the extracts were read as the reciprocal of the last dilution showing agglutination
Statistical analysis
Antioxidant and antiplatelet experiments were done in triplicate. The results are presented as mean ± standard error of the mean (SEM). A one-way analysis of variance (ANOVA) was used for comparison of means using Microsoft Office Excel. A difference was considered statistically significant when p ≤ 0.05.
Results
The extraction yields of acetone extracts of plants used in this study are presented in . C. vendae gave the highest yield of 15.7%, while K. wilmsii possessed the lowest yield (6.9%). The TLC DPPH method of qualitative antioxidant detection showed that the acetone extracts of P. longifolia, K. wilmsii, O. natalitia, L. alata, C. harveyi, and C. vendae displayed antioxidant compounds due to their DPPH free radical scavenging activity. Antioxidant compounds were seen as yellow bands against a purple background. The acetone extract of K. anthotheca, however, did not show any antioxidant compound on the TLC plate ().
Figure 1. Chromatogram of 100 μg acetone extracts of the leaves of P. longifolia (PL), K. wilmsii (KW), O. natalitia (ON), K. anthotheca (KA), L. alata (LA), C. harveyi (CH), and C. vendae (CV), separated with CEF mobile phase and sprayed with 0.2% DPPH. Antioxidant compounds are indicated by yellow areas.
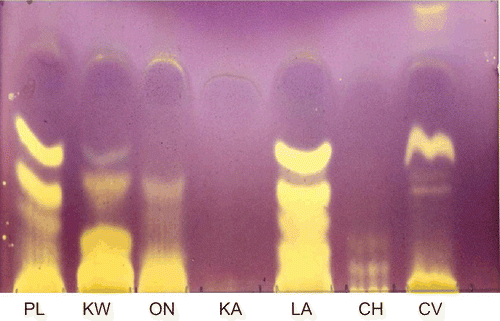
Table 1. Antioxidant and antiplatelet activity of acetone extracts of seven South African plants.
In the TEAC antioxidant assay, extracts of P. longifolia, L. alata, and C. vendae showed superior free radical scavenging activity when compared with other extracts and the standard antioxidant (Trolox) used in this study, with respective TEAC values of 1.39, 1.94, and 2.08 (). The results corroborate those of the qualitative assay where the extracts showed the presence of antioxidant compounds. Extracts of O. natalitia, K. wilmsii, C. harveyi, and K. anthotheca showed lower TEAC values of 0.79, 0.67, 0.15, and 0.10, respectively.
In the quantitative DPPH assay (), L-ascorbic acid had an EC50 (1.59 ± 0.80 µg/mL) value lower than all the extracts; however, L-ascorbic acid showed no significant (p ≤ 0.05) difference in its free radical scavenging effect when compared with L. alata (EC50, 3.58 ± 0.23 µg/mL) and K. wilmsii (EC50, 3.57 ± 0.41 µg/mL). K. anthotheca showed a higher EC50 (176.40 ± 26.56 µg/mL) value, and differed significantly (p ≤ 0.05) from all the other extracts and l-ascorbic acid. Extracts of P. longifolia, C. vendae, C. harveyi, and O. natalitia had EC50 values of 6.57 ± 0.23, 4.41 ± 0.14, 10.47 ± 1.96, and 7.50 ± 0.13 µg/mL, respectively. The lower the EC50 of a substance, the more effective is its free radical scavenging effect.
The antiplatelet actions of C. vendae and C. harveyi did not differ significantly (p ≤ 0.05) from that of aspirin (standard agent) (). The low EC50 shown by C. vendae (0.06 ± 0.01 µg/mL) and C. harveyi (0.19 ± 0.00 µg/mL) depicts good antiplatelet activity when compared with that of aspirin (0.04 ± 0.00 µg/mL).
The cytotoxic activities of the extracts in the Vero monkey kidney cell line assay are provided in . In the assay, the extracts of O. natalitia, K. anthotheca, L. alata, C. harveyi, and C. vendae at the highest concentration used were relatively toxic; they caused complete death of the Vero cells. However, at lower concentrations, all the extracts showed relatively lower toxicity when compared with the reference agent berberine (cytotoxic agent). Over 90% of Vero cells treated with berberine at the concentration of 100 µg/mL were not viable. The effects of the extracts on fixed equine erythrocytes are shown in . All the plant extracts exhibited very low hemagglutination assay (HA) titer values with a wide range of concentrations at which agglutination occurred. However, the acetone extract of C. harveyi showed a very high HA titer value and a very low concentration at which agglutination occurred.
Figure 2. Viability of Vero monkey kidney cell line treated with different concentrations of acetone extracts of seven South African plants. Values are mean ± SEM.
