Abstract
Phytochemical investigations on the ethyl acetate soluble fraction of the whole plant of Isatis costata Linn. (Brassicaseae) led to the isolation of the oxindole alkaloids costinones A (1), B (2), isatinones A (3), B (4), indirubin (5), and trisindoline (6). Compounds 1–6 displayed significant to moderate inhibition against xanthine oxidase enzyme with IC50 values ranging from 90.3 ± 0.06 to 179.6 ± 0.04 µM, whereas the standard inhibitor of xanthine oxidase (allopurinol) had an IC50 value of 7.4 ± 0.07 µM. Compounds 1 (IC50 7.21 ± 0.05 µM), 2 (IC50 9.40 ± 0.03 µM), 3 (IC50 11.51 ± 0.07 µM), 4 (IC50 12.53 ± 0.06 µM), 5 (IC50 14.29 ± 0.09 µM), and 6 (IC50 17.34 ± 0.04 µM) exhibited pronounced activities when compared with the standard tyrosinase inhibitor l-mimosine (IC50 3.70 ± 0.03 µM), along with DPPH radical scavenging activity with IC50 226, 270, 300, 320, 401, and 431 µM, respectively. The crude extract and compounds 1, 2, 5, and 6 showed significant antifungal activity against Trichophyton schoen leinii, Aspergillus niger, Candida albicans, Trichophyton simii, and Macrophomina phaseolina.
Introduction
The genus Isatis (Brassicaseae) comprises 50 species, mainly distributed in the Irano-Turanian region. In Pakistan it is represented by seven species (CitationNasir & Ali, 1989). Isatis tinctoria L. or woad is a common plant cultivated throughout the centuries to produce the blue dye indigo. Nowadays, woad is also used in Chinese folk and modern medicine (CitationPinkas et al., 1996). “Ban-Lan-Gen” is one of the most commonly used traditional Chinese medicines for antipyretic, anti-inflammatory, antiviral, antimicrobial, and detoxifying purposes. Its original source was considered to be the dried roots of three plants, Isatis indigotica Fort. (Brassicaseae), Isatis tinctoria L., and Strobilanthes cusia (Nees) O. Kuntze (Acanthaceae) (CitationJiangsu New Medical College, 1985; CitationZhong, 1979). Now the roots of Isatis indigotica have been identified as the main source of “Ban-Lan-Gen” and recorded as such in the Chinese Pharmacopoeia (CitationChinese Pharmacopoeia Committee of Ministry of Public Health, 1990). The ethno-pharmacological importance of the genus Isatis prompted us to investigate the chemical constituents of Isatis costata Linn., which is an annual or biennial herb, found in northern parts of Pakistan.
Xanthine oxidase (E.C. 1.2.3.2) is the enzyme responsible for the formation of uric acid from the purines hypoxanthine and xanthine, and is responsible for the medical condition known as gout. Gout is caused by the deposition of uric acid in the joints leading to painful inflammation, with inhibition of xanthine oxidase (XO) leading to a remission in gout (CitationChiang et al., 1994). XO also serves as an important biological source of oxygen-derived free radicals that contribute to oxidative damage to living tissues that are involved in many pathological processes such as inflammation, atherosclerosis, cancer, and aging (CitationChiang et al., 1994; CitationCos et al., 1998). Therefore, in vitro bioassays are used to examine test materials for XO inhibition, as inhibitors of XO may be potentially useful for the treatment of gout or other XO-induced diseases (CitationGoodman et al., 1990).
Tyrosinase (E.C. 1.14.18.1) is a multifunctional Cu-containing enzyme widely distributed in plants and animals. It catalyzes the o-hydroxylation of monophenols and also the oxidation of o-diphenols to o-quinones. It is known to be a key enzyme for melanin biosynthesis in plants and animals. Therefore, tyrosinase inhibitors are clinically useful for the treatment of some dermatological disorders associated with melanin hyperpigmentation. Moreover, these are also important in cosmetics for whitening and depigmentation after sunburn. In addition, tyrosinase is known to be involved in the molting process of insects and adhesion of marine organisms (CitationShiino et al., 2001).
