Abstract
Cell suspension cultures of Silybum marianum L. Gaertn (Compositae) produce silymarin, a mixture of flavonolignans. In an attempt to increase cell growth and silymarin production, we exposed cell cultures to various levels of Ag+ (0.2, 0.4, 0.8, 1, and 2 mM) for different exposure times (12, 24, 48, 72, 144, and 216 h). A dramatic increase in cell growth was observed after 12 h in media supplemented with 0.2, 0.4, 0.8, and 1 mM Ag+, and the value in medium treated by 1 mM Ag+ after 72 h was 7.21 g, which was about two-fold that of the control (3.32 g). The highest silymarin production reached about 56 μg g−1 DW, 24 h after treatment with Ag+ (0.8 mM), which was 30-fold that of the control. Silybin, isosilybin, silychristin, silydianin, and taxifolin were abundant flavonolignans. Ag+ in low concentrations is a positive elicitor for cell growth and silymarin production in cell suspension cultures of Silybum marianum.
Introduction
Silymarin (SLM) is an antihepatotoxic polyphenolic substance isolated from the fruits of the milk thistle plant (Silybum marianum L. Gaertn, Compositae). Derivatives of milk thistle have been used as herbal remedies for almost 200 years (CitationKvasnicka et al., 2003). It has been shown that SLM consists of a large number of flavonolignans including silybin (SBA, SBB), isosilybin (ISBA, ISBB), silydianin (SD), silychristin (SC), and taxifolin (TXF) (Sanchez-Sampedro et al., Citation2005a) (). These metabolites have shown other interesting applications, such as anticancer and cancer protective agents, for the treatment of neurodegenerative and neurotoxin processes, gastrointestinal problems, neuropathy, and cardiopulmonary problems, and for skin protection (CitationKatiyar, 2002; CitationVan Erp et al., 2005; CitationVeladimir & Walterova, 2005). Tissue cultures derived from this species could be an alternative for the production of flavonolignans; however, few studies have been conducted, and in all of them production was lower than in the fruits (CitationSanchez-Sampedro et al., 2007) and none showed production of all known flavonolignans from S. marianum (SB, SC, SD, and TXF). In plant cell cultures, the use of elicitors has been an important strategy for improving the production of plant secondary metabolites. Elicitors can trigger an array of defense or stress responses and activate specific genes for the enzymes involved in secondary metabolite biosynthesis, and improve the production of plant secondary metabolites (CitationNamdeo, 2007; CitationRajendran et al., 1994; CitationXu et al., 2007). In general, elicitors are classified on the basis of their origin and molecular structure. The elicitors may be biotic or abiotic. The biotic elicitors have biological origin, derived from the pathogen or from the plant itself. The abiotic elicitors do not have a biological origin and are grouped into physical factors and chemical compounds (CitationVasconsuelo & Boland, 2007).
Figure 1. Chemical structure of silybin A (1), silybin B (2), isosilybin A (3), isosilybin B (4), silychristin (5), and silydianin (6).
A number of elicitors, such as yeast extract, chitin, chitosan, and methyl jasmonate, have been investigated for the enhancement of flavonolignan production in cell cultures of S. marianum (Sanchez-Sampedro et al., Citation2005b). Results showed that yeast extract stimulated production. Jasmonic acid (JA) and methyl jasmonate (MeJA) enhanced the yeast extract effect.
To date, there is no published work on the effects of abiotic elicitors in S. marianum cell suspension culture. Therefore, we conducted this study to find an elicitor that can be effective on SLM production in cell suspension cultures of S. marianum. For this, we tested the effects of various levels of Ag+ (0.2, 0.4, 0.8, 1, and 2 mM) for different exposure times (12, 24, 48, 72, 144, and 216 h), and growth index and SLM production were studied.