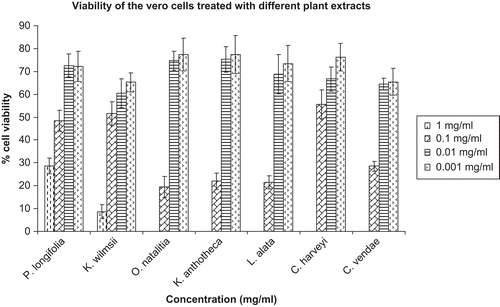
Table 2. Cytotoxic effect of acetone extracts of seven South African plants on formaldehyde-fixed equine erythrocytes.
Discussion
In this study, we applied established in vitro assays in order to evaluate the antioxidant, antiplatelet, and cytotoxic actions of seven South African tree leaves. These plants had shown promising antifungal and bacterial activities in previous studies. This study is the first to report the antioxidant, antiplatelet, and cytotoxicity activities of these plants. These natural products were able to scavenge free radicals in a concentration-dependent fashion in two separate antioxidant assays. DPPH is a free radical, stable at room temperature, which produces a violet solution in ethanol. It is reduced in the presence of an antioxidant molecule, giving rise to uncolored solutions. The use of DPPH provides an easy and rapid way to evaluate antioxidants (CitationMensor et al., 2001). The extracts that showed antioxidant activity separated poorly from the origin when applied and eluted on TLC. This could have been the result of overloading the TLC plates or due to the presence of polyphenolic compounds (CitationNaidoo et al., 2006). Polyphenols do not move well from the origin due to their high polarity, leading to tight binding with normal phase silica (CitationDavidson, 1964).
The ABTS+ assay described here involves direct production of the blue/green ABTS.+ chromophore through the reaction between ABTS and potassium sulfate, which has absorption maxima at wavelengths 645 nm, 734 nm, and 815 nm (CitationMiller et al., 1993; CitationRe et al., 1999).
Both Trolox and l-ascorbic acid have been used as standards in the quantification of antioxidant activity (Fukomoto & Mazza, 2000). The EC50 values of P. longifolia, K. wilmsii, O. natalitia, L. alata, C. harveyi, and C. vendae were lower than that of Ginkgo biloba extract (EGb 761), whose EC50 is 40.72 μg/mL (CitationAderogba et al., 2004; CitationBridi et al., 2001; CitationMensor et al., 2001). The extract of Ginkgo biloba (EGb 761) has been widely employed for its significant benefit as an antioxidant for the prevention of neurodegenerative disorders (CitationBridi et al., 2001). Similarly, these extracts showed free radical scavenging in the DPPH TLC assay.
It has been suggested that more than one method of antioxidant testing should be used to obtain detailed knowledge of the antioxidant activity of test substances, and in addition, the extrapolation of in vitro data to in vivo situations is often difficult (CitationAruoma, 2003). The TEAC assay is used commonly for screening compounds, food products, and extracts for antioxidant activity, and is particularly useful in providing a ranking order of antioxidants (Citationvan den Berg et al., 1999), despite the limitations it carries.
The agonist adrenaline (epinephrine) induced platelet aggregation in equine platelets. The extracts of P. longifolia, K. wilmsii, O. natalitia, K. anthotheca, L. alata, C. harveyi, and C. vendae inhibited platelet aggregation induced by this agonist with different potencies. However, only the extract of C. vendae showed a statistically (p < 0.05) significant platelet inhibitory effect.
Platelet activation is usually accompanied by a rise in cytosolic Ca2+ levels and this occurs through stimulation of the enzymes that are not fully functional at the low Ca2+ concentration present in the resting platelets (CitationBerridge, 1993; CitationHeemskerk & Sage, 1994). In platelets, either the stimulation of phospholipase C (PLC) or the activation of inhibitory G-protein (Gi)-linked receptors elevates the cytosolic Ca2+ levels (CitationPuri et al., 1995). This takes place through the release of Ca2+ from internal stores or through the entry of Ca2+ across the plasma membrane from an external medium (CitationBerven et al., 1995; CitationObberghen-Schilling & Pouyssegur, 1993).
An alternative pathway of increasing the Ca2+ influx is through activation of the Gi-linked pathway. Agonists such as adrenaline are known to inhibit adenylyl cyclase activity in platelets, leading to a decrease in intracellular cyclic adenosine monophosphate (cAMP) levels (CitationPuri et al., 1995). Multiple studies have shown that agents that decrease cAMP levels stimulate platelet aggregation (CitationNieuwland et al., 1994; CitationSiess et al., 1993). This occurs through activation of α2-adrenergic receptors Citation(Siess & Lapetina, 1989). α2-Adrenoceptors in platelets are known to be coupled to the guanine nucleotide-binding protein G, which mediates inhibition of adenylate cyclase. This mainly takes place either through an increase in Ca2+ influx (CitationShah et al., 1996) or as a result of activation of some other proteins (CitationMusgrave & Seifert, 1995). It is possible that the extracts in this study may contain components which block these Ca2+ channels or act via an unknown mechanism(s) to cause increased levels of intracellular cAMP in platelets, as demonstrated through the inhibition of platelet aggregation.
