Abstract
The antinociceptive activities of the petroleum ether fraction (PEF) from the aqueous ethanol extract of Vitex negundo Linn. (Verbenaceae) seeds have been evaluated in several nociceptive mouse models. Given orally, the PEF (at doses of 12, 24, and 48 mg/kg body weight) produced significant dose-related inhibitions on chemical nociception induced by intraperitoneal acetic acid and sub-plantar formalin injections and on thermal nociception in the hot-plate test. Naloxone (1 mg/kg bw subcutaneously), a non-selected opioid receptor antagonist, notably reversed the analgesic effect caused by the PEF (48 mg/kg bw) when assessed against the first phase of the formalin test, but this effect was less significant for the PEF in the second phase. Our observations suggest that the PEF probably interacted with the opioid system and may be more effective on inflammatory pain. In addition, potent anti-inflammatory activity of the PEF was observed in the xylene-induced ear edema test, which further indicates that the analgesic effects of the PEF may be partially mediated by its anti-inflammatory activity. Further chemical analysis suggests that the analgesic activities of the PEF could be mostly due to the abundance of fatty acids with synergetic effects in the present work.
Introduction
Vitex negundo Linn. (Verbenaceae) is a small aromatic plant with typical five foliolate leaf pattern. It flourishes abundantly in wastelands and is widely distributed in tropical to temperate regions, native of South Asia, China, Indonesia, and the Philippines, up to an altitude of 1500 m. Flavonoids, lignans, terpenoids, iridoid glycosides, and alkaloids are the major classes of compounds isolated from this plant according to the former phytochemical studies on V. negundo (CitationDiaz et al., 2003; CitationOno et al., 2004; CitationChawla et al., 1992; CitationSrinivas & Raju, 2002).
All parts of V. negundo have been commonly used as folk medicine. The extracts of V. negundo leaves possess anti-inflammatory, analgesic and hypouricemic activity as well as antihyperglycemic activity (CitationDharmasiri et al., 2003; CitationUmamaheswari et al., 2007; CitationVillasenor & Lamadrid, 2006). Antioxidant and antiandrogenic properties were reported from the flavonoid-rich fraction of the seeds (CitationZheng et al., 1999; CitationBhargava, 1989; CitationDas et al., 2004). V. negundo has also been found useful for CNS depressant effects, antihistamine release and hepatopreventive purposes (CitationGupta et al., 1999; CitationNair et al., 1994; CitationAvadhoot & Rana, 1991).
Previous studies have already demonstrated the significant analgesic activity of aqueous extract from V. negundo seeds (CitationZhong et al., 1996), consistent with its traditional use in southern China for the treatment of various pain disorders, such as stomach ache, hernia ache, dysmenorrhea, arthralgia, and piles. In our preliminary experiment we compared the antinociceptive activity of the aqueous ethanol extract with that of the aqueous extract and found that the aqueous ethanol extract was more effective in inhibiting the abdominal constriction induced by acetic acid in mice (data not shown). In order to characterize the main analgesic fraction of V. negundo seeds, we further examined the antinociceptive activity of some fractions with different polarity from the aqueous ethanol extract (the petroleum ether, dichloromethane, acetoacetate, and n-butanol fractions), and found that the petroleum ether fraction had a powerful antinociceptive activity. The present study was designed to further examine the effects of the PEF on nociception mouse models induced by both the chemical and the thermal stimuli so as to elucidate the analgesic activity and the possible mechanism of the PEF, and provide scientific basis for the clinical use of Vitex negundo seeds.
Materials and methods
Plant material
The seeds of Vitex negundo (Chinese name “Huang-Jing-zi”), which were identified by Han-Chen Zheng of the School of Pharmacy, Second Military Medical University, were obtained from the Wanglang National Nature Reserve, Sichuan province in October 2006. A voucher specimen (#2006-168) has been deposited in the herbarium of the Department of Pharmacognosy, School of Pharmacy, Second Military Medical University.
Animal
Experimental groups consisted of 10 ICR (Institute of Cancer Research) mice (18-22 g) per group. They were housed at 21° ± 1°C under a 12 h light/12 h dark cycle and had free access to standard pellet diet (Purina chow) and tap water. The animals were deprived of food for 15 h before the experiment, with free access to drinking water. Each animal was used only once in the experiment. The experimental protocols were approved by the Animal Care and Use Committee of our institute (May 14, 2008; Approval number: 2008LY029) and complied with the recommendations of the International Association for the Study of Pain (CitationZimmermann, 1983).
Drugs and chemicals
The following reagents and drugs were used: EtOH (Analytical Reagent), petroleum ether (AR), dichloromethane (AR), ethyl acetate (AR), n-butanol (AR), formalin (AR), xylene (AR), and acetic acid (AR) (Sinopharm Chemical Reagent, Shanghai, China), morphine hydrochloride, indomethacin, dexamethasone, naloxone hydrochloride (Chengdu Pharmaceutical Factory, Chengdu, China).
Morphine hydrochloride, indomethacin, dexamethasone, and naloxone hydrochloride were dissolved in physiological saline (0.9% NaCl). The vehicles used alone had no effect on the nociceptive responses in mice.
The sample preparation
The dried powders of Vitex negundo seeds (1 kg) were extracted with 80% aqueous ethanol using a Soxhlet apparatus. The ethanol extract was concentrated under reduced pressure to obtain a residue (89.4 g), which was subsequently extracted with petroleum ether, dichloromethane, ethyl acetate, and n-butanol. The PEF was concentrated under reduced pressure to obtain a residue. The residue was further transferred to an evaporating dish (250 mL) and put in a drying oven (50° ± 1°C for 2 h) and afterwards in a desiccator (cooled for 0.5 h). These processes were repeated for three times to confirm that the PEF extract was dried sufficiently (9.6 g, with a yield of 0.96%, w/w) for bioactivity determination.
Gas chromatography-mass spectrometry (GC-MS)
GC/MS analyses were performed using the Finnigan Voyager equipped with a VF-5ms fused-silica capillary column (30 × 0.25 mm i.d., film thickness 0.25 μm, Varian Medical, CA, USA). The column temperature was programmed at 100°C (2 min), increasing to 300°C at a rate of 15°C/min, injector temperature was 250°C; carrier gas (helium) was set at a flow rate of 1 mL/ min; ionization energy 70 eV, and scan mode EI. One μL of sample was injected with split ratio of 1:30 (v/v) and the compounds were identified by matching their mass spectra and retention times with the standard obtained from NIST (National Institute of Standards and Technologies) whenever possible.
Protocol
The antinociceptive activity of the PEF of Vitex negundo seeds was evaluated on the chemical nociception in the test models of acetic acid-induced writhing, formalin-hind paw licking, and on the thermal nociception in the hot-plate test. In all of the nocifensive tests, conscious (unanesthetized) mice were used. The doses of the positive drugs were determined on the basis of the principle of their pharmacokinetics and clinical use. The PEF of Vitex negundo seeds was administered orally. The doses (12, 24, and 48 mg/kg bw) selection for the fraction was based on the results of preliminary experiments in line with what traditional healers use for treatments. Control groups were treated with a similar volume of vehicle that had been used to dilute the fraction.
Abdominal constriction induced by acetic acid
In the acetic acid-induced writhing test (CitationGarcia et al., 2004), groups of overnight fasted mice (n = 10) were treated with the PEF of V. negundo seeds, vehicle or indomethacin (IND), 1 h before the administration of acetic acid (0.7%, 10 mL/kg bw, i.p.). The number of writhings was counted for each animal, starting 3 min after acetic acid injection over a period of 12 min.
Formalin test
In the formalin test (CitationSantos & Calixto, 1997), groups of mice were treated as above with the PEF of V. negundo seeds or vehicle and after 60 min, each mouse was given 20 μL of 5% formalin (in 0.9% saline, sub-plantar) into the right hind-paw. The duration of paw licking as an index of painful response was determined at 0-5 min (early phase, neurogenic) and 20-25 min (late phase, inflammatory) after formalin injection. Morphine was used as a positive control drug, which was administrated at a dose of 10 mg/kg bw s.c., 30 min before the test. In order to verify the possible mechanism of the PEF of V. negundo seeds, anti-nociception (48 mg/kg bw) animal groups pretreated with naloxone (a non-selected opioid receptor antagonist) were used. Naloxone was administered 15 min before the PEF of V. negundo seeds or morphine.
Hot-plate test
The hot-plate test (CitationFranzotti et al., 2000) was carried out on groups of female mice using a hot-plate apparatus (model YLS-6B, Shanghai, China), maintained at 55° ± 1°C. Only mice that showed initial nociceptive responses between 5 and 30 s were selected for the experiment. The latency to first sign of hind paw licking or jumping to avoid heat nociception was taken as an index of nociceptive threshold. In this test, pre-treatment latencies were determined three times with intervals of 20 min. The groups of mice were pre-treated with the fraction or vehicle and 1h later the measurement started. A morphine-treated (10 mg/kg bw s.c. 30 min before the test) animal group was included as a positive control. The cut-off time was 60 s in the hot-plate test in order to minimize tissue injuries.
Assay of xylene-induced inflammation in mice
PEF, vehicle, or dexamethasone were administered orally 1 h separately before each xylene topical application to the right ear. The edema was measured 1 h after xylene treatment, and the method is the same as reported in the literature (CitationWei et al., 2004). The ear swelling was measured by subtracting the weight of the left ear from that of the right. The inhibitory ratio (IR) was calculated as follows, edema A: edema was induced by xylene alone, and edema B: edema was induced by xylene plus sample. Each value was the mean of individual determinations in 10 mice. The formula was:
Open-field test
The effect of PEF on spontaneous locomotor activity and exploratory behavior was assessed by the open-field test (CitationTsuda et al., 1996). The photoelectrical spontaneous locomotor activity apparatus (model ZZ-6, Chengdu, China) was a round arena (34 cm in diameter) with the floor divided into 21 equal areas. Immediately after evaluation, each animal was transferred to the apparatus and observed for 5 min. The number of rearing responses, the number of areas crossed by all paws, and the total time spent on being immobilized (immobility) were recorded. One hour before the test, the groups of mice were pre-treated with the fraction or vehicle. A diazepam-treated (1 mg/kg bw i.p.) animal group was included as a positive control.
Sodium pentobarbitone-induced sleeping time
In this test (CitationSantos et al., 2005), groups of mice (n = 10) were treated orally with the fraction, and vehicle 60 min before the injection of sodium pentobarbitone. Diazepam was used as the reference drug. The time between losing and regaining righting reflex was considered as the duration of sleep time in seconds.
Statistical analysis
All data were expressed as the mean ± SEM. Data were subjected to ANOVA followed by Dunnett’s multiple comparison test. P ≤ 0.05 was considered significant.
Results
Chemical compounds in the PEF of V. negundo seeds
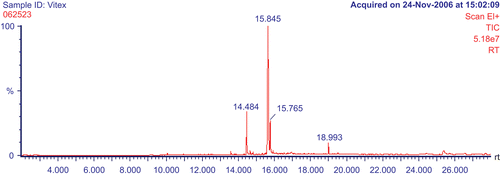
The results of GC-MS analysis on the PEF showed predominance of fatty acids (, ). A total of 11 constituents representing 99.79% of the fraction were identified. n-Hexadecanoic acid (11.75%), linoleic acid (57.22%), oleic acid (14.42%), and stearic acid (10.46%) were found to be the major constituents. Among the remaining constituents (6.15%), 3, 7, 11, 15-tetramethyl-2-hexadecen-1-ol, palmitic acid ethyl ester, 7α-isopropenyl-4, 5-dimethyloctahydroindene-4-carboxylic acid, and 4, 4-dimethyl-5α-androstan-3α-ol were detected in percentages ranging from 0.77% to 3.12%.
Table 1. The chemical compounds in the PEF (GC-MS analysis).