Materials and methods
Cell line and cultivation conditions
The cell line was established from cotyledon segments from 10-day-old seedlings of dried fruits (Hungarian seeds) of the milk thistle that were supplied by the Institute of Medical Plants, Iranian Academic Center for Education, Culture and Research (ACECR). Calli were developed from explants after 30 days of culture in darkness. The calli were subcultured every 15 days in Petri dishes containing 25 mL Murashige and Skoog medium (MS) (CitationMurashige & Skoog, 1962) supplemented with 30 g L−1 sucrose, 3 mg L−1 picloram (Pic), and 0.4 mg L−1 Kinetin (Kin) (Hasanloo et al., Citation2007b). All cultures were grown in the dark at 25 ± 1°C. Cell suspensions were established from cultures in the same medium and were maintained in 250 mL Erlenmeyer flasks with 50 mL of medium. The suspensions were shaken at 120 rpm in darkness and subcultured after 2 weeks in the same medium and conditions (CitationHasanloo et al., 2008).
Elicitation
Suspension media (10 mL) containing about 1 g of cells were subcultured to flasks containing 40 mL MS medium. Treatments were done 3 days after transfer, when cells had started the active growth phase. Elicitation was carried out with AgNO3 (0, 0.5, 1, 2.5, and 5 mg L−1). AgNO3 was dissolved in MS medium and prepared as a concentrated stock solution and added to cultures after filter-sterilization according to the above concentrations. Control received an equivalent volume of culture medium. Dry weight (DW) and TXF, SC, SD, SB, and ISB were determined for different exposure times (24, 48, 72, 144, and 216 h).
Extraction
Cells from the suspension were separated from the medium and the samples were defatted with ethyl acetate. The flavonolignans were extracted from the dried residue with 10 mL of methanol at 40°C for 8 h. The methanol solution was concentrated to a dry residue. The extract was dissolved in 2 mL of methanol and kept at 4°C in darkness (CitationCacho et al., 1999; Hasanloo et al., Citation2005b, Citation2007a).
Determination and quantification of flavonolignans
The amounts of flavonolignans were determined by high-performance liquid chromatography (HPLC) according to Hasanloo et al. (Citation2005a) and CitationRahnama et al. (2008) on a Knauer liquid chromatograph equipped with an injector with a 20 μL loop, a Nucleosil C18 5 μ (250 × 4.6 mm) column, K2600A ultraviolet (UV) detector, and ChromGate software for peak integration. The mobile phase consisted of the solvents, with a gradient program. All solvents and chemicals were of HPLC grade (Merck). The elution time and flow rate were 30 min and 1 mL min−1 and peaks were detected at 288 nm.
Identification was achieved by comparison of retention times (Rt) of standards of SC, SD, SB, TXF, and a standard mixture of SLM. Quantification of these metabolites, expressed in mg g−1 of dry weight, was accomplished using a known concentration of standard and peak areas. The data obtained from the analysis of each solution allowed us to plot a calibration curve showing good linearity (correlation coefficient = 0.999).
Chemicals
Standards of SLM, SB, and TXF were purchased from Sigma; SC and SD from Phytolab; 2,4-D (dichlorophenoxyacetic acid), Kin, and Pic from Sigma; agar and solvents from Merck.
Statistical analyses
All experimental analyses were carried out on a minimum of three independent samples for each treatment and three replications of each sample were assayed. Statistical significance was calculated using the Duncan test for unpaired data (ά ≤ 0.05) and the analysis of variance (ANOVA) method was used for comparison of means. Statistical analysis was done using SAS software (Version 6.2).
Results
Time course of S. marianum cell growth in suspension culture
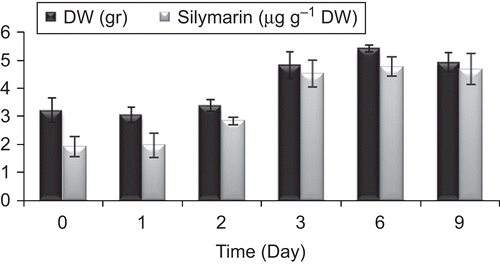
The time courses of cell growth and SLM production in cell suspension culture are shown in . The lag phase of cell growth ranged from the beginning to the third day. From day 3 to day 9, the cell growth phase was the exponential phase. In this phase, cell growth was fast and SLM production increased. The maximum cell DW and SLM production were 5.43 g and 4.8 µg g−1 DW.