Anti-inflammatory drugs could affect oxidant damage in several ways. First, they might directly scavenge such reactive oxidants as •OH and HOCl. Most, if not all, anti-inflammatory drugs are capable of reacting quickly with .OH. Hence, drugs with good anti-inflammatory activity could be of use as free radical scavengers (CitationHalliwell et al., 1988).
Platelet aggregation plays a pathophysiological role in a variety of thromboembolic disorders. Therefore, prevention of platelet aggregation by drugs should provide effective prophylactic and/or therapeutic treatments for such diseases (CitationHsiao et al., 2003).
Studies have demonstrated that botany and medicine are related. Free radicals and lipid peroxidation have been suggested as potentially important causative agents of several diseases in animals and humans (CitationNair et al., 2003). It would be worthwhile to explore and find safer and efficacious remedies from natural sources, particularly from the relatively undiscovered and unexplored rich flora of South Africa.
The antioxidant and antiplatelet effects of these extracts, and the additional benefit of their low cytotoxicity, provide strong motivation for the development of these plants as possible drugs for the control of diseases in animals and humans. Most considerably, these properties substantiate the use of plant screening exercises for detecting disease control agents.
In conclusion, these plant species appear to be potential sources of antioxidant and anti-inflammatory agents. From this study, we can rightly infer that the South African flora offers great potential in the search for natural compounds with disease controlling agents. The bioassay-guided fractionation procedure to isolate and characterize active compounds from those plant extracts that show good activity is currently being undertaken in our laboratory.
Acknowledgements
The permission granted to us by the University of Pretoria Botanical Garden and the Lowveld National Botanical Garden, Nelspruit to collect the plant leaves is also greatly acknowledged.
Declaration of interest
We have acknowledged funds received from the SA-NRF for the study. The funds were provided to one of the authors (MM Suleiman) as PhD bursary at the University of Pretoria.
References
- Aderogba MA, Okoh EK, Adelanwa TA, Obuotor EM (2004): Antioxidant properties of the Nigerian Piliostigma species. J Biol Sci 4: 501–503.
- Aruoma OI (2003): Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat Res 20: 523–524
- Berridge MJ (1993): Inositol triphosphate and calcium signalling. Nature 361: 315–325
- Berven LA, Crouch MF, Katsis F, Kemp BE, Harland LM, Berritt GJ (1995): Evidence that the pertussis toxin-sensitive trimeric GTP-binding protein Gi2 is required for agonist- and store-activated Ca2+ inflow in hepatocytes. J Biol Chem 25: 893–897.
- Bors W, Saran M, Eltsner EF (1992): Screening of plant antioxidants. Mod Methods Plant Anal 13: 277–295.
- Bridi R, Crossetti FP, Steffen VM, Henriques AT (2001): The antioxidant activity of standardised extracts of Ginkgo biloba (EGb 761) in rats. Phytother Res 15: 449–451.
- Cao Y, Cao R (1999): Angiogenesis inhibited by drinking tea. Nature 398: 381.
- Dall’Acqua S, Cervellati R, Cecilia Loi M, Innocenti G (2008): Evaluation of in vitro antioxidant properties of some traditional Sardinian medicinal plants: Investigation of the high antioxidant capacity of Rubus ulmifolius. Food Chem 106: 745–749.
- Davidson RL (1964): An experimental study of succession in the Transvaal Highveld. In: Davis DHS, ed., Ecological studies in Southern Africa. The Hague, Junk, pp. 113–125.
- Fratantoni JC, Poindexter BJ (1990): Characterization of the platelet response to exogenous arachidonic acid. Thromb Res 22: 157–166.
- Fukumoto LR, Mazza G (2000): Assessing antioxidant and prooxidant activities of phenolic compounds. J Agric Food Chem 48: 3597–3604.
- Halliwell B, Hoult JR, Blake DR (1988): Oxidants, inflammation, and anti-inflammatory drugs. FASEB J 2: 2867–2873.
- Heemskerk JWM, Sage O (1994): Calcium signalling in platelets and other cells. Platelets 5: 295–316.
- Holmsen H, Sal Janicoff L, Fukami MH (1977): Platelet behaviours and biochemistry. In: Ogston D, Bennett B, eds., Haemostasis: Biochemistry, Physiology and Pathology. New York, Wiley, pp. 239–319
- Hsiao G, Shen MY, Lin KH, Chou CY, Tzu NH, Lin CH, Chou DS, Chen TF, Sheu JR (2003): Inhibitory activity of kinetin on free radical formation of activated platelets in vitro and on thrombus formation in vivo. Eur J Pharmacol 465: 281–287.
- Iwalewa EO, Adewunmi CO, Omisore NOA, Adebanji OA, Azike CK, Adigun AO, Adesina OA, Olowoyo OG (2005): Pro- and antioxidant effects and cytoprotective potentials of nine edible vegetables in southwest Nigeria. J Med Food 8: 539–544.
- Kehrer JP (1993): Free radicals as mediators of tissue injury and disease. Crit Rev Toxicol 23: 21–48
- McCord JM (2000): The evolution of free radicals and oxidative stress. Am J Med 108: 652–659
- Mensor LL, Menezes FS, Leitão GG, Reis AS, Santos TC, Coube CS, Leitão SG (2001): Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res 15: 127–130.
- Miller NJ, Rice-Evans CA, Davies MJ, Gopinathan V, Milner A (1993): A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci 84: 407–412.
- Mosmann T (1983): Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55–63
- Musgrave IF, Seifert R (1995): α2A-Adrenoceptors mediate activation of non-selective cation channels via Gi-proteins in human erythroleukaemia (HEL) cells: No evidence for functional role of imidazoline receptors in modulating calcium. Biochem Pharmacol 49: 187–196
- Naidoo V, Chikoto H, Bekker LC, Eloff JN (2006): Antioxidant compounds in Rhoicissus tridentata extracts may explain their antibabesial activity. South African J Sci 102: 198–200.
- Nair U, Bartsch H, Nair J (2003): Prevention of degenerative diseases; clues from studies investigating oxidative stress, Brussels, 13 November 2002. Mutagenesis 18: 477–483.
- Nieuwland R, Wijburg OL, van Willigen G, Akkerman JW (1994): α2A-Adrenergic receptors activate protein kinase C in human platelets via a pertussis toxin-sensitive G-protein. FEBS Lett 339: 79–83.
- Obberghen-Schilling EV, Pouyssegur J (1993): Signalling pathways of the thrombin receptor. Thromb Haemost 70: 163–167.
- Page CP, Archer CB, Paul W, Morley J (1984): PAF: A mediator of inflammation and asthma. Trends Pharmacol Sci 5: 239–241.
- Pekoe G, Van Dyke K, Peden D, Mengoli H, English D (1982): Antioxidation theory of non-steroidal anti-inflammatory drugs based upon the inhibition of luminol-enhanced chemiluminescence from the myeloperoxidase reaction. Inflamm Res 12: 371–376.
- Puri RN, Kumar A, Chen H, Colman RF, Colman RW (1995): Inhibition of ADP-induced platelet responses by covalent modification of aggregin, a putative ADP receptor, by 8-(4-bromo-2,3-dioxobutylthio) ADP. J Biol Chem 270: 482–488.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999): Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26: 1231–1237.
- Sadique J, Al-Rqobab NA, Bughaith MF, El-Gindy AR (1989): The bioactivity of certain medicinal plants on the stabilization of RBC membrane system. Fitoterapia LX: 525–532
- Saeedu SA, Gilani AH, Majoo RU, Shah BH (1997): Anti-thrombotic and anti-inflammatory activities of protopine. Pharmacol Res 36: 1–7.
- Shah BH, Shamim G, Khan S, Saeed SA (1996): Protein kinase C inhibitor, chelerythrine, potentiates the adrenaline-mediated aggregation of human platelets through calcium influx. Biochem Mol Biol Int 38: 1135–1141.
- Siess W, Lapetina EG (1989): Platelet aggregation induced by α2-adrenoceptor and protein kinase C activation A novel synergism. Biochem J 263: 377–385.
- Siess W, Grunberg B, Luber K (1993): Functional relationship between cyclic AMP-dependent protein phosphorylation and platelet inhibition. Adv Exp Med Biol 344: 229–235.
- Silva GL, Lee I, Kinghorn D (1998): Special problems with the extraction of plants. In: Cannell RJP, ed., Natural Products Isolation, 1st ed. New Jersey, Humana Press, pp. 343–363.
- Takao T, Kitatani F, Watanabe N, Yagi A, Sakata K (1994): A simple screening method for antioxidants and isolation of several antioxidants produced by marine bacteria from fish and shellfish. Biosci Biotech Biochem 58: 1780–1783.
- van den Berg R, Haenen GRMM, van den Berg H, Bast A (1999): Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem 66: 511–517.
- Vane JR, Botling RM (1995): New insights into the mode of action of anti-inflammatory drugs. Inflamm Res 44: 1–10.
- Vincent JE, Bonta IL, Ziglstra FJ (1977): Accumulation of blood platelets in carrageenan rat paw oedema. Possible role in inflammatory process. Agents Actions 8: 291–295.
- Weiss SJ (1989): Tissue destruction by neutrophils. N Engl J Med 320: 365–376.