Antioxidants, which scavenge active oxygen species (free radicals), are found in a variety of foodstuffs and are commonly referred to as scavengers (CitationBeckman et al., 1990; CitationBohme et al., 1993). Many oxidants are plant based, and play an important role in protecting plants that are exposed to sunlight and live under severe oxygen stress. Antioxidants also play an important role in human health, because the biologic defense mechanism cannot operate under severe oxygen stress. According to recent research, activated oxygen is thought to be a major factor in aging, hardening of the arteries, diabetes, cancer, and tissue injury in skin (CitationBeckman et al., 1994; CitationIto & Hirose, 1989). Indeed, approximately 90% of age-related diseases are linked to activated oxygen. Free radicals have significant relevance in the inflammation process, cardiovascular disease (CitationHertog et al., 1993; CitationHollman et al., 1996; CitationMoure et al., 2001), arteriosclerosis, malaria, rheumatoid arthritis, and neurodegenerative disease (CitationHunt et al., 2001; CitationMeyer et al., 1998).
Research on plants used traditionally for the treatment of systemic and topical infections has shown that many inhibit the growth of a wide range of microorganisms. Such plants may contain antiparasitic, antifungal, antibacterial, and/or antihistamine compounds (CitationFicker et al., 2003; CitationIslam et al., 2001; CitationJones et al., 2000; CitationOmar et al., 2000). Some of these traditionally used plants may lead to the development of new antifungal agents which are in increasing demand due to resistance to conventional drugs (CitationWhite et al., 1998). It is also important to document the phytochemical and pharmacological bases of traditional treatments to evaluate their efficacy and safety and to document the biological activity of the species.
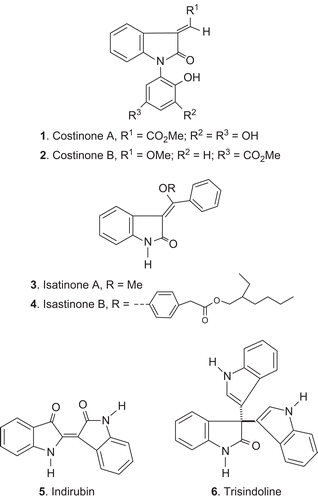
In the present study we have described the xanthine oxidase/tyrosinase inhibitory, antioxidant, and antifungal activities of the oxindole alkaloids (1–6) () that were isolated from Isatis costata and published previously by our research group, in which we did not report the stereochemistry of the isatinones A (3) and B (4) (CitationFatima et al., 2006, Citation2007). The alkaloids 1, 2, 5, and 6 showed significant antifungal activities against various strains. The antifungal activity of the crude extract and compounds 3 and 4 were reported by us previously (CitationFatima et al., 2007).
Materials and methods
Plant material
The whole plant material was collected in April 2004 from North-West Frontier Province (N.W.F.P.) Swat and identified as Isatis costata Linn. by Dr. Ghosia Lutfullah, Center of Biotechnology, University of Peshawar, Pakistan. A voucher specimen (BPU-105) is deposited in the herbarium of the Department of Botany, University of Peshawar, Peshawar, Pakistan.
Extraction and isolation
The shade-dried whole plants (17 kg) were chopped and extracted three times with EtOH (60 L) at room temperature for 96 h. The ethanolic extract was evaporated in vacuo to give a dark greenish residue (400 g), which was partitioned between AcOEt and water. The water fraction was basified with 10% NH4OH and extracted with CH2Cl2. The CH2Cl2 fraction (40 g) was subjected to column chromatography eluting with hexane:AcOEt in increasing order of polarity to obtain six fractions. The fraction obtained from hexane:AcOEt (5:5) was rechromatographed over silica gel using hexane:AcOEt (8:2–3:7) as solvent system to afford two successive fractions. The second fraction was further purified by column chromatography over silica gel using hexane:AcOEt (5:5) as eluent to afford compound (1) (19 mg). Compound (2) (16 mg) was purified by column chromatography of the first fraction using the solvent system hexane:AcOEt (6:4). Silica gel column chromatography of the fraction eluted with 7:3 hexane:AcOEt and elution with mixtures of hexane:AcOEt provided two sub-fractions, respectively. Slow evaporation of the first fraction deposited pale yellow crystals of isatinone A (3, 11 mg). The second fraction was rechromatographed over silica gel, again eluting with hexane:AcOEt mixtures. The eluent obtained from 3:7 hexane:AcOEt provided isatinone B (4, 17 mg).
The fraction obtained from hexane:AcOEt (6:4) was rechromatographed over silica gel using hexane:AcOEtO (8.5:1.5–5:5) as an eluent to afford two successive fractions. The second fraction on purification by column chromatography over silica gel provided compound (5) by using hexane:AcOEt (7:3) as eluent. The first fraction on purification by column chromatography over silica gel and elution with hexane:AcOEt (7:3) afforded trisindoline (6) (25 mg).
Costinone A (1)
Pale yellow crystals, mp 207–208°C (EtOH), [α]D +110.0° (c = 0.12 MeOH); UV λmax (MeOH) nm (log ϵ) 275 (2.25), 230 (3.95), 207 (4.21); IR νmax cm−1: 3470, 1720, 1690, 1600, 1575, 1510; EIMS m/z [M]+ 327 (27), 267 (100), 143 (45), 126 (37), 98 (17), 90 (60), 60 (35), 28 (30), 27 (12); HREIMS calcd for C17H13NO6: 327.0742. Found: 327.0739. For 1H- and 13C-NMR data see the reference (CitationFatima et al., 2006).
Costinone B (2)
Pale yellow crystals, mp 195–197°C (EtOH); [α]D +99.7° (c = 0.12 MeOH); UV λmax (MeOH) nm (log ϵ) 279 (2.50), 230 (4.10), 210 (4.47); IR νmax cm−1: 3460, 1715, 1687, 1610, 1560, 1505; EIMS m/z [M]+ 325 (21), 293 (100), 152 (41), 143 (47), 115 (50), 98 (15), 90 (57); HREIMS calcd for C18H15NO5: 325.0950. Found: 325.0947. For 1H- and 13C-NMR data see the reference (CitationFatima et al., 2006).
Isatinone A (3)
Pale yellow amorphous solid; mp 178–179°C; UV (MeOH) λmax 208, 231, 270 nm; IR νmax (KBr): 3301, 1680, 1600, 1565, 1460 cm−1; EIMS m/z 251 [M]+, 236, 209, 160, 131, 117, 92, 77; HREIMS: 251.0946 (calcd for C16H13NO2: 251.0943). For 1H- and 13C-NMR data see the reference (CitationFatima et al., 2007).
Isatinone B (4)
Pale yellow amorphous solid; mp 189–191°C; [α]D18 +89.7° (c = 0.02 MeOH); UV (MeOH) λmax 205, 232, 275 nm; IR νmax (KBr): 3305, 1685, 1715, 1610, 1560, 1450 cm−1; EIMS m/z 483 [M]+, 349, 321, 311, 293, 236, 160, 131, 116, 91, 77. Negative HRFABMS: 482.2328 (calcd 482.2331 for C31H32NO4). For 1H- and 13C-NMR data see the reference (CitationFatima et al., 2007).
Indirubin (5)
Red crystals, mp 400–403°C (EtOH); [α]D +99.7° (c = 0.12 MeOH); UV λmax (log ϵ) (MeOH) nm 207 (4.03), 239 (2.25), 290 (3.02), 360 (2.80), 540 (4.97); IR νmax cm−1: 3455, 1685, 1615, 1560; EIMS m/z (rel. int. %) [M]+ 262 (47), 235 (100), 219 (72), 119 (40), 91 (45), 77 (85); HREIMS m/z 262.0742 (calcd. for C16H10N2O2: 262.0739). For 1H- and 13C-NMR data see the reference (CitationFatima et al., 2006).
Trisindoline (6)
Colorless amorphous solid; UV (MeOH) λmax 290, 280, 274, 254, 219 nm; IR νmax (KBr): 3200, 1705, 1472 cm−1. HREIMS: 363.1371 (calcd for C24H17N3O: 363.1401). For 1H- and 13C-NMR data see the reference (CitationFatima et al., 2007).
Xanthine oxidase inhibition assay
The xanthine oxidase inhibition activity was assayed in phosphate buffer (0.1 M, pH 7.5). Xanthine oxidase (0.003 unit/well) in 20 mL and test samples in 10 mL dimethylsulfoxide (DMSO) were mixed in a 96-well microplate and pre-incubated for 10 min at room temperature. The reaction was initiated by adding 20 mL of 0.1 mM xanthine, and the resulting uric acid formation was measured spectrophotometrically at 295 nm using a microplate reader (SpectraMax 384; Molecular Devices) (CitationLee et al., 1998).
Tyrosinase inhibition assay
Tyrosinase inhibition assay was performed with l-mimosine as standard inhibitor for tyrosinase in a SpectraMax 340 microplate reader (Molecular Devices). The compounds were screened for the o-diphenolase inhibitory activity of tyrosinase using l-DOPA as substrate (CitationHearing, 1987). IC50 studies were carried out with a 3.3% solution in MeOH. Thirty units of mushroom tyrosinase (28 nM) were preincubated with the compound in 50 nM Na-phosphate buffer (pH 6.8) for 10 min at 25°C. Then, l-DOPA (0.5 mM) was added to the mixture, and the enzyme reaction was monitored for the formation of DOPAchrome by measuring the change in absorbance at 475 nm (at 37°C) for 10 min. The percent inhibition of the enzyme was calculated as follows:
where ABSBlank is absorbance for the blank, and ABSSample is absorbance for the sample.
DPPH (1,1-diphenyl-2-picryl hydrazyl) free radical scavenging activity
The reaction mixture containing 5 μL of test sample was dissolved in DMSO and 95 μL of DPPH (300 μM; Sigma) in ethanol. The reaction mixture was taken in a 96-well microtiter plate (Molecular Devices) and incubated in ELISA (enzyme-linked immunosorbent assay) at 37°C for 30 min; the absorbance was measured at 515 nm. Percent radical scavenging activity was determined by comparison with a DMSO-containing control (). The IC50 value represents the concentration of compound to scavenge 50% of the DPPH radical. BHA (3-tert-butyl-4-hydroxyanisole) was used as a positive control. All the chemicals used were of analytical grade (Sigma, USA) (CitationFujita et al., 1998; CitationSmith et al., 1987).
Table 1. In vitro xanthine oxidase and tyrosinase inhibitory and antioxidative activity of compounds 1–6.
Bioassays
The antifungal bioassay was performed on human, animal, and plant pathogens. The crude extract, compounds 1, 2, 5, and 6, and the standard drugs (each at a concentration of 400 µg/mL of Sabouraud dextrose agar) were subjected to antifungal activity assays against Trichophyton schoen leinii ATCC 22775, Aspergillus niger ATCC 1015, Pseudallescheria boydii ATCC 44330, Candida albicans ATCC 10231, Microsporum canis ATCC 36299, Trichophyton mentagrophytes ATCC 28185, Trichophyton simii ATCC 25923, Fusarium solan ATCC 36031, Macrophomina phaseolina ATCC 53789, and Rhizoctonia solani ATCC 76131, according to the established protocol (CitationChoudhary et al., 1995).
Results and discussion
Xanthine oxidase inhibitory activities of alkaloids 1–6
Xathine oxidase is a highly versatile enzyme (CitationNishino, 1994). It catalyzes the hydroxylation of purines, particularly the conversion of xanthine to uric acid (CitationMassey et al., 1969) and also the reduction of O2 (McCord, Citation1985; CitationZweier et al., 1988). The compounds 1–6 were tested for inhibition against the xanthine oxidase enzyme. Compounds 1 and 2 showed significant inhibitory potential against xanthine oxidase, with IC50 values 90.3 ± 0.06 and 101.7 ± 0.02 µM, respectively. Compounds 3–6 displayed moderate inhibitory activity against xanthine oxidase (), whereas the standard inhibitor of xanthine oxidase (allopurinol) had an IC50 value of 7.4 ± 0.07 µM. The inhibitory activities of compounds 1 and 2 were comparable; the former was slightly more potent than the latter.
Tyrosinase inhibitory activities of alkaloids 1–6
The inhibitory activity of the isolated compounds 1–6 on mushroom tyrosinase was studied (). In these experiments, compounds 1 (IC50 7.21 ± 0.05 µM), 2 (IC50 9.40 ± 0.03 µM), 3 (IC50 11.51 ± 0.07 µM), 4 (IC50 12.53 ± 0.06 µM), 5 (IC50 14.29 ± 0.09 µM), and 6 (IC50 17.34 ± 0.04 µM) exhibited pronounced activities when compared with the standard tyrosinase inhibitor l-mimosine (IC50 3.70 ± 0.03). Hyperpigmentation is associated with increased plasma melanocyte-stimulating hormone activity with insufficient production of glucocorticoids (Addison’s disease). Recently, a number of inhibitors of natural origin have been used in cosmetics (CitationLida et al., 1995).
Antioxidant activities of alkaloids 1–6
Compounds 1–6 were subjected to a DPPH radical scavenging assay and showed a varying degree of activity. The results of scavenging activities are depicted in . Compounds 1 and 2 exhibited IC50 values of 226 ± 0.38 and 270 ± 0.42 µM, respectively, suggesting a significant free radical-scavenging activity; compounds 3–6 also showed moderate activity with IC50 values in the range of 300 ± 0.09 to 431 ± 0.09 µM. The antioxidant potentials of the test samples were compared with 3-(tert-butyl)-4-hydroxyanisol (BHA), used as a positive control () (CitationSmith et al., 1987).
Biological activity
The antifungal activities of compounds 1, 2, 5, and 6 were determined by the agar tube dilution method, and significant activity was observed against Trichophyton schoen leinii, Aspergillus niger, Candida albicans, Trichophyton simii, Macrophomina phaseolina; moderate activity against Pseudallescheria boydii, Trichophyton mentagrophytes, Rhizoctonia solani; and weak activity against Microsporum canis and Fusarium solani (). It is important to note that compounds 1 and 2 were more potent than 5 and 6, which may probably be due to the presence of additional hydroxyl, methoxy, and ester moieties.
Table 2. In vitro fungicidal bioassay of crude extract and compounds 1, 2, 5, and 6.
Conclusion
Compounds 1–6 could be lead compounds in the treatment of oxidative-stress related human diseases and hyperpigmentation related diseases associated with overproduction of melanocytes, and as antifungals. However, further in vivo study would help in exploring the pharmacological properties of these compounds.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alexander BD, Perfect JR (1997): Antifungal resistance trends towards the year 2000. Implications for therapy and new approaches. Drugs 54: 657–678.
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA (1990): Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 87: 1620–1624.
- Beckman JS, Ye YZ, Anderson PJ, Chen J, Accavitti MA, Tarpey MM, White CR (1994): Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe-Seyler 375: 81–88.
- Bohme GA, Bon C, Lemaire M, Reibaud M, Piot O, Stutzmann JM, Doble A, Blanchard JC (1993): Altered synaptic plasticity and memory formation in nitric oxide synthase inhibitor-treated rats. Proc Natl Acad Sci USA 90: 9191–9194.
- Chiang HC, Lo YJ, Lu FJ (1994): Xanthine oxidase inhibitors from the leaves of Alsophila spinulosa (Hook) Tryon. J Enzyme Inhib Med Chem 8: 61–71.
- Chinese Pharmacopoeia Committee of Ministry of Public Health (1990): Chinese Pharmacopoeia, Part 1. Beijing, People’s Health Press, p. 172.
- Choudhary MI, Dur-e-Shahwar Parveen, Z, Jabbar A, Ali I, Atta-ur-Rahman (1995): Antifungal steroidal lactones from Withania coagulance. Phytochemistry 40: 1243–1246.
- Cos P, Ying L, Calomme M, Hu JP, Cimanga K, Van Poel B, Pieters L, Vlietinck AJ, Berghe DV (1998): Structure activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J Nat Prod 61: 71–76.
- Fatima I, Ahmad I, Anis I, Malik A, Afza N (2007): Isatinones A and B, new antifungal oxindole alkaloids from Isatis costata. Molecules 12: 155–162.
- Fatima I, Ahmad I, Nawaz SA, Malik A, Afza N, Luttfullah G, Choudhary MI (2006): Enzyme inhibition studies of oxindole alkaloids from Isatis costata. Heterocycles 68: 1421–1428.
- Ficker CE, Arnason JT, Sanchez-Vindas P, Poveda-Alvarez L, Akpagana K, Gbéassor M, De Souza C, Smith ML (2003): Inhibition of diverse human pathogenic fungi by ethnobotanically selected plant extracts. Mycoses 46: 29–37.
- Fujita Y, Uehara I, Morimoto Y, Nakashima M, Hatano T (1998): Studies on inhibition mechanism of autooxidation by tannins and flavonoids. II. Inhibition mechanism of coffee tannins isolated from leaves of Artemisia species on lipoxygenase dependent lipid peroxidation. Yakugaku Zasshi 108: 129–135.
- Goodman GA, Wall TW, Nies AS, Taylor P, eds. (1990): Pharmacological Basis of Therapeutics, 5th ed. MacMillan Press, New York. pp. 674–681.
- Hearing VJ Jr (1987): Mammalian monophenol monooxygenase (tyrosinase): Purification, properties, and reactions catalyzed. Methods Enzymol 142: 154–165.
- Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D (1993): Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 342: 1007–1011.
- Hollman PC, Hertog MG, Katan M (1996): Analysis and health effects of flavonoids. Food Chem 57: 43–46.
- Hunt EJ, Lester CE, Lester PA, Tackett RL (2001): Effect of St. John’s wort on free radical production. Life Sci 69: 181–190.
- Islam A, Sayeed A, Bhuiyan MSA, Mosaddik MA, Islam MAU, Astaq Mondaotl Khan GRM (2001): Antimicrobial activity and cytoxicity of Zanthoxylum budrunga. Fitoterapia 72: 428–430.
- Ito N, Hirose M (1989): Antioxidants – carcinogenic and chemopreventive properties. Adv Cancer Res 53: 247–302.
- Jiangsu New Medical College (1985): Dictionary of Chinese Crude Drugs. Shanghai, Shanghai Scientific and Technological Press, pp. 1250–1252.
- Jones NP, Arnason JT, Abou-Zaid M, Akpagana K, Sanchez-Vindas P, Smith ML (2000): Antifungal activity of extracts from medicinal plants used by First Nations Peoples of eastern Canada. J Ethnopharmacol 73: 191–198.
- Lee SK, Mbwambo ZH, Chung H, Luyengi L, Gamez EJC, Mehta RJ, Kinghorn D, Pezzuto JM (1998): Evaluation of the antioxidant potential of natural products. Comb Chem High T Scr 1: 35–46.
- Lida K, Hase K, Shimomura K, Sudo S, Kdota S, Namba T (1995): Potent inhibitors of tyrosinase activity and melanin biosynthesis from Rheum officinale. Planta Med 61: 425–428.
- Massey V, Brumby PE, Komai H (1969): Studies on milk xanthine oxidase. Some spectral and kinetic properties. J Biol Chem 244: 1682–1691.
- McCord JM (1985) Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 312: 159–163.
- Meyer AS, Heiononen M, Frankel EN (1998): Antioxidant interactions of catechin, cyanidin, caffeic acid, quercetin, and ellagic acid on human LDL oxidation. Food Chem 61: 71–75.
- Moure A, Cruz J, Franco D, Dominguez M, Sineiro J, Dominguez H, Nunez MJ (2001): Natural antioxidants from residual sources. J Food Chem 72: 145–171.
- Nasir YJ, Ali SI (1989): Flora of Pakistan, No. 191. Islamabad, National Herbarium/Pakistan Agriculture Research Council, p. 94.
- Nishino T (1994): The conversion of xanthine dehydrogenase to xanthine oxidase and the role of the enzyme in reperfusion injury. J Biochem 116: 1–6.
- Omar S, Lemonnier B, Jones N, Ficker C, Smith ML, Neema C, Towers GHN, Goel K, Arnason JT (2000): Antimicrobial activity of extracts of eastern North American hardwood trees and relation to traditional medicine. J Ethnopharmacol 73: 161–170.
- Pinkas M, Peng W, Torck M, Trotin F (1996): Plants Medicinal Chinoises. Paris, Maloin, p. 86.
- Shiino M, Watanabe Y, Umezawa K (2001): Synthesis of N-substituted N-nitrosohydroxylamines as inhibitors of mushroom tyrosinase. Bioorg Med Chem 9: 1233–1240.
- Smith RC, Reeves JC, Dage RC, Schnettler RA (1987): Antioxidant properties of 2-imidazolones and 2-imidazolthiones. Biochem Pharmacol 36: 1457–1460.
- White TC, Marr KA, Bowden RA (1998): Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev 11: 382–402.
- Zhong YZ (1979): Institute of Materia Medica, Chinese Academy of Medical Sciences. Beijing, People’s Health Press, Vol. 1, p. 453.
- Zweier JL, Kuppusamy P, Lutty GA (1988): Measurements of endothelial cell free radical generation: evidence for a central mechanism of free radical injury in postischemic tissues. Proc Natl Acad Sci USA 85: 4046–4050.