Figure 2. Time courses of S. marianum cell growth (▪) and silymarin production (□) in cell suspension culture. 10 mL suspension culture containing about 1 g Fresh Weight cells was incubated in 40 mL medium in a 100 mL flask on a rotary shaker (120 rpm). Data are the average of three experiments each in triplicate (mean ± SD).
Effects of feeding time and elicitor on S. marianum cell growth
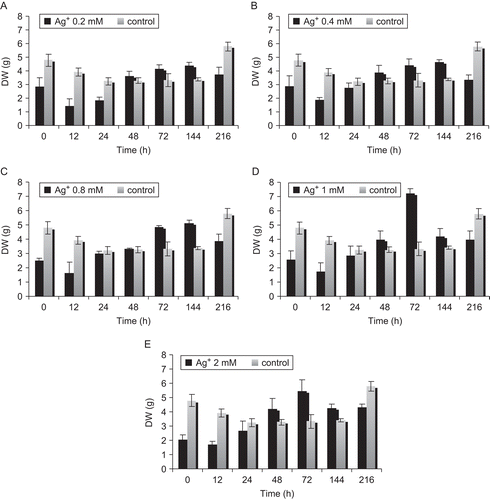
Different feeding time had significant effects on cell growth, and Ag+ was detrimental to growth, particularly at 72 h after treatment. The cell growth phase was from 24 to 72 h, which was the exponential phase. In this time cells grew fast, and cell DW reached a maximum at the end of 72 h. The highest DW was in medium treated with 1 mM Ag+, which reached 7.21 g, and was 2.3-fold that of the control ().
Figure 3. Effects of feeding time in S. marianum cell cultures. Three-day-old cultures were treated with Ag+ (A 0.2, B 0.4, C 0.8, D 1, and E 2 mM). Cells were analyzed for dry weight at the indicated times; (▪) treated cultures, (□) control cultures. Data are the average of three experiments each in triplicate (mean ± SD).
Effects of feeding time and elicitor concentration on silymarin production of S. marianum cells
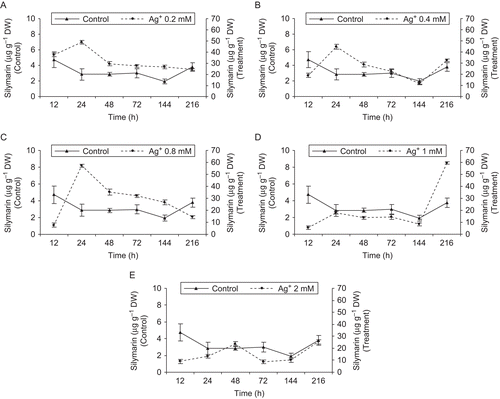
SLM content in S. marianum cell cultures supplied with different concentrations of Ag+ changed significantly, and no marked change was observed in the control experiments. However, the addition of elicitor induced an increase of SLM in a short time.
When the treatment time was 24 h, the SLM content reached 49, 45, and 56 μg g−1 DW in cell cultures supplied with 0.2, 0.4, and 0.8 mM, respectively. The highest SLM content was in medium treated with 0.8 mM Ag+. After 24 h the SLM content decreased, but was still higher than that of the control. On the other hand, in media treated with 1 and 2 mM Ag+, the SLM content was about 18 and 23 µg g−1 DW, respectively. It is interesting to note that the production of SLM increased after 144 h when elicitor concentrations were 1 and 2 mM (60 and 24 μg g−1 DW, respectively), as shown in .
Figure 4. Effects of feeding time in S. marianum cell cultures. Three-day-old cultures were treated with Ag+ (A 0.2, B 0.4, C 0.8, D 1, and E 2 mM). Cells were analyzed for silymarin production at the indicated times; (•) treated cultures, (▴) control cultures. Data are the average of three experiments each in triplicate (mean ± SD).
