Abstract
Chemical constituents as well as cytotoxic and insecticidal activity of the crude methanol extract from the leaves of Phyllanthus reticulatus Poir. (Euphorbiaceae) were investigated. (5R*,6R*)-4,6-Dimethoxycarbonyl-5-[2′,3′,4′-trihydroxy-6′-(methoxycarbonyl) phenyl]-5,6-dihydro-2H-pyran-2-one (1) along with 3,4,3′-tri-O-methylellagic acid, and methyl gallate were isolated from the dichloromethane extract. Determination of their structures was based on spectroscopic analysis. Compound 1 possessed a very weak insecticidal activity against Spodoptera frugiperda (Sf9) with an IC50 value of 27.27 μg/mL.
Introduction
Phyllanthus reticulatus Poir. (Euphorbiaceae) is a shrub easily grown and distributed widely in Thailand. It has been used as a folklore medicine in Thailand for the treatment of asthma, anemia, fever, and thirst, or used as a diuretic (CitationPangthong et al., 1986), astringent, and anti-inflammatory agent (CitationPoopatanapong & Wongprasert, 1987). An infusion of dried leaves has been used as a diuretic and external astringent for sores, burns, suppuration, chafes, and venereal sores in human adults in East Africa (CitationHedberg et al., 1983), Tanzania (CitationChhabra et al., 1984), and Malaysia (CitationIlham et al., 1995). In India, the leaf juice has been used as human adult antivenin (CitationSelvanyagam et al., 1994) and for diarrhea in children (CitationJayaweera, 1980). Previously, there have been some reports on antibacterial (CitationSawhney et al., 1978; CitationKhan et al., 1980) and antimalarial (CitationOmulokoli et al., 1997) activity from the leaf extract of this plant. Compounds reported earlier from various parts of P. reticulatus were such as pyrogallic acid, ellagic acid, p-coumaric acid (CitationNeves & Neves, 1966), betulinic acid, glochidonol (CitationHui et al., 1976), epi-friedelanol (CitationChandler & Hooper, 1979), taraxerone (CitationJoshi et al., 1981), 3,3′,4-tri-O-methylellagic acid, pirorisinol (CitationSangkasila, 1998), and tricin (CitationJain & Nagpal, 2002). This article presents the isolation and structure elucidation of chemical constituents from the leaves of P. reticulatus as well as their cytotoxic and insecticidal activity.
Materials and methods
General experimental procedures
1H-Nuclear magnetic resonance (NMR) (300 MHz) and 13C-NMR (75.4 MHz) experiments were performed on an Ultrashield™ 300 spectrometer, Bruker®. Chemical shifts were recorded as parts per million (ppm) on the δ scale, using tetramethylsilane (TMS) as internal standard (Scientific and Technological Research Equipment Center, Silpakorn University).
Plant collection and authentication
P. reticulatus leaves were collected from Nakhon Pathom Province, Thailand in September 2004. Authentication was performed by comparison with a herbarium specimen (collection no. BKF 127336) at the Forest Herbarium, Royal Forest Department, Bangkok, Thailand. A voucher specimen has been deposited at the Department of Pharmacognosy, Faculty of Pharmacy, Silpakorn University, Thailand.
Extraction and isolation
The dried ground leaves of P. reticulatus (2.2 kg) were macerated with methanol (10 L) three times to furnish the crude methanol extract (610.4 g). The crude methanol extract was mixed with kieselguhr (1.8 kg), and continuous extraction was performed to yield hexane (68.2 g), dichloromethane (12.9 g), and methanol (41.8 g) extracts, respectively. The dichloromethane extract was subjected to column chromatography using Sephadex LH-20 and a mixture of CH2Cl2:MeOH (1:1) as a mobile phase to yield five main fractions (C1–C5). The second fraction (C2, 3.4856 g) was submitted to Sephadex LH-20 column chromatography, employing a gradient system of 50% hexane–CH2Cl2 to 50% CH2Cl2–acetone as eluent, to give three fractions (C21, C22, C23). The second fraction (C22) was further purified on a silica gel column using the gradient system of 50% hexane–CH2Cl2 to 50% CH2Cl2–acetone, and compound 1 (64.0 mg) was crystallized from the saturated acetone solution by adding hexane and allowing it to stand for 24 h in a cool place. Fraction C21 (0.6 g) was chromatographed on a silica gel column and eluted with 3–100% EtOAc in CHCl3 to yield 3,4,3′-tri-O-methylellagic acid (4.9 mg). Successive column chromatography of fraction C4 from the dichloromethane extract on Sephadex LH-20 with a mixture of hexane and acetone (50:50) yielded methyl-3,4,5-trihydroxybenzoate (methyl gallate) (81.8 mg).