Effect on abdominal constriction induced by acetic acid
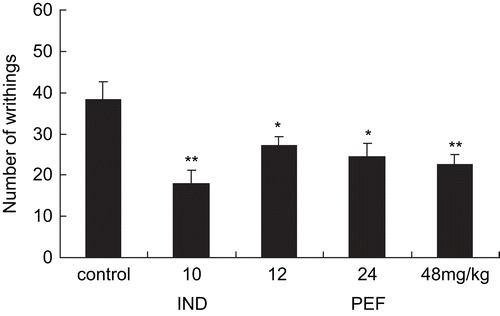
In the acetic acid-induced writhing test, the treatment with fraction decreased significant amount of inhibition on the mean number of writhes (). These were in the order of 38.3 ± 4.42, 27.23 ± 2.05, 24.49 ± 3.16, and 22.5 ± 2.47, respectively, for the controls and the fraction at the tested doses of 12, 24, and 48 mg/kg. The positive drug, indomethacin (10 mg/kg bw), also manifested the significantly diminished number of writhes (17.87 ± 3.36). Therefore, the data showed that the fraction had a dose-dependent inhibition of writhing on nociception.
Effect on the formalin rest
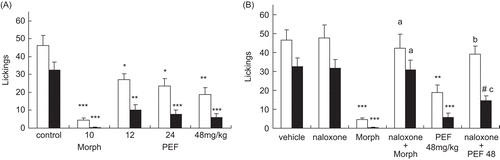
In the formalin test, the vehicle-treated animals showed mean licking times of 46.33 ± 5.57 in the first phase and 32.36 ± 4.73 in the second phase (). Pretreatment with the fraction caused significant diminutions of both the first phase (27.25 ± 3.29, 23.67 ± 4.14, and 18.83 ± 3.87 s) and the second phase (10.07 ± 3.08, 7.63 ± 2.41, and 5.83 ± 2.17 s) pain responses, at the tested doses of 12, 24, and 48 mg/ kg bw, respectively. Morphine (10 mg/kg bw), the reference drug also significantly suppressed the formalin response in both phases (first phase, 4.5 ± 1.05 and second phase, 0.35 ± 0.11 s). When used alone, naloxone (1 mg/kg bw, s.c.) failed to modify the formalin-induced nociceptive responses in a significant manner () (naloxone: first phase, 47.67 ± 6.82 and second phase, 31.67 ± 4.41 s). In the combination studies the antinociceptive effect of morphine was fully reversed by prior treatment of the animals with naloxone against both phases of formalin-induced pain (P < 0.001), whereas pretreatment of the animals with naloxone completely reversed the antinociceptive effect of the PEF from V. negundo seeds only during the early phase () (P < 0.01). During the second phase the reverse was significant, but less marked (P < 0.05) (naloxone + morphine: first phase 42.33 ± 7.27, and second phase 30.83 ± 5.19 s; naloxone + dose (48 mg/kg): first phase 39.18 ± 4.12, and second phase 14.43 ± 2.76 s).
Figure 3. (A) Effects of the PEF and morphine (Morph) on formalin-induced nociception in mice. (B) Effects of naloxone on the PEF and morphine antinociception in the formalin test. The total time spent in licking the injected hind-paw was measured in the early phase (0-5 min, white column) and the late phase (20-25 min, black column). Each bar represented the mean ± SEM (n = 10). Asterisks indicate significant difference from control. *P < 0.05; **P < 0.01; ***P < 0.001 versus control, #P < 0.05 versus the naloxone; aP < 0.001 versus Morph; bP < 0.01 versus the fraction; cP < 0.05 versus the fraction (ANOVA followed by Dunnett’s test).
Effect on the hot-plate test
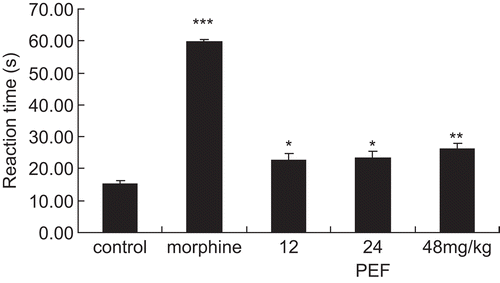
Treatment of animals with the PEF from Vitex negundo seeds (12, 24, and 48 mg/kg bw) or morphine (10 mg/ kg bw) caused a significant increase in the latency response in the hot-plate test (from 15.1 ± 1.13 s in the control group to 22.74 ± 2.12, 23.45 ± 2.05, and 26.14 ± 1.19 s in the PEF-treated groups, respectively, and to 59.63 ± 0.74 s in the morphine-treated group). The results showed that the PEF fraction had potent and graded central analgesic effect ().
