Abstract
The hypoglycemic and antioxidant effects of ethanol extract from the roots and rhizomes of Rheum franzenbachii Münt. (Polygonaceae) were evaluated in streptozotocin-induced diabetic rats. Effects of repeated oral administration of ethanol extract (125, 250, and 500 mg/kg body weight) on the plasma glucose level (PGL), oral glucose tolerance test (OGTT), malondialdehyde (MDA), reduced glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT) in diabetic rats were examined. It was found that administration of ethanol extract (125, 250, and 500 mg/kg) produced a significant fall in PGL, AUC, and MDA, while elevating the GSH levels and SOD and CAT activities in diabetic rats. The dose of 500 mg/kg was identified as the most effective dose, with a decrease of 65.8 and 44.0% in PGL and MDA, and elevation of 72.6, 75.0, and 51.5% in GSH level and SOD and CAT activities, respectively, after 14 days of ERF administration in diabetic rats. Moreover, the OGTT studies showed a maximum reduction in PGL and AUC. From the active extract of Rheum franzenbachii, two stilbenes, desoxyrhapontigenin (1) and desoxyrhaponticin (2), were isolated as major constituents. The present study concludes that the ethanol extract of roots and rhizomes from Rheum franzenbachii had significant hypoglycemic and antioxidant effects.
Introduction
Diabetes mellitus (DM) is a chronic metabolic condition characterized by a disorder of glucose homeostasis. Despite the great efforts made in the understanding and management of diabetes, the disease and disease-related complications remain unabated (CitationTiwari & Madhusudana Rao, 2002). An excess of free radicals and a sharp reduction of antioxidant defense systems have been found to be mainly due to oxidative stress, which results in several adverse effects commonly seen in diabetes mellitus such as neuropathy, nephropathy, retinopathy, and vascular disorders (CitationOberley, 1988). Recent observations have shown that many of these complications are diminished upon supplementation with appropriate antioxidants. An investigation of the antioxidant activity of 35 medicinal plants used by the indigenous people of the North American boreal forest supported the contribution of these traditional medicines in complications of diabetes mellitus (CitationMcCune & Johns, 2002).
Rhubarb, one of the traditional and best-known Chinese herbal medicines, has been used for thousands of years in China. As described in the Chinese Pharmacopoeia, rhubarb consists of the roots and rhizomes of Rheum officinale Baill. (Polygonaceae), Rheum palmatum L., and Rheum tanguticum Maxim. ex Balf., all of which belong to Sect. Palmata. Aside from Sect. Palmata, species from Sect. Rhapontica are also used for rhubarb drugs in local areas of China. These unofficial species include Rheum franzenbachii Münt. (Polygonaceae), Rheum hotaoense C. Y. Cheng et C. T. Kao, and Rheum emodi Wall. (CitationChina Pharmacopoeia Committee, 2005; CitationXiao et al., 1984). Recently there have been many reports of various Rheum pharmacological activities. The petroleum ether and chloroform extracts from R. emodi rhizomes have shown a moderate degree of antibacterial and antifungal activity (CitationSuresh Babu et al., 2003). R. palaestinum extracts exhibited significant antiplatelet activity on human platelet-rich plasma (PRP) aggregation induced by collagen and adenosine diphosphate (ADP) (CitationAburjai, 2000). The methanol extract from rhizomes of R. undulatum showed a vasorelaxant effect (CitationYoo et al., 2007). However, there are no reports on the antihyperglycemic activity of Rheum franzenbachii. Therefore, our study investigated the hypoglycemic and antioxidant effects of the ethanol extract from the roots and rhizomes of Rheum franzenbachii in streptozotocin-induced diabetic rats.
Materials and methods
Preparation of Rheum franzenbachii extract and isolation of compounds
The fresh roots and rhizomes of Rheum franzenbachii were obtained from Gansu Province and the Ningxia Autonomous Region of China in October 2007, and were identified by Meihua Yang of the Institute of Medicinal Plant Development, China Academy of Medical Science. A voucher specimen has been deposited in the author’s research laboratory at the College of Food Engineering and Biotechnology, Tianjin University of Science & Technology. The dried and powdered roots and rhizomes of Rheum franzenbachii (500 g) were extracted with 95% ethanol (11 L) at room temperature three times, for 2 h each time. The combined extracts were filtered and evaporated under reduced pressure. The concentrated extract of Rheum franzenbachii was lyophilized to afford a powder (ERF, yield: 11.4% w/w).
The ethanol extract was fractionated by high performance liquid chromatography (HPLC). Chromatographic separation was performed on a Waters 600E system using a Kromasil™ C18 analytical column (250 × 4.6 mm, i.d., 5 µm; Eka Nobel, Sweden). The extract was eluted with MeOH/AcCN/H2O 35:5:60, 1 mL/min, and ultraviolet (UV) detection was at 320 nm.