(5R*,6R*)-4,6-Dimethoxycarbonyl-5-[2′,3′,4′-trihydroxy-6′-(methoxycarbonyl) phenyl]-5,6-dihydro-2H-pyran-2-one (1)
Colorless needles, [α]![]() = +171.5° (c 0.52, Me2CO). EIMS (70 eV): m/z 396 [M]+ (33.6), 364 (30.3), 332 (75.2), 305 (100), 273 (75.2), 245 (56.3), 217 (21.8). 1H-NMR (acetone-d6, 300 MHz): 7.14 (1H, s, H-5′), 6.82 (1H, d, J = 0.9 Hz, H-3), 5.41 (1H, dd, J = 0.9, 1.8 Hz, H-5), 5.28 (1H, d, J = 1.8 Hz, H-6), 3.68 (3H, s, COOCH3-6), 3.64 (3H, s, COOCH3-4), 3.63 (3H, s, COOCH3-6′). 13C-NMR (acetone-d6, 75 MHz) ().
= +171.5° (c 0.52, Me2CO). EIMS (70 eV): m/z 396 [M]+ (33.6), 364 (30.3), 332 (75.2), 305 (100), 273 (75.2), 245 (56.3), 217 (21.8). 1H-NMR (acetone-d6, 300 MHz): 7.14 (1H, s, H-5′), 6.82 (1H, d, J = 0.9 Hz, H-3), 5.41 (1H, dd, J = 0.9, 1.8 Hz, H-5), 5.28 (1H, d, J = 1.8 Hz, H-6), 3.68 (3H, s, COOCH3-6), 3.64 (3H, s, COOCH3-4), 3.63 (3H, s, COOCH3-6′). 13C-NMR (acetone-d6, 75 MHz) ().
Table 1. NMR chemical shifts (ppm) of geraniinic acid (CitationFoo, 1995) and compound 1 in acetone-d6.
3,4,3′-tri-O-Methylellagic acid
Colorless needles. EIMS (70 eV): m/z 344 [M]+ (66.4), 329 (13.4), 286 (12.6), 273 (5.04), 258 (4.2), 241 (4.2), 149 (8.4), 129 (9.2). 1H-NMR (CDCl3, 300 MHz): 7.77 (1H, s), 7.68 (1H, s), 6.26 (1H, s, OH), 4.41 (3H, s, OCH3), 4.24 (3H, s, OCH3), 4.04 (3H, s, OCH3). 13C-NMR (CDCl3, 75 MHz): 111.58, 107.76, 62.17 (OCH3), 61.99 (OCH3), 56.84 (OCH3).
Methyl-3,4,5-trihydroxybenzoate (methyl gallate)
Pale yellow amorphous solid. EIMS (70 eV): m/z 185 [M + 1]+ (21.8), 184 [M+] (49.6), 153 (100.0), 125 (21.0). 1H-NMR (acetone-d6, 300 MHz): 7.12 (2H, s, H-2,4), 3.79 (3H, s, OCH3-7). 13C-NMR (acetone-d6, 75 MHz): 166.37 (C-7), 145.17 (C-3,5), 137.85 (C-4), 120.84 (C-1), 108.89 (C-2,6), 51.04 (OCH3-7).
Cytotoxicity assay
The cytotoxicity assay was performed by the National Center for Genetic Engineering and Biotechnology (BIOTEC, Thailand), using human tumor cell lines: small cell lung cancer (NCI-H187), mouth carcinoma (KB), and breast cancer (MCF7). The tumor cell lines were plated overnight in 96-well microplates. Serial dilutions of the test samples were added and cells were incubated for 4–6 days. Cell growth was measured by colorimetric methods using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (CitationMosmann, 1983) and sulforhodamine B (SRB) assay (CitationRubinstein et al., 1990). Positive controls were doxorubicin (IC50 of 33.7 × 10−2 μg/mL, 17.3 × 10−2 μg/mL, and 8.3 × 10−2 μg/mL for NCI-H187, KB, and MCF7, respectively) and ellipticine (IC50 of 33.2 × 10−2 μg/mL, 30.3 × 10−2 μg/mL, and 5.2 × 10−2 μg/ mL for NCI-H187, KB, and MCF7, respectively).