Figure 4. Effects of the PEF and morphine on thermal-induced antinociception in the hot-plate test. The time in seconds (s) of first sign of hind-paw licking or jump response to avoid heat nociception was recorded. Cut-off time was 60 s. Each column represented the mean ± SEM (n = 10). Asterisks indicate significant difference from control. *P < 0.05, **P < 0.01, ***P < 0.001 (ANOVA followed by Dunnett’s test).
Effect on the xylene-induced ear edema test
The anti-inflammatory activity of PEF was evaluated as inhibition of xylene-induced ear edema in mice. The percentage inhibition edema of PEF was significant (p < 0.05) at the doses tested (33.22%, 38.76%, and 49.51% at 12, 24, and 48 mg/kg bw, respectively) in a dose-dependent manner, as compared with the control (). As a positive control, dexamethasone (1 mg/ kg bw) significantly inhibited the ear edema by 45.97%. These results demonstrate the potent anti-inflammatory properties of the PEF from V. negundo seeds.
Table 2. Effects of PEF from Vitex negundo seeds on ear edema induced by xylene in mice.
Effect on the open-field test
The fraction (12, 24, and 48 mg/kg bw) did not affect the motor coordination in mice. The mean permanence time of animals and the length of the route in the apparatus, obtained in the fraction-treated groups, weren’t statistically different from those of vehicle-treated control group over a 5-min period. Only diazepam (1 mg/kg, i.p.) significantly (P < 0.01, data not shown) affected the mobile performance in comparison with the control group.
Effect on sodium pentobarbitone -induced sleeping time
The effects of the fraction and diazepam on sodium pentobarbitone-induced sleeping time were as follows: vehicle-treated controls: 44.35 ± 2.95 s; the dose (12, 24, and 48 mg/kg bw): 39.86 ± 5.74, 40.37 ± 4.23, and 40.22 ± 6.18 s, respectively; diazepam: 158.62 ± 8.96 s. Not the fraction but diazepam significantly prolonged the sleeping time induced by pentobarbitone sodium (P < 0.001).
Discussion
In the presented experiments, the PEF (at doses of 12, 24, and 48 mg/kg body wt) of V. negundo seeds demonstrated dose-related and marked antinociception against several models of chemical nociception in mice, namely acetic acid-induced abdominal constriction and formalin-induced licking response. Furthermore, PEF was found to produce significant analgesic activity in the hot-plate assay.
The acetic acid-induced writhing method was widely used for the evaluation of peripheral antinociceptive activity, which was able to determine the antinociceptive effect of compounds or dose levels that might appear inactive in other methods like the hot-plate test (CitationBentley et al., 1983). However, it was known that constriction induced by acetic acid was considered to be a non-selective antinociceptive model, since acetic acid indirectly induced the release of endogenous mediators stimulating the nociceptive neurons that were sensitive to non-steroidal anti-inflammatory drugs (NSAIDs), narcotics, and other centrally acting drugs (CitationSanchez-Mateo et al., 2006). Our results indicated that the PEF of V. negundo seeds reduced the number of writhings in the animal model, showing powerful antinociceptive effects. However, the results of this writhing test alone didn’t ascertain whether the antinociceptive effect was central or peripheral.
In order to confirm this, the formalin test was carried out. The advantage of the formalin model of nociception was that it could discriminate pain in its central and/or peripheral components. The test consists of two different phases which can be separated in time: the first one is generated in the periphery through the activation of nociceptive neurons by the direct action of formalin and the second phase occurs through the activation of the ventral horn neurons at the spinal cord level (CitationTjolsen et al., 1992). Central analgesic drugs such as narcotics inhibited equally in both phases, while peripherally acting drugs such as steroids (hydrocortisone, dexamethasone) and NSAIDs (aspirin) suppressed mainly in the later phase (CitationTrongsakul et al., 2003). In this test, the PEF of V. negundo seeds could reduce the duration of the paw licking obviously in both the first phase (neurogenic) and the second phase (inflammatory) of the formalin test. Therefore, our results suggest that the PEF possesses notable central analgesic activity and probably acts on the central nervous system when systemically administrated. In addition, the significant effect of the PEF of V. negundo seeds on the hot-plate response provided a further confirmation of its central effect, since the hot-plate test was predominantly a spinal reflex and was considered to be selective for centrally acting analgesic compounds (CitationSakurada et al., 2003), thus indicating that the PEF includes component(s) of action with a central mechanism.
To verify possible antinociceptive mechanisms of the PEF which are still not completely understood, we had examined the effect of naloxone, a non-selective opioid receptor antagonist, on the antinociceptive activity of the PEF of Vitex negundo seeds. Since the antinociception produced by PEF was naloxone-sensitive in the formalin test, the central effect of the fraction is probably occurring either through the opioid receptors or by promoting the release of endogenous opiopeptides.
Furthermore, the analgesic effect of the PEF was more prominent during the second inflammatory phase than that during the first neurogenic phase in the formalin test. And partial reversal of naloxone against the second inflammatory phase was also observed in the formalin test. These results indicate that the PEF may be more effective on inflammatory pain, in which anti-inflammatory processes may be involved. As we know, prostaglandins play an important role in pain progress in chemical nociception models (CitationDeraedt et al., 1980; CitationSantos et al., 1998) and are the target of action of commonly used anti-inflammatory drugs. Several other inflammatory mediators, such as sympathomimetic amines, tumor necrosis factor-α, interleukin-1β, and interleukin-8 are also involved in the nociceptive response to chemical stimulus in mice (CitationFerreira et al., 1988, Citation1993; CitationDuarte et al., 1988; CitationSantos et al., 1998; CitationRibeiro et al., 2000). Therefore, the xylene-induced ear edema test was further employed to evaluate the anti-inflammatory activity of PEF. Our results showed that the PEF exhibited significant anti-inflammatory activity, consistent with the indications revealed in the second phase (inflammatory) of the formalin test, implying that the analgesic activity of the PEF may be partly mediated by its anti-inflammatory action, probably in inhibiting COX-2 enzyme that selectively regulated the production of prostaglandins, since linoleic acid, a major constituent in PEF (57.22%), had been reported to show significant COX-2 inhibitory effects (CitationRingbom et al., 2001). Conjugated linoleic acid has also been reported to modulate the inhibition of nitric oxide (NO) production and tumor necrosis factor α (TNF α) (CitationLi et al., 2006).
The antinociception caused by the PEF of V. negundo seeds seemed to be unrelated to motor impairment or sedation since the mice tested in open-field and sodium pentobarbitone-induced sleeping time tests showed no significant effect on these behaviors.
The chemical studies revealed that the PEF was a complex mixture of terpenoids and fatty acids, which comprised a great part of the fraction, including n-hexadecanoic acid (11.75%), linoleic acid (57.22%), oleic acid (14.42%), and stearic acid (10.46%) as the major constituents. Fatty acids have unique roles as precursor molecules of chemical mediators of inflammation and regulators of immune function such as the leukotrienes and prostaglandins. These compounds are synthesized and released by almost all tissue in the body, and participate in many biological functions, including the inflammatory and immune processes (CitationEndres & Klinik, 1996). A previous study reported that a mixture of 9-octadecanoic (oleic acid), hexadecanoic, 9,12-octadecanoic (linoleic acid) and 9,12,15-octadecanoic acid could, significantly and dose-dependently, reduce acetic acid-induced abdominal constrictions and increase the reaction time in the hot-plate test (CitationLedon et al., 2003). Due to complexity of the chemical compounds from the PEF, it would be difficult to attribute the observed activity to any single chemical component present in it. So it might equally be true that the antinociceptive activity of the PEF was related to the combination of four major chemical compounds: n-hexadecanoic acid, cis-9, cis-12-octadecadienoic acid (linoleic acid), oleic acid, and stearic acid.
In conclusion, the study demonstrated the notable antinociceptive activity of the PEF of V. negundo seeds in the test models of nociception induced by chemical and thermal stimuli, and further suggested that the antinociceptive activity might be related to the interaction with the opioids system and to the inhibition on COX-2 enzyme, which merited further studies regarding the isolation of major bioactive components responsible for the observed effect and the precise site and the mechanism of action.
Acknowledgement
The authors are indebted to Hu Yaoming, Center of Analysis and Measurement (CAM), Fudan University, for the GC/MS analysis and technical assistance.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Avadhoot Y, Rana AC (1991): Hepatoprotective effect of Vitex negundo against carbon tetrachloride-induced liver damage. Arch Pharm Res 14: 96–98.
- Bentley GA, Newton SH, Starr J (1983): Studies on the antinociceptive action of a-agonist drugs and their interaction with opioid mechanisms. Br J Pharmacol 79: 125–134.
- Bhargava SK (1989): Antiandrogenic effects of a flavonoid-rich fraction of Vitex negundo seeds: A histological and biochemical study in dogs. J Ethnopharmacol 27: 327–339.
- Chawla AS, Sharma AK, Handa SS (1992): Chemical investigation and anti-inflammatory activity of Vitex negundo seeds. J Nat Prod 55: 163–167.
- Das S, Parveen S, Kundra CP, Kundra CP, Pereira BMJ (2004): Reproduction in male rats is vulnerable to treatment with the flavonoid-rich seed extracts of Vitex negundo. Phytother Res 18: 8–13.
- Deraedt R, Jourquey S, Delevallee F, Flahaut M (1980): Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur J Pharmacol 61: 17–24.
- Dharmasiri MG, Jayakody JRAC, Galhena G, Liyanage SSP, Ratnasooriya WD (2003): Anti-inflammatory and analgesic activities of mature fresh leaves of Vitex negundo. J Ethno-pharmacol 87: 199–202.
- Diaz F, Chavez D, Lee D, Mi Q, Chai HB, Tan GT, Kardono LBS, Riswan S, Fairchild CR, Wild R, Farnsworth NR, Cordell GA, Pezzuto JM, Douglas Kinghorn A (2003): Cytotoxic flavone analogues of vitexicarpin, a constituent of the leaves of Vitex negundo. J Nat Prod 66: 865–867.
- Duarte IDG, Nakamura M, Ferreira SH (1988): Participation of the sympathetic system in acetic acid-induced writhing in mice. Braz J Med Biol Res 21: 341–343.
- Endres S, Klinik M (1996): N-3 Polyunsaturated fatty acids and human cytokine synthesis. Lipids 31: S239-S242.
- Ferreira SH, Lorenzetti BB, Bristow AF, Poole S (1988): Interleukin-1β as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature 334: 698–700.
- Ferreira SH, Lorenzetti BB, Poole S (1993): Bradykinin initiates cytokine mediated inflammatory hyperalgesia. Br J Pharmacol 110: 1227–1231.
- Franzotti EM, Santos CV, Rodrigues HM, Mourao RH, Andrade MR, Antoniolli AR (2000): Anti-inflammatory, analgesic activity and acute toxicity of Sida cordifolia L. (Malva-branca). J Ethnopharmacol 72: 273–277.
- Garcia MD, Fernandez MA, Alvarez A, Saenz MT (2004): Antinociceptive and anti-inflammatory effect of the aqueous extract from leaves of Pimenta racemosa var. ozua (Mirtaceae). J Ethnopharmacol 91: 69–73.
- Gupta M, Mazumder UK, Bhawal SR (1999): CNS activity of Vitex negundo Linn. in mice. Indian J Exp Biol 37: 143–146.
- Ledon N, Casaco A, Rodriguez V, Cruz J, Gonzalez R, Tolon Z, Cano M, Rojas E (2003): Anti-inflammatory and analgesic effects of a mixture of fatty acids isolated and purified from sugar cane wax oil. Planta Med 69: 367–369.
- Li GM, Butz D, Dong BY, Park Y, Pariza MW, Cook ME (2006): Selective conjugated fatty acids inhibit guinea pig platelet aggregation. Eur J Pharmacol 545: 93–99.
- Nair AM, Tamhankar CP, Saraf MN (1994): Studies on the mast cell stabilising activity of Vitex negundo Linn. Indian Drugs 32: 277–282.
- Ono M, Nishida Y, Masuoka C, Li JC, Okawa M, Ikeda T, Nohara T (2004): Lignan derivatives and a norditerpene from the seeds of Vitex negundo. J Nat Prod 67: 2073–2075.
- Ribeiro RA, Vale ML, Thomazzi SM, Paschoalato ABP, Poole S, Ferreira SH, Cunha FQ (2000): Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur J Pharmacol 387: 111–118.
- Ringbom T, Huss U, Stenholm A, Flock S, Skatteb L, Perera P, Bohlin L (2001): COX-2 inhibitory effects of naturally occurring and modified fatty acids. J Nat Prod 64: 745–749.
- Sakurada T, Matsumura T, Moriyama T, Sakurada C, Ueno S, Sakurada S (2003): Differential effects of intraplantar capsazepine and ruthenium red on capsaicin-induced desensitization in mice. Pharmacol Biochem Behav 75: 115–121.
- Sanchez-Mateo CC, Bonkanka CX, Hernandez-Perez M, Rabanal RM (2006): Evaluation of the analgesic and topical anti-inflammatory effects of Hypericum reflexum L. fil. J Ethnopharmacol 107: 1–6.
- Santos AR, Vedana EM, De Freitas GA (1998): Antinociceptive effect of meloxicam in neurogenic and inflammatory nociceptive models in mice. Inflamm Res 47: 302–307.
- Santos ARS, Calixto JB (1997): Further evidence for the involvement of tachykinin receptor subtypes in formalin and capsaicin models of pain in mice. Neuropeptides 31: 381–389.
- Santos FA, Jeferson FA, Santos CC, Silveira ER, Rao VSN (2005): Antinociceptive effect of leaf essential oil from Croton sonderianus in mice. Life Sci 77: 2953–2963.
- Srinivas K, Raju MBV (2002): Chemistry and pharmacology of Vitex negundo. Asian J Chem 14: 565–569.
- Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K (1992): The formalin test: An evaluation of the method. Pain 51: 5–17.
- Trongsakul S, Panthong A, Kanjanapothi D, Taesotikul T (2003): The analgesic, antipyretic and anti-inflammatory activity of Diospyros variegata Kruz. J Ethnopharmacol 85: 221–225.
- Tsuda M, Suzuki T, Misawa M, Nagase H (1996): Involvement of the opioid system in the anxiolytic effect of diazepam in mice. Eur J Pharmacol 307: 7–14.
- Umamaheswari M, AsokKumar K, Somasundaram A, Sivashanmugam T, Subhadradevi V, Ravi TK(2007): Xanthine oxidase inhibitory activity of some Indian medical plants. J Ethnopharmacol 109: 547–551.
- Villasenor IM, Lamadrid MRA (2006): Comparative anti-hyperglycemic potentials of medicinal plants. J Ethno-pharmacol 104: 129–131.
- Wei F, Ma LY, Jin WT, Ma SC, Han GZ, Khan IA, Lin RC (2004): Antiinflammatory triterpenoid saponins from the seeds of Aesculus chinensis. Chem Pharm Bull 52: 1246–1248.
- Zheng GM, Luo ZM, Chen DM (1999): Studies on the compositions of Vitex negundo L. seeds with antioxidant activity. Guangdong Gongye Dexue Xuebao 16: 41–47.
- Zhong ST, Qiu GY, Liu YB, Wang FC (1996): Comparative studies on pharmacological activity of the fruits of Vitex trifolia L.var. simplicifolia Cham, Vitex trifolia L., Vitex negundo L. and Vitex negundo L.var. cannabifolia. Pharmacol Clin Chin Mater Med 1: 37–39.
- Zimmermann M (1983): Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16: 109–110.