Our results showed that Ag+ can elicit the production of SB in cell suspension cultures of S. marianum. The addition of Ag+ at concentrations less than 1 mM to the culture medium of S. marianum cells enhanced SB production. When the elicitor concentrations were 0.2, 0.4, and 0.8 mM, the contents of SB were 11, 4, and 7 μg g−1 DW after 24 h, and the maximum SB content was 10 times that of the control. We observed that treatment with 1 mM Ag+ triggered greater SB (9.5 μg g−1 DW), when treatment time was >144 h, almost nine-fold that of the control. However, no marked change was observed in production from medium treated with 2 mM Ag+ ().
Table 1. Effects of feeding time in S. marianum cell cultures.
The highest concentration of TXF was observed in medium supplemented with 0.8 mM Ag+ after 24 h, and was almost 14-fold that of the control. No significant changes were observed in medium treated with 0.2 mM Ag+ until 144 h, when the content was higher than that of the control. TXF content significantly increased in media supplemented by 0.4, 1, and 2 mM Ag+, when the elicitor treatment time was >144 h. SC and SD contents of S. marianum cells were increased in media treated by 0.2, 0.4, and 0.8 mM Ag+ when elicitor treatment time was 24 h. After this time, their levels were decreased. However, they were higher than that of the control (). The SC content of S. marianum cells increased when the elicitor treatment time was >144 h in media treated by 1, 2, and 0.4 mM Ag+ (31, 14, and 17 μg g−1 DW, respectively). The SD content in media supplemented by Ag+ (all concentrations), when treatment time was 216 h, was about 3 μg g−1 DW. In the present study, the levels of analytes were consistent in all of several control growths, while the production of flavonolignans was stimulated by Ag+ treatments.
Discussion
Optimization of calli induction and cellular proliferation in cell suspension cultures is the first step toward establishing large-scale production of active compounds (CitationWalker et al., 2002). In an earlier report we showed that the growth and production of SLM in calli cultures were increased by the use of Pic in media. It has also been reported that the growth and production of SLM in cell suspension cultures were strictly regulated by dark conditions (Hasanloo et al., Citation2007b, Citation2008). The data presented in this study demonstrated that media supplemented with 3 mg L−1 picloram with JA in dark conditions could be useful for the production of silymarin and growth in cell suspension cultures of S. marianum. Hairy root cultures of S. marianum produce silymarin. It has been observed that hairy roots of S. marianum are susceptible to elicitation by an elicitor, and yeast extract increased silybin production (CitationRahnama et al., 2008).
Metals, such as Co2+, Ni2+, Fe2+, Ag+, and V, all stimulate the biosynthesis of a wide range of plant secondary metabolites (CitationNeil et al., 1994; CitationTumova et al., 2001; CitationZhao et al., 2001). Therefore, we examined the effects of Ag+ on the growth and production of SLM in cell suspension cultures of S. marianum.
In this experiment, it has been observed that cell suspension cultures of S. marianum are susceptible to elicitation by AgNO3, with increased SB production after 72 h. This can be extremely helpful in increasing productivities in large-scale processes. A similar observation was obtained in suspension cultures of Saussurea medusa Maxim and Linum album Boiss (CitationXu et al., 2007; CitationShams-Ardakani et al., 2005). In view of the positive effects of manipulation of the components of culture medium, the growth and flavonolignan production of S. marianum cell suspension were tested using different concentrations of KNO3, KH2PO4, iron, and calcium. Only the removal of calcium ions promoted flavonolignan accumulation (CitationCacho et al., 1999). Thus, the positive effect of AgNO3 in our experiment is related to Ag+.
The mechanisms of elicitation of secondary metabolite production by cell suspension cultures are not fully understood. However, many studies suggest that an elicitor signal is perceived by a receptor on the surface of the plasma membrane and initiates a signal transduction network, which regulates the expression of biosynthesis genes involved in plant secondary metabolism and further key enzymes that catalyze the biosynthesis of the target secondary metabolites (CitationXu et al., 2007; CitationZhao et al., 2005).