Insecticidal assay
Compound 1 was tested for insecticidal activity against Spodoptera frugiperda (Sf9) by a colorimetric method, using the 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay with some modification (CitationSalehzadeh et al., 2002; CitationFornelli et al., 2004). The Sf9 cells were cultured at 27°C in Grace’s insect medium supplemented with 10% fetal calf serum (FCS) (v/v); 5 × 104 cells/well (100 μL) were plated in 96-well tissue culture plates and incubated at 27°C for 24 h. Dilutions of compound 1 ranging from 0.005 to 5 g/well were added into the wells and incubated at 27°C for 24 h. The wells were washed with phosphate buffered saline (PBS). New Grace’s insect medium (100 μL) was added, co-cultured with 10 μL of 5 mg/mL MTT reagent in the dark, and incubated at 27°C for 3 h until a purple precipitate was visible. The medium was discarded and washed with PBS twice. Dimethylsulfoxide (DMSO) (100 μL) was added and left at room temperature in the dark for 20 min. This was then shaken by tapping and absorbance determined at 550 nm using a Fusion Universal Microplate Analyzer (Packard Bioscience Company). The 50% inhibiting concentration (IC50) was calculated from dose–response curves to evaluate the cytotoxicity of compound 1 against Sf9. The assay was performed in triplicate, including blank wells containing medium only and untreated control cells with the presence of 0.5% DMSO instead of compound 1. The positive control was rotenone at IC50 = 0.1 μg/mL.
Results and discussion
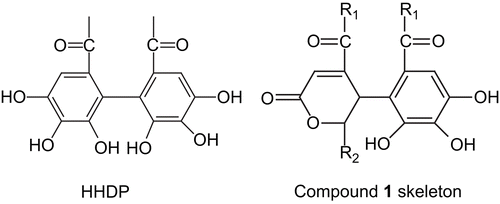
The hexane and methanol extracts of P. reticulatus leaves were inactive in the in vitro cytotoxicity study. The dichloromethane extract showed IC50 values of 11.89 μg/mL in KB and 16.08 μg/mL in MCF7, but was inactive in the NCI-H187 human tumor cell line. The dichloromethane extract was then further purified using column chromatography, resulting in the separation of compound 1 and other known compounds. Compound 1 was isolated as colorless needles. From the 13C-NMR spectrum (), there were 17 carbons, consisting of four carbonyl carbons (169.56, 166.02, 165.17, 162.90), eight aromatic carbons (145.06, 142.69, 141.85, 137.98, 128.89, 117.53, 115.01, 107.73), three methoxyl carbons (52.27, 51.95, 51.51), an oxygenated methine carbon (78.27), and an aliphatic methine carbon (34.34). The 1H-NMR spectrum showed the presence of two aromatic protons (7.14, 6.82), two methine protons (5.41, 5.28), and three methoxyl protons (3.68, 3.64, 3.63). In electron impact mass spectrometry (EIMS), the molecular ion at m/z 396 suggested the possible molecular formula of C17H16O11. Heteronuclear multiple quantum coherence (HMQC) spectra indicated four methine groups, the proton (5.28) associated with the oxygenated carbon (78.27), and the proton (5.41) associated with the aliphatic carbon (34.34). The deshielded proton at 6.82 was attributed to olefinic carbon (128.89) and the sharp singlet signal at 7.14 ppm was bonded to aromatic carbon (107.73). A small vicinal coupling (J = 1.8 Hz) between these two methine protons indicated a trans relationship with respect to their relative orientation. Moreover, the splitting pattern of doublets of doublets at 5.41 of H-5 with J = 0.9 Hz indicated a meta coupling with the olefinic proton (H-3, 6.82) which was attributed to the “W conformation” of the four bonds between them. These spectral data were consistent with a cyclic lactone structure linked to gallate as shown in . This cyclohexenone portion was derived from the hexahydroxydiphenoyl (HHDP) moiety, which is a characteristic structural element found in the biosynthesis of tannins (CitationKhanbabaee & Ree, 2001).
Heteronuclear multiple bond coherence (HMBC) showed a correlation between the protons of methoxyl ester groups and carbonyl carbons that attached to C-4, C-6, and C-6′, respectively. Compound 1 consisted of two chiral carbons at C-5 and C-6. The small vicinal coupling (J = 1.8 Hz) between them indicated the trans relative configuration as found in positions 5 and 6 of geraniinic acid, reported by CitationFoo (1995) recently. Consequently, the structure of compound 1 was unambiguously confirmed as (5R*,6R*)-4,6-dimethoxycarbonyl-5-[2′,3′,4′-trihydroxy-6′-(methoxycarbonyl) phenyl]-5,6-dihydro- 2H-pyran-2-one (), a methylester subunit of geraniinic acid. The comparison of the NMR data between geraniinic acid and compound 1 is shown in .
Figure 2. The structure of (5R*,6R*)-4,6-dimethoxycarbonyl-5-[2′,3′,4′-trihydroxy-6′-(methoxycarbonyl) phenyl]-5,6-dihydro-2H-pyran-2-one (compound 1).
![Figure 2. The structure of (5R*,6R*)-4,6-dimethoxycarbonyl-5-[2′,3′,4′-trihydroxy-6′-(methoxycarbonyl) phenyl]-5,6-dihydro-2H-pyran-2-one (compound 1).](/cms/asset/6b9ef00f-4a48-4783-aed4-469764e5cdc9/iphb_a_427563_f0002_b.gif)
Two other compounds isolated from the dichloromethane extract were identified as 3,4,3′-tri-O-methylellagic acid, and methyl-3,4,5-trihydroxybenzoate (methyl gallate) by the spectrometric methods.
Compound 1 was inactive (IC50 > 20 μg/mL) in the human tumor cell line cytotoxic assays (NCI-H187, KB, and MCF7). However, this compound exhibited a very weak insecticidal activity against Spodoptera frugiperda (Sf9) with an IC50 of 27.27 μg/mL, compared with rotenone (IC50 = 0.1 μg/mL).
Acknowledgements
The authors are grateful to the Graduate School of Silpakorn University and Silpakorn University Research and Development Institute (SURDI) for financial support.
Declaration of interest
The authors are grateful to the Faculty of Pharmacy for research facilities, and the Scientific and Technological Research Equipment Center for EIMS and NMR experiments.
References
- Chandler RF, Hooper SN (1979): Friedelin and associated triterpenoids. Phytochemistry 18: 711–724.
- Chhabra SC, Uiso FC, Mshiu EN (1984): Phytochemical screening of Tanzanian plants. I. J Ethnopharmacol 11: 157–179.
- Foo LY (1995): Amariinic acid and related ellagitannins from Phyllanthus amarus. Phytochemistry 39: 217–224.
- Fornelli F, Minervini F, Logrieco A (2004): Cytotoxicity of fungal metabolites to lepidopteran (Spodoptera frugiperda) cell line (SF-9). J Invertebr Pathol 85: 74–79.
- Hedberg I, Hedberg O, Madati PJ, Mshigeni KE, Mshiu EN, Samuelsson G (1983): Inventory of plants used in traditional medicine in Tanzania. II. Plants of the families Dilleniaceae – Opiliaceae. J Ethnopharmacol 9: 105–127.
- Hui WH, Li MM, Wong KM (1976): Examination of the Euphor-biaceae of Hong Kong. Part 12. A new compound, 21-alpha-hydroxyfriedel-4(23)-en3-one and other triterpenoids from Phyllanthus reticulatus. Phytochemistry 15: 787–798.
- Ilham M, Yaday M, Norhanom AW (1995): Tumour promoting activity of plants used in Malaysian traditional medicine. Nat Prod Sci 1: 31–42.
- Jain R, Nagpal S (2002): Chemical constituents of the roots of Kirganelia reticulate. J Indian Chem Soc 79: 776–777.
- Jayaweera DMA (1980). Medicinal Plants (Indigenous and Exotic) used in Ceylon. Part II. Colombo, M.D. Gunasena, pp. 230–231.
- Joshi KC, Singh P, Mehra A (1981): Crystalline compounds of the roots of Phyllanthus reticulatus. J Indian Chem Soc 58: 102–103.
- Khan MR, Ndaalio G, Nkunya MHH, Wevers H, Sawhney AN (1980): Studies on African medicinal plants. Part I. Preliminary screening of medicinal plants for antibacterial activity. Planta Med Suppl 40: 91–97.
- Khanbabaee K, Ree T (2001): Tannins: Classification and definition. Nat Prod Rep 18: 641–649.
- Mosmