Abstract
The present study evaluates the antioxidative and radical scavenging potential of the tuber extract of Amorphophallus paeoniifolius (Dennst.) Nicolson, (Araceae). The ethanol extract of A. paeoniifolius (APE) was studied for the inhibition of lipid peroxidation estimated in terms of thiobarbituric acid reactive substances (TBARS) and the levels were reduced by 4.3% to 67.2% in a dose-dependent manner. Further, APE was analyzed for scavenging capacities based on 1,1-diphenyl-2-picrylhydrazyl-2-radical (DPPH) assay and percentage inhibition activity based on 2,2-azinobis-(-3-ethyl) benzo-thiozoline-6-sulfonate (ABTS+) and H2O2. The A. paeoniifolius extract showed a maximum of 68.6% of DPPH scavenging activity and the maximum inhibition of 74% and 67.2% in the case of ABTS and H2O2, respectively. The antioxidant efficiency and inhibition of oxidation of the extract was found to be dose-dependent at the tested concentrations of 1-50 µg/mL. High-performance thin layer liquid chromatography (HPTLC) profile of the extract suggests the presence of polyphenols such as gallic acid, resveratrol, quercetin and two unidentified compounds. The results suggest that the ethanol extract of A. paeoniifolius has a potent antioxidant activity in vitro and can be utilized as an effective and safe source of antioxidants.
Introduction
India is considered to be a rich emporium of medicinal plants, mainly used in preventive and curative medicine. Over 50% of all modern clinical drugs are of natural product origin and they play an important role in drug development programs of the pharmaceutical industry (CitationBaker et al., 1995). In recent years, the popularity of complementary medicine has increased. Dietary measures and traditional plant therapies as prescribed by Ayurvedic and other indigenous systems of medicine are used commonly in India (CitationWarier, 1995). The World Health Organization (CitationWHO, 2002) has also recommended the evaluation of the plants effective in conditions where safe modern drugs are lacking. An intensive search for novel types of antioxidants has been carried out from numerous plant materials (CitationScartezzini & Speroni, 2000; CitationNg et al., 2000).
A large number of synthetic antioxidants, such as Butylated hydroxytoluene (BHT) and Butylated hydroxyanisole (BHA) have been reported as chemopreventive agents for cancer, but there have been reports of adverse effects (CitationKahl & Kappus, 1993; CitationBotterweck et al., 2000). Hence, the natural antioxidants available in our food source have been suggested as safe for therapy (CitationAthar, 2002). A number of antioxidants have been derived from plants and have been widely studied (CitationBalunas & Kinghorn, 2005; CitationDehghan et al., 2007; CitationPuvanendran et al., 2008; CitationRosidah et al., 2008). Numerous studies have also indicated that many plant products, including flavonoids, anthraquinones, tannins, proanthocyanidins, and other phenolics, as well as substances derived from fruit, vegetables, and various plant or herbal extracts, have radical-scavenging activity (CitationBorek, 1997). Therefore, these substances could be proposed as health-beneficial products.
Amorphophallus paeoniifolius (Dennst.) Nicolson (Araceae) is a commonly available tuber in South India, widely used in folk medicine for treatment of acute rheumatism, tumors, lung swelling, asthma, vomiting, and abdominal pain (CitationBogner, 1995). So far, no attempts have been made to evaluate the chemical composition and medicinal properties of A. paeoniifolius. Hence, the present study was performed to investigate the antioxidant potential of ethanol extract of A. paeoniifolius using different in vitro models, and its polyphenolic composition was also determined.
Materials and methods
Chemicals
1,1-Diphenyl-2-picrylhydrazyl (DPPH), 2,2-azinobis-(-3-ethyl) benzo-thiozoline-6-sulphonate (ABTS), butylated hydroxy toluene (BHT) and nitroblue tetrazolium (NBT) were obtained from Sigma, St. Louis, MO. All other chemicals and solvents used in the study were obtained from SD-Fine, Mumbai, India.
Plant material
Amorphophallus tuber was collected from a local market in Coimbatore, Tamil Nadu during July, 2004. The plant specimen was authenticated by R. Gopal (Botanical Survey of India, Southern Circle, Coimbatore, Tamil Nadu, India). A voucher specimen (SP No. 1) was deposited at the herbarium unit of the Biotechnology Department, Bharathiar University, Coimbatore (BU/BT/SPE-24).
Tuber extract preparation
Amorphophallus tuber (250 g) was dried under shade and the yield obtained was 32.5% w/w. The dried material was ground to a fine powder (particle size ∼0.25 mm) using a laboratory mill and first defatted with 750 mL petroleum ether (60°–80°C) for 2-3 h in a Soxhlet apparatus, the resulting extract was dried and extracted with 1 L ethanol (80%) in a Soxhlet apparatus for 5 h. The extract was filtered, subjected to concentration at a reduced pressure using a rotary evaporator and subjected to lyophilization to obtain a crude ethanol extract (CitationWilson et al., 2005). The percentage yield was found to be 6.2%. The extracted fraction was used for analyzing the antioxidant properties.
Inhibition of free radical generation by oxidation
The oxidation inhibiting effect of the extract to prevent free radical formation was tested by performing the lipid peroxidation assay in vitro using rat brain homogenate.
Preparation of rat brain homogenate
Adult male Wistar rats weighing about 200-250 g were used. The animals were fed ad libitum with normal laboratory pellet diet (Hindustan Lever, Mumbai, India) and water. Animals were maintained under a constant 12 h light and dark cycle and at an environmental temperature of 21–23°C. The animals used in the present study were maintained in accordance with the guidelines of the National Institute of Nutrition, Indian Council of Medical Research, Hyderabad, India. The rats were deprived of food 12 h prior to sacrifice. The animals were euthanized and sacrificed by cervical decapitation, dissected and the whole brain except cerebellum was removed quickly. It was further processed to get 10% homogenate in 0.15 M KCl using a Teflon homogenizer (CitationAuddy et al., 2003). The homogenate was filtered to get a clear solution and used as a source of polyunsaturated fatty acids for determining the extent of lipid peroxidation.
In vitro lipid peroxidation determination
The extent of lipid peroxidation in rat homogenate was measured in vitro in terms of formation of thiobarbituric acid reactive substances (TBARS). Different concentrations of the extract were made up with ethanol and expressed in terms of dry weight (µg/mL). The samples were individually added to the brain homogenate (0.5 mL). This mixture was incubated with 0.15 M KCl (100 μL). Lipid peroxidation was initiated by adding 100 μL of 15 mM FeSO4 solution. The reaction mixture was incubated at 37°C for 30 min. An equal volume of TBA: TCA (1:1) reagent was added to the above solution followed by the addition of 1 mL BHT. This final mixture was heated over a water bath for 20 min at 80°C and cooled, then centrifuged and the absorbance was read at 523 nm using a spectrophotometer (CitationOhkawa et al., 1979). The percentage inhibition of lipid peroxidation was calculated by comparing the absorbance of the test with those of controls not treated with the extract as per the formula:
Antioxidant potential
Antioxidant potential of the extract of A. paeoniifolius tuber was analyzed based on its capacity to scavenge DPPH, ABTS and H2O2. The extract was tested at different concentrations from1-50 µg/mL. In our preliminary studies, we examined antioxidant properties of the extracts obtained using different solvents and found that ethanol extract showed higher antioxidant efficiency along with the high conten)t of phenolic compounds. Hence, we carried out the study using several in vitro model systems to study the antioxidant activity of ethanol extract of this plant.