Ethylene at high concentrations may inhibit the biosynthesis of secondary metabolites, whereas at low concentration it promotes the production of secondary metabolites (CitationPan et al., 2000). The effects of ethylene biosynthesis inhibitors, including α-amino isobutyric acid, CoCl2 and NiCl2, and an inhibitor of ethylene action, Ag+, on fungal elicitor-induced paclitaxel production were assessed in cell suspension cultures of Taxus chinensis, T. chinensis var. mairei, and T. yunnanensis to elucidate the role of ethylene in paclitaxel synthesis. All these ethylene inhibitors enhanced the paclitaxel yield, and most significantly (up to three-fold) by the combination of CoCl2 (20 μM) and Ag+ (30 μM). Ag+ is a potent inhibitor of the ethylene signal transduction pathway. So, a positive effect of Ag+ on secondary metabolite production may be attributed to its inhibitory effect on ethylene synthesis or action (CitationZhang & Wu, 2003).
It is recognized that abiotic elicitors such as heavy metals, as well as osmotic and salt stress, often induce the synthesis and accumulation of the same defense-related secondary metabolites (CitationZhao et al., 2005).
Oxidative burst is a significant event in defense responses when plant cells are exposed to abiotic or biotic stress such as elicitor treatment (CitationZhao et al., 2005). How reactive oxygen species (ROS) mediate elicitor-induced production of secondary metabolites is still not clear, but it is known that ROS induce the expression of many defense and secondary metabolite biosynthesis genes, such as phenylalanine ammonia lyase, that lead to the biosynthesis of flavonoids (CitationXu et al., 2007). Certainly, H2O2-mediated non-enzymatic or enzymatic lipid peroxidation can inhibit the octadecanoid pathway, leading to biosynthesis of JA and related compounds and other oxylipids, which have been reported to function in the induction of plant secondary metabolites (CitationThoma et al., 2003).
In the present work, the positive effects of AgNO3 on growth and, specifically, SLM production would make this treatment an interesting candidate for improving productivity and studying the biochemical pathway of biosynthesis of SLM. Therefore, it can be concluded that optimizing the media conditions can be extremely helpful in increasing productivities in large-scale processes.
Acknowledgements
We are thankful to Mrs. Roshanak Sepehrifar for her help with HPLC measurements.
Declaration of interest
This work was made possible by financial support from the Agricultural Biotechnology Research Institute of Iran (ABRII).
References
- Cacho M, Moran M, Corchete P, Fernandez-Tarrago J (1999): Influence of medium composition on the accumulation of flavonolignans in cultured cells of Silybum marianum (L.) Gaertn. Plant Sci 144: 63–68.
- Hasanloo T, Khavari Nejad RA, Majidi E (2007a): Evaluation of phenotypic coefficient and flavonolignan content in dried fruits of cultivated and endemic Silybum marianum (L.) Gaertn. J Med Plants 22: 77–90.
- Hasanloo T, Khavari Nejad RA, Majidi E, Shams-Ardakani MR (2005a): Analysis of flavonolignans in dried fruits of Silybum marianum (L.) Gaertn from Iran. Pak J Biol Sci 8: 1778–1782.
- Hasanloo T, Khavari Nejad RA, Majidi E, Ziai SA, Shams-Ardakani MR (2005b): Determination of flavonolignan of dried fruits of Silybum marianum L. Gaertn collected from different areas of Iran by spectrophotometer, TLC and HPLC. J Med Plants 4: 25–32.
- Hasanloo T, Khavari Nejad RA, Majidi E (2007b): Media optimization for flavonolignans accumulation and growth index of callus culture of Silybum marianum (L.) Gaertn. J. Plant Res 1: 7–12.
- Hasanloo T, Khavari Nejad RA, Majidi E, Shams-Ardakani MR (2008): Flavonolignan production in cell suspension culture of Silybum marianum (L.) Gaertn. Pharm Biol 46: 1–6.
- Katiyar SK (2002): Treatment of silymarin, a plant flavonoid, prevents ultraviolet light-induced immune suppression and oxidative stress in mouse skin. Int J Oncol 21: 1213–1222.