Animals
Male Wistar rats, 8 weeks old (weighing 180–220 g) were used for the study. All animals were obtained from the Experimental Animal Center of the Academy of Military Medical Science, Beijing. The rats were maintained under standard conditions at 24 ± 2°C, relative humidity 50 ± 5%, and a 12 h light/dark cycle. A commercial pellet diet and water were provided ad libitum. The experimental protocol was approved by the Experimental Animal Committee of the Academy of Military Medical Science.
Induction of diabetes
Animals were fasted for 16 h and made diabetic by injecting streptozotocin (STZ) solution intraperitoneally (Sigma) (60 mg/kg in citrate buffer 0.5 mM, pH 4.5). Control rats received only the buffer. After 8 weeks, diabetic rats were used for the experiment when the plasma glucose level (PGL) was greater than 200 mg/dL.
Estimation
Blood samples were obtained from the tail vein in fasting (16 h) rats. Collected blood samples were centrifuged at 3000 rev/min for 15 min to separate the plasma and erythrocytes. The PGL was measured by an enzymatic–spectrophotometric glucose oxidase/peroxidase assay kit (BioSystem Development LLC, Middleton, WI). Erythrocytes were washed three times with phosphate buffered saline (PBS), pH 7.4 (0.02 M phosphate; 0.123 M NaCl). The packed erythrocytes after the final wash were used for estimation of malondialdehyde (MDA) (CitationOhkawa & Yagi, 1979), reduced glutathione (GSH) (CitationBeutler, 1975), superoxide dismutase (SOD) (CitationLee et al., 1975), and catalase (CAT) (CitationAebi, 1987).
Hypoglycemic and antioxidant effects of ERF
STZ-induced diabetic rats were divided into five groups of 10 animals each (groups 2–6). Group 1, as normal control, received 2 mL of 0.5% sodium carboxymethyl cellulose (CMC) (vehicle); group 2 as the diabetic control also received 2 mL of 0.5% sodium CMC; group 3 (diabetic rats) were given the standard oral hypoglycemic agent metformin (500 mg/kg body weight (bw)); groups 4–6 (diabetic rats) received ERF 125 mg/kg bw, 250 mg/kg bw, and 500 mg/kg bw, respectively, dissolved in 2 mL of 0.5% sodium CMC. This treatment was given once a day for 14 days. The fasting (16 h) PGL, MDA, GSH, SOD, and CAT levels were measured before treatment and after treatment for 14 days.
Oral glucose tolerance test of ERF
The animals were fasted for 2 h after the last treatment on the 14th day and an oral glucose tolerance test (OGTT) was performed. Glucose (2 g/kg bw) was orally administered to the rats. The PGLs were measured at 0, 30, 60, and 120 min after glucose load, respectively. Values of the area under the curve (AUC) from the basal level at time 0 during the course of 120 min were calculated.
Statistical analysis
All data are expressed as mean ± standard error of the mean (SEM). The data were statistically analyzed by Student’s t-test; p < 0.01 or p < 0.05 was considered to be significant.
Results
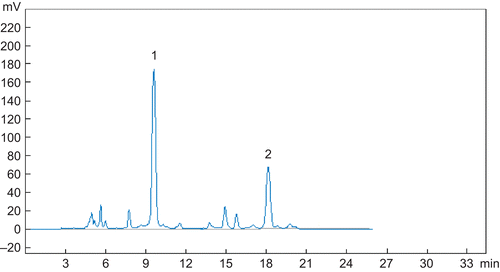
The extract constituents were analyzed by HPLC (). The ethanol extract of the roots and rhizomes of Rheum franzenbachii gave two main stilbenes, desoxyrhapontigenin (1) and desoxyrhaponticin (2). The structures of the isolated compounds () were established by spectroscopic methods.
Figure 2. Structure of stilbenes 1 and 2 isolated from the roots and rhizomes of Rheum franzenbachii.
The PGLs of the diabetic control group were found to be increased significantly (p < 0.01) when compared with the normal control group of rats (), which indicates successful induction of diabetes in rats. At the same time, the PGLs of the diabetic rats were found to be decreased significantly after administration of metformin (500 mg/kg) and ERF (125, 250, and 500 mg/kg) for 14 days, when compared with the diabetic control group of rats (p < 0.01). The rats treated with 500 mg/kg of metformin and 500 mg/kg of ERF produced a fall of 70.1 and 65.8%, respectively, in PGL after 14 days of ERF administration. However, the ERF doses of 125 and 250 mg/kg caused a decrease of only 27 and 58.7% after 14 days of administration, respectively.
Table 1. Hypoglycemic effect of extract of Rheum franzenbachii (ERF) on plasma glucose levels (PGLs) in experimental rats.
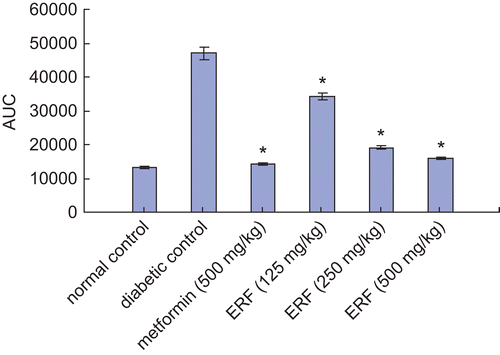
The PGLs and values of AUC of control, drug-treated (different doses of Rheum franzenbachii extract), and reference drug metformin-treated animals on the OGTT are presented in and 4. The PGLs of all groups of rats increased significantly after glucose load (p < 0.01) (). The maximum level was reached in 30 min; after that, the PGLs decreased with time. A significant (p < 0.01) fall of 13.3, 14.7, and 21.3% in PGLs of diabetic rats was observed after 120 min of glucose administration with 125, 250, and 500 mg/kg of ERF, respectively. The dose of 500 mg/kg of metformin produced a significant (p < 0.01) reduction of 27.4% in PGL at 120 min during OGTT in diabetic rats. The values of the AUC from the basal level during the 120 min experimental period after ERF (125, 250, and 500 mg/kg) and metformin administration were significantly smaller compared with the diabetic control group (p < 0.01) (). The value of the AUC produced by 500 mg/kg of ERF was close to that produced by 500 mg/kg of metformin. Therefore, 500 mg/kg of ERF is more effective than the doses of 125 and 250 mg/kg on improving OGTT by reducing PGLs and values of AUC in STZ-induced diabetic rats.
Figure 3. Effect of extract of Rheum franzenbachii (ERF) on plasma glucose levels (PGLs) during oral glucose tolerance test in streptozotocin (STZ)-induced diabetic rats. *p < 0.01 compared with initial value, **p < 0.01 compared with value of 30 min.
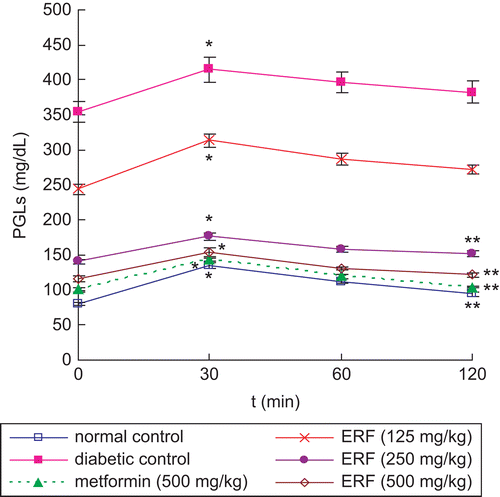
Figure 4. Values of area under the curve (AUC) from basal level at time 0 during the course of 120 min in STZ-induced diabetic rats. *p < 0.01 compared with diabetic control group.
The antioxidant effect of ERF is presented in . Diabetic rats showed a significant increase in MDA levels when compared with normal rats (p < 0.05). The MDA levels were significantly lower in the ERF- and metformin-treated diabetic rats when compared with the untreated diabetic control group after 14 days of administration of ERF and metformin (p < 0.05). After the induction of diabetes, all diabetic rats exhibited a significant decrease in GSH levels and SOD and CAT activities, when compared with normal rats. The GSH levels and SOD and CAT activities were elevated significantly in the ERF- and metformin-treated diabetic rats in comparison with the untreated diabetic control group of rats at the end of the 14-day treatment period (p < 0.05). The dose of 500 mg/kg of ERF was found to be most effective, with a reduction of 44.0% in MDA, and elevation of 72.6, 75.0, and 51.5% in GSH level and SOD and CAT activities, respectively. However, the dose of 500 mg/kg of metformin showed a reduction of 45.7% in MDA, and elevation of 80.1, 68.8, and 47.8% in GSH level and SOD and CAT activities, respectively.
Table 2. Antioxidant effect of ERF on MDA, GSH, SOD, and CAT in experimental rats.
Discussion
The results of our study suggest that an ethanol extract of roots and rhizomes of Rheum franzenbachii not only possesses a significant hypoglycemic effect, but also has a remarkable antioxidant effect in STZ-induced diabetic rats. The antioxidant activity seems to be associated with the hypoglycemic effect in this study. As presented in the “Results” section, the ethanol extract produced dose-dependent hypoglycemic and antioxidant effects in the diabetic rats. The administration of ethanol extract at 125, 250, and 500 mg/kg produced a fall in PGLs, AUC, and MDA, while elevating the GSH levels and SOD and CAT activities in diabetic rats. The hypoglycemic and antioxidant effects of the 500 mg/kg dose were comparable to those of metformin (500 mg/kg).
Previously, it was reported that stilbenes from the rhizome of Rheum undulatum L. such as rhapontigenin, desoxyrhapontigenin, rhaponticin, desoxyrhaponticin, piceatannol, and resveratrol showed antioxidant activity (CitationMatsuda et al., 2001). In another study, it was demonstrated that desoxyrhapontigenin from Rheum undulatum rhizomes inhibited postprandial hyperglycemia in ICR mice (CitationChoi et al., 2005). From the present results, it can be seen that Rheum franzenbachii contained desoxyrhapontigenin and desoxyrhaponticin. As a conclusion, it could be considered that the hypoglycemic and antioxidant activities of Rheum franzenbachii might be related to the desoxyrhapontigenin and desoxyrhaponticin.
Further studies should be undertaken to isolate and identify the active hypoglycemic and antioxidant compounds and investigate the mechanisms of hypoglycemic and antioxidant action of the ethanol extract of the roots and rhizomes of Rheum franzenbachii.
In conclusion, this study demonstrates that the ethanol extract of Rheum franzenbachii has strong hypoglycemic and antioxidant effects, when compared with metformin. Thus, the active ingredients of this plant may be very useful in reducing the complications usually resulting from oxidative stress in diabetes mellitus.
Acknowledgements
We are thankful to Tianjin University of Science & Technology for the partial support of this work. We also thank Meihua Yang of the Institute of Medicinal Plant Development, China Academy of Medical Science, for identification of the plant material, and the Academy of Military Medical Science for providing pharmacological laboratory facilities.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aburjai TA (2000): Anti-platelet stilbenes from aerial parts of Rheum palaestinum. Phytochemistry 55: 407–410.
- Aebi BE (1987): Catalase in methods of enzymatic analysis. In: Bergmayer HU, ed., Methods in Enzymology. Weinheim, VCH Verlagsgesselschaft mbH, pp. 273–286.
- Beutler E (1975): Glutathione. In: Beutler E, ed., Red Cell Metabolism: A Manual of Biochemical Methods. New York, Grune and Stratton, pp. 105–107.
- China Pharmacopoeia Committee (2005): Pharmacopoeia of the People’s Republic of China. Beijing, Chemical Industry Press, pp. 17.
- Choi SZ, Lee SO, Jang KU, Chung SH, Park SH, Kang HC, Yang EY, Cho HJ, Lee KR (2005): Antidiabetic stilbene and anthraquinone derivatives from Rheum undulatum. Arch Pharm Res 28: 1027–1030.
- Lee KT, Tan IK, Seet AM (1975): A new method for determination of glutathione reductase in erythrocyte and plasma. Clin Chim Acta 58: 101–105.
- Matsuda H, Morikawa T, Toguchida I, Park JY, Harima S, Yoshikawa M (2001): Antioxidant constituents from rhubarb: structural requirements of stilbenes for the activity and structures of two new anthraquinone glucosides. Bioorg Med Chem 9: 41–50.
- McCune LM, Johns T (2002): Antioxidant activity in medicinal plants associated with the symptoms of diabetes mellitus used by the Indigenous Peoples of the North American boreal forest. J Ethnopharmacol 82: 197–205.
- Oberley LW (1988): Free radicals and diabetes. J Biol Chem 5: 113–124.
- Ohkawa H, Yagi K (1979): Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351–358.
- Suresh Babu K, Srinivas PV, Praveen B, Hara Kishore K, Suryanarayana Murty UJ, Madhusudana Rao J (2003): Antimicrobial constituents from the rhizomes of Rheum emodi. Phytochemistry 62: 203–207.
- Tiwari AK, Madhusudana Rao J (2002): Diabetes mellitus and multiple therapeutic approaches of phytochemicals: present status and future prospects. Curr Sci 83: 30–38.
- Xiao P, He L, Wang L (1984): Ethnopharmacologic study of Chinese rhubarb. J Ethnopharmacol 10: 275–293.
- Yoo MY, Oh KS, Lee JW, Seo HW, Yon GH, Kwon DY, Kim YS, Ryu SY, Lee BH (2007): Vasorelaxant effect of stilbenes from rhizome extract of rhubarb (Rheum undulatum) on the contractility of rat aorta. Phytother Res 21: 186–189.