Antioxidant assay with 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical
DPPH is a free radical which when dissolved in ethanol has a blue-violet color. When it reacts with the reducing agent, the solution loses color which depends upon the number of electrons taken up. Hence, the loss of color indicates radical scavenging activity of test material. DPPH was measured by the method of CitationCervato et al. (2000). Three ml of 60 μM DPPH in ethanol was added to 1 mL of the extract and then incubated at room temperature for 15 min. Absorbance was read at 517 nm using a Genesys-5-spectrophotometer (Spectronic Instruments, Rochester, NY). The antioxidant activity was calculated as inhibition (%) of DPPH radical formation:
ABTS+ cation radical and antioxidant activity
The total antioxidant activity of the extract was measured by the ABTS radical cation-decolorization assay (CitationPellegrini et al., 2003). ABTS was prepared by reacting 5 mL of 7 mM ABTS and 88 μL of 140 mM potassium persulfate and the mixture was allowed to stand in the dark at room temperature for 12-16 h before use. ABTS (1 mL) was added to glass test tubes containing 50 μL of the extract and mixed by vortex mixer for 30 s. Absorbance was measured at 734 nm after 2 min. The percentage inhibition was calculated by:
Determination of H2O2 scavenging activity
The ability of extracts to scavenge H2O2 was determined according to the method of CitationRuch et al. (1989). Extracts at different concentrations in ethanol were added to H2O2 solution (0.6 mL, 40 mM). Absorbance of H2O2 at 230 nm was determined 10 min later against a blank solution containing the phosphate buffer without H2O2. The percentage of H2O2 scavenging of both the extracts and standard compounds were calculated:
Chemical characterization of A. paeoniifolius using HPTLC
Total phenolic assay
Total phenolics were determined according to the method of CitationRajeshwar et al. (2005). One mL of the extract was taken into test tubes and mixed with 1 mL 95% ethanol, 5 mL water and then 0.5 mL 1 N Folin-Ciocalteu reagent was added. After 5 min, 1 mL of 5% Na2CO3 was added and the reaction mixture was allowed to stand for 60 min before the absorbance at 765 nm was measured. A standard curve was established for each assay using 50 to 500 μg of gallic acid in 95% ethanol and expressed as gallic acid equivalent (1mg gallic acid equivalent per g leaf extract) (CitationRajeshwar et al., 2005; CitationTabernero et al., 2006).
Estimation of polyphenolics using HPTLC
The extract was dissolved in ethanol at a concentration of 1 mg/mL and 10 µL of the aliquot was spotted over HPTLC silica gel plate with a 100 µM Silica coating (Merck KGaA-silica gel 60F 254, Darmstadt, Germany) at 12 mm from the lower edge using an automatic TLC Sampler III apparatus (winCATS Planar Chromatography Manager) as bands of 10 mm length. After 10 min, the chromatogram was developed with toluene: ethyl acetate: glacial acetic acid (12.5: 7.5: 0.5 v/v) as mobile phase. The chromatogram was run at 48°C until the mobile phase traveled approximately 16 cm in distance. Then the plates were air dried for 10 min and scanned at 260 nm. Gallic acid, resveratrol, and quercetin were used as standards and run along with the sample. The compounds were quantified using a calibration curve obtained by plotting the concentration of standard against the peak area on the scanned chromatogram (CitationVanhaelen-Fastre et al., 2000).
Results
Antioxidant activity of A. paeoniifolius tuber extract
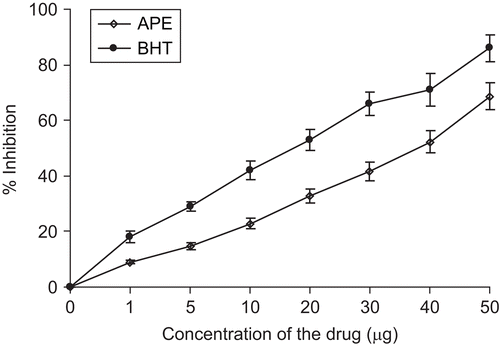
The ethanol extract of A. paeoniifolius tuber shows moderate inhibitory activity against lipid peroxidation which was substantiated by the low levels of TBARS value. The extract showed a percentage inhibition ranging between 4.3 to 67.2% in a dose-dependent manner ().
Table 1. Effect of alcoholic extract of Amorphophallus paeoniifolius on inhibition of lipid peroxidation and H2O2 scavenging activity. (Values are mean of three replicates.)
Radical scavenging activity of A. paeoniifolius tuber extract
DPPH scavenging activity
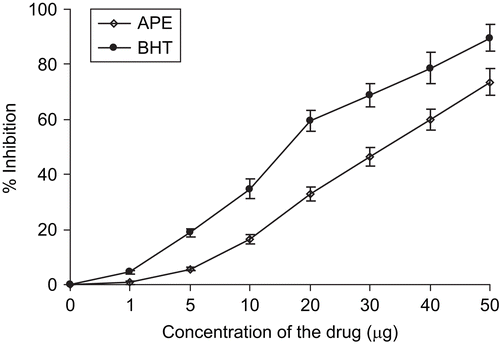
The scavenging activity on DPPH was found to be dose-dependent (). The extract at the concentration of 50 µg/mL had higher inhibitory activity of 68.6%. The IC50 value was observed to be 30 µg/mL. The results reveal that extracts contain powerful inhibitor compounds, which may act as primary antioxidants that react with free radicals.
ABTS+ scavenging activity
The percentage inhibition of ABTS+ was also found to be dose-dependent () suggesting the sensitivity of the assay towards ethanol-soluble antioxidants. The IC50 value of the extract was found to be 35 µg/mL. The results were compared with the standard BHT, as a reference compound.
H2O2 scavenging activity
Tuber extract at 40 µg/mL exhibited 50% scavenging activity. The same patterns of results were observed as earlier, which may suggest the possible high level of lipophilic phenolic antioxidants in the extract ().
Chemical characterization of A. paeoniifolius tuber extract
Total phenolic composition
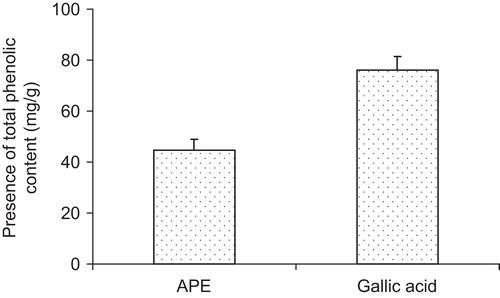
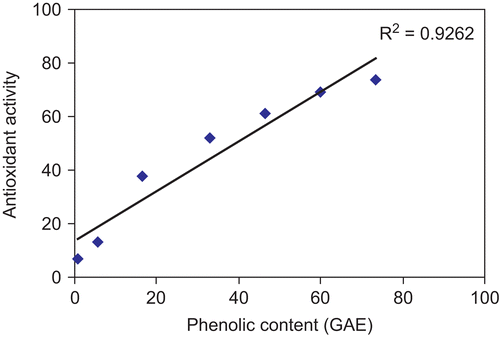
The total phenolic content in A. paeoniifolius at different concentrations was analyzed by the Folin-Ciocalteu method (). The phenolic content of the extract was found to be 44.58 mg GAE/g powder. The total phenolic content was correlated with the inhibition of lipid peroxidation and was represented in . A highly significant positive correlation was observed between the phenolic content and the antioxidant activity measured by in vitro lipid peroxidation.
HPTLC analysis
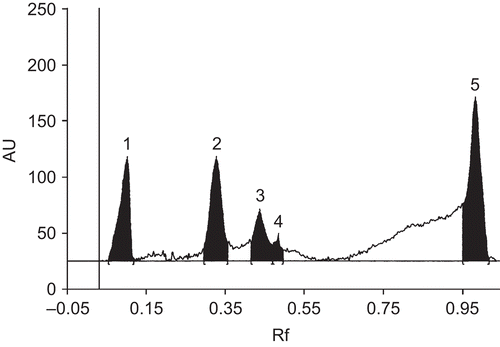
The preliminary phytochemical testing indicated the presence of high amounts of phenolics along with flavonoids and alkaloids (unpublished data). The HPTLC analysis showed five major peaks having Rf values 0.06, 0.29, 0.41, 0.47, and 0.95 (). The Rf values of 0.06, 0.41, and 0.47 peaks are consistent with those of gallic acid, resveratrol, and quercetin standards respectively. The content of gallic acid was found to be 6.41% w/w resveratrol was 1.8% w/w and quercetin was 0.96% w/w. The other peaks are remained to be identified.
Figure 5. The HPTLC chromatogram of Amorphophallus paeoniifolius leaf extract at 260 nm. The chromatogram shows five major peaks when scanned at 260 nm using toluene: ethyl acetate: glacial acetic acid (12.5: 7.5: 0.5 v/v) as mobile phase. The three peaks (1, 3 and 4) of Rf values are gallic acid (0.06), resveratrol (0.41) and quercetin (0.47), respectively, and two others (2 and 5) remain to be identified.
Discussion
Plant substances continue to serve as a viable source of drugs for the world population and several plant-based drugs are in extensive clinical use (CitationRoja & Rao, 2000). For the past few decades, a number of plants have been widely used for the treatment of various diseases due to their antioxidant properties (CitationSaeed et al., 2005; CitationKahkonen et al., 1999). The plants described contain antioxidant principles that can explain and justify their use in traditional medicine in the past as well as the present. For the preparation of the herbal mixture of Indian traditional medicine, the antioxidant activity arising from their content of plants due to their antioxidant principles is very important (CitationScartezzini & Speroni, 2000). A. paeoniifolius has already been listed as a medicinal plant and widely used in Ayurveda therapy (CitationPal, 2000). In order to identify the plants with antioxidant activity in Indian traditional medicine, this study was undertaken to examine the antioxidant properties of A. paeoniifolius tuber, which has been used traditionally for many years.
In this research, the TBARS assay of the ethanol extract of A. paeoniifolius tuber measured the ability of the antioxidant to prevent the oxidative degradation of lipids. The observed inhibition of the oxidation suggests the presence of a biologically functional compound preventing the oxidative degradation of membrane lipids.
It has been suggested that the free radical responsible for toxicity is the hydroxyl radical, formed via the metal catalyzed Haber-Weiss reaction or Fenton reaction (CitationLubec et al., 1996; CitationRinne et al., 2003). In this process, the ferric iron is reduced by superoxide, with subsequent oxidation of ferrous iron by H2O2 forming a hydroxyl radical thereby initiating the series of oxidative reactions. The antioxidative property of this plant may be attributed to a number of reasons including the scavenging of hydroxyl or superoxide radical, by changing the ratio of Fe3+/Fe2+, reducing the rate of conversion of ferrous to ferric or by chelation of iron (CitationBraughler et al., 1986).
The antioxidant potential of the extract was measured by its scavenging activity of DPPH, ABTS+, and H2O2. The DPPH radical scavenging activity suggested that the physico-chemical nature of the individual phenolics present in the extract may be more important in contributing to the antioxidant activity than the total phenolic content measured by the Folin-Ciocalteu assay. The scavenging of DPPH is representative of the capacity of test compounds to scavenge free radicals independently from any enzymatic activity. The phenolic compounds present in the extract such as gallic acid, resveratrol and quercetin, possibly contributes to the scavenging activity on DPPH radicals. BHT, a reference drug, presented the highest DPPH radical-scavenging activity at all concentrations with IC50 value of 17 µg ().
In the ABTS+ radical cation-decolorization assay, the extract showed potent scavenging ability that suggests the presence of phenolic antioxidants in the extract. However, the activity was found to be less than the reference drug. The scavenging effect on DPPH radicals and ABTS represent direct radical-scavenging activity. Moreover, the scavenging effect of the extract was directly correlated with their suppressive effect on lipid peroxidation.
The antioxidant activity of plant extracts varies with assay methods (CitationSun & Ho, 2005). Therefore, a single assay may be inadequate (CitationYen et al., 2005). For this reason we cross checked antioxidant activities of A. paeoniifolius tuber with different antioxidant activity assays based on different mechanisms. In our preliminary studies we examined the antioxidant property of the extracts obtained using different solvents and found that ethanol extract showed higher antioxidant efficiency along with the high content of phenolic compounds. This finding coincides with a previous study that reported the extraction using ethanol or methanol exhibited the highest total phenolic content, followed by water and ethyl acetate (CitationJayaprakasha et al., 2008). Based on the difference in polarity of the extraction solvents, the solubility of phenolic compounds and the rate of mass transfer could be different (CitationBi et al., 2009). As flavonoids and phenolic acids are more soluble in ethanol, it is reasonable to obtain a higher extraction yield when ethanol is used (CitationMarkham, 1982).
The antioxidative effect of this extract is mainly due to the presence of phenolic components, such as flavonoids, phenolic acids, and phenolic diterpenes. The antioxidant activity of phenolic compounds is possibly due to their redox properties, which plays an important role in absorbing and neutralizing free radicals, quenching singlet and triplet oxygen, or decomposing peroxides. Many of these phytochemicals possess significant antioxidant capacity that may be associated with lower incidence and lower mortality rates of cancers and other oxidative stress-related disorders in human populations. Numerous phenolic phytochemicals have been reported for antioxidative, anticarcinogenic, antimicrobial, antiallergic, antimutagenic, antidiabetic, and anti-inflammatory activities (CitationHo, 1992). Among the numerous polyphenols, gallic acid, resveratrol, and quercetin are widely distributed in the plant kingdom and reported to possess antioxidant, antidiabetic, and anticancer properties (CitationBravo, 1998). The content of gallic acid was found to be 6.41% w/w, resveratrol was 1.8% w/w, and quercetin was 0.96% w/w. The phenolic antioxidants may contribute directly to antioxidative property as free radical terminators as well as metal chelators (CitationRice-Evans et al., 1997). The total phenolic content of 1 g of the extract was found to be 44.58 mg GAE. The phenolic composition present in the extract may be a possible reason for the antioxidant potential.
Based on our observations, it may be concluded that the A. paeoniifolius tuber possesses significant antioxidant potential and these effects were attributed to their phenolic constituents. Hence, ethanol extracts may be used as a potential dietary antioxidant to retard aging and prevent diseases caused by ROS or ameliorating oxidative damage.
Acknowledgements
We thank Dr. Jayabal Panneer Selvam, Department of Biochemistry and Biotechnology, Annamalai University, Annamalai Nagar, for scientific contributions and helpful discussions.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Athar M (2002): Oxidative stress and experimental carcinogenesis. Ind J Exp Biol 40: 656–667.
- Auddy B, Ferreira M, Blasina F, Lafon L, Arredondo F, Dajas F, Tripathi PC, Seal T, Mukherjee B (2003): Screening of antioxidant activity of three Indian medicinal plants, traditionally used for the management of neurodegenerative diseases. J Ethnopharmacol 84:131–138.
- Baker JT, Borris RP, Carte B, Cordell GA, Soejarto DD, Cragg GM, Gupta MP, Iwu MM, Madulid DR, Tyler VE (1995): Natural product drug discovery and development: New perspectives on international collaboration. J Nat Prod 58: 1325–1357.
- Balunas MJ, Kinghorn AD (2005): Drug discovery from medicinal plants. Life Sci 78: 431–441.
- Bi HM, Zhang S, Liu C, Wang CZ (2009): High hydrostatic pressure extraction of salidroside from Rhodiola Sachalinensis. J Food Proc Eng 32: 53–63.
- Bogner J (1995): A remarkable new Amorphophallus (Araceae) from India. Kew Bull 50: 397–400.
- Borek C (1997): Antioxidants and cancer. Sci Med 4: 51–62.
- Botterweck AA, Verhagen H, Goldbohm RA, Kleinjans J, van den Brandt PA (2000): Intake of butylated hydroxyanisole and butylated hydroxytoluene and stomach cancer risk: Results from analyses in the Netherlands Cohort Study. Food Chem Toxicol 38: 599–605.
- Braughler JM, Duncan LA, Chase RL (1986): The involvement of iron in lipid peroxidation. Importance of ferric to ferrous ratios in initiation. J Biol Chem 261: 10282–10289.
- Bravo L (1998): Polyphenols: Chemistry, dietary sources, metabolism and nutritional significance. Nutr Rev 56: 317–333.
- Cervato C, Carabelli M, Gervasio S, Cittera A, Cazzola R, Cestaro B (2000): Antioxidant properties of oregano (Origanum vulgare) leaf extracts. J Food Biochem 24: 453–465.
- Dehghan G, Shafiee A, Ghahremani MH, Ardestani SK, Abdollahi M (2007): Antioxidant potential of various extracts from Ferula szovitsiana in relation to their phenolic content. Pharm Biol 45: 691–699.
- Ho CT (1992): Phenolic compounds in food: An overview, in: Huang MT, Ho CT, Lee CY, eds, Phenolic Compounds in Food and Health II: Antioxidants and Cancer Prevention. American Chemical Society Symposium Series 507. Washington DC, American Chemical Society, pp. 2–7.
- Jayaprakasha GK, Girennavar B, Patil BS (2008): Radical scavenging activities of rio red grapefruits and sour orange fruit extracts in different in vitro model systems. Bioresource Technol 99: 4484–4494.
- Kahkonen MP, Hopia AI, Vuorela H, Rauha JP, Pihlaja K, Kujala TS, Heinonen M (1999): Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem 47: 3954–3962.
- Kahl R, Kappus H (1993): Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. Z Lebensm Unters Forsch 196: 329–38.
- Lubec B, Hayn M, Denk W, Bauer G (1996): Brain lipid peroxidation and hydroxyl radical attack following intravenous administration. Free Radic Biol Med 21: 219–223.
- Pal DC (2000): Some plants used in homeopathic systems of medicine in India, in: Maheshwari JK, eds, Ethnobotany Medicinal Plants of Indian Subcontinent. New Delhi, Scientific Publishers, pp. 79–83.
- Markham KR (1982): Techniques of Flavonoid Identification. London, Academic Press, pp. 86–90.
- Ng TB, Liu F, Wang ZT (2000): Antioxidative activity of natural products from plants. Life Sci 66: 709–723.
- Ohkawa H, Ohishi N, Yagi K (1979): Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351–358.
- Pellegrini N, Del Rio D, Colombi B, Bianchi M, Brighenti F (2003): Application of the 2, 2’-azobis (3-ethylenebenzothiazoline-6-sulfonic acid) radical cation assay to a flow injection system for the evaluation of antioxidant activity of some pure compounds and beverages. J Agric Food Chem 51: 260–264.
- Puvanendran H, Wickramasinghe A, Nedra Karunaratne D, Carr G, Wijesundara DSA, Andersen R, Karunaratne V (2008): Antioxidant constituents from Xylopia championii. Pharm Biol 46: 352–355.
- Rajeshwar Y, Gupta M, Mazumder UK (2005): Antitumor activity and in vivo antioxidant status of Mucuna pruriens (Fabaceae) seeds against Ehrlich ascites sarcinoma in Swiss albino mice. Iran J Pharmacol Therap 4: 46–53.
- Rice-Evans CA, Miller NJ, Paganga G (1997): Antioxidant properties of phenolic compounds. Trends Plant Sci 2: 152–159.
- Rinne JO, Kaasinen V, Jarvenpaa T, Nagren K, Roivainen A, Yu M, Oikonen V Kurki, (2003): Brain acetylcholinesterase activity in mild cognitive impairment and early Alzheimer’s disease. J Neurol Neurosur Psychiatry 74: 113–115.
- Roja G, Rao PS (2000): Anticancer compounds from tissue cultures of medicinal plant. J Herbs Spices Med Plants 7: 71–102.
- Rosidah Yam, MF, Sadikun A, Asmaw MZ (2008): Antioxidant potential of Gynura procumbent. Pharm Biol 46: 616–662.
- Ruch RJ, Cheng SJ, Klaunig JE (1989): Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 10: 1003–1008.
- Saeed SA, Urfy MZS, Ali TM, Khimani FW, Gilani AH (2005): Antioxidants: Their role in health and disease. Int J Pharmacol 1: 226–233.
- Scartezzini P, Speroni E (2000): Review on some plants of Indian traditional medicine with antioxidant activity. J Ethnopharmacol 71: 23–43.
- Sun T, Ho CT (2005): Antioxidant activities of buckwheat extracts. Food Chem 90: 743–749.
- Tabernero M, Serrano J, Saura-Calixto F (2006): The antioxidant capacity of cocoa products: Contribution to the Spanish diet. Int J Food Sci Tech 41: 28–32.
- Vanhaelen-Fastre RJ, Faes ML, Vanhaelen MH (2000): High-performance thin-layer chromatographic determination of six major ginsenosides in Panax ginseng. J Chromatogr A 868: 269–276.
- Warier PK (1995): Eugenia jambolana Linn., in: Warier PK, Nambiar VPK, Ramankutty C, eds, Indian Medicinal Plants. Madras, Orient Longman, p. 45.
- WHO (2002): Expert consultation on diet, nutrition and the prevention of chronic diseases. WHO Technical Report Series 916. Geneva, World Health Organization, pp. 13-29.
- Wilson B, Abraham G, Manju VS, Mathew M, Vimala B, Sundaresan S, Nambisan B (2005): Antimicrobial activity of Curcuma zedoaria and Curcuma malabarica tubers. J Ethnopharmacol 99: 147–151.
- Yen GC, Duh PD, Su HJ (2005): Antixodiant properties of lotus seed and its effect on DNA damage in human lymphocytes. Food Chem 89: 379–385.