- Kvasnicka F, Biba B, Sevcik R, Voldrich M, Kratka J (2003): Analysis of the active components of silymarin. J Chromatogr A 990: 239–245.
- Murashige T, Skoog F (1962): A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497.
- Namdeo AG (2007): Plant cell elicitation for production of secondary metabolites: A review. Pharmacog Rev 1: 69–79.
- Neil SJ, Lenton JR, Wibberely MS (1994): Differential effects of elicitors on secondary metabolism in hairy root cultures of tobacco (Nicotiana tabacum). Biochem Soc Trans 22: 383–388.
- Pan Z, Wang H, Zhong J (2000): Scale up study on suspension cultures of Taxus chinensis cells for production of taxane diterepene. Enzyme Microb Technol 27: 714–723.
- Rahnama H, Hasanloo T, Shams, MR, Sepehrifar R (2008): Silymarin production in hairy root culture of Silybum marianum (L.) Gaertn. Iran J Biotechnol 6: 113–118.
- Rajendran L, Suvarnalatha G, Ravishankar GA, Venkataraman LV (1994): Enhancement of anthocyanin production in callus cultures of Daucus carota L., under the influence of fungal elicitors. Appl Microbial Biotechnol 42: 227–231.
- Sanchez-Sampedro MA, Fernandez-Tarago J, Corchete P (2005a): Yeast extract and methyl jasmonate induced silymarin production in cell culture of Silybum marianum L. Gaerth. J Biotechnol 119: 60–69.
- Sanchez-Sampedro MA, Fernandez-Tarago J, Corchete P (2007): Silymarin synthesis and degradation by peroxidases of cell suspension cultures of Silybum marianum. J Plant Physiol 164: 669–674.
- Sanchez-Sampedro MA, Fernandez-Tarrago J, Corchete P (2005b): Enhanced silymarin accumulation is related to calcium deprivation in cell suspension cultures of Silybum marianum L. Gaertn. Plant Physiol 162: 1177–1182.
- Shams-Ardakani MR, Hemmati S, Mohagheghzadeh A (2005): Effect of elicitors on the enhancement of podophylotoxin biosynthesis in suspension cultures of Linum album. Daru 13: 56–60.
- Thoma I, Loeffler C, Sinha AK, Gupta M, Krischke M, Steffan B, Roitsch T, Mueller MJ (2003): Cyclopentenone isoprostanes induced by reactive oxygen species trigger defense gene activation and phytoalexin accumulation in plants. Plant J 34: 363–375.
- Tumova L, Gallova K, Rimakova J (2001): Silybum marianum. In vitro. Ceska Slov Farm 53: 135–140.
- Van Erp NPH, Baker S, Zhao M, Rudek MA, Guchelaar HJ, Nortier JWR, Sparreboom A, Gelderblom H (2005): Effect of milk thistle (Silybum marianum) on the pharmacokinetics of irinotecan. Clin Cancer Res 11: 7800–7806.
- Vasconsuelo A, Boland R (2007): Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci 172: 861–875.
- Veladimir K, Walterova D (2005): Silybin and silymarin new effects and applications. Biomed Papers 149: 29–41.
- Walker TS, Pal Bais H, Vivanco JM (2002): Jasmonic acid-induced hypericin production in cell suspension cultures of Hypericum perforatum L. (St. John’s wort). Phytochemistry 60: 289–293.
- Xu C, Zho B, Ou Y, Wang X, Yung X, Wang Y (2007): Elicitor-enhanced syringin production in suspension cultures of Sussurea medusa. World J Microbiol Biotechnol 23: 965–970.
- Zhang CZ, Wu JY (2003): Ethylene inhibitors enhance elicitor-induced paclitaxel production in suspension cultures of Taxus spp. cells. Enzyme Microb Technol 32: 71–77.
- Zhao J, Davis LC, Verpoorte R (2005): Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23: 283–333.
- Zhao J, Hu Q, Zhu WH (2001): Enhanced catharanthine production in Catharanthus roseus cell cultures by combined elicitor treatment in shake flasks and bioreactors. Enzyme Microb Technol 28: 673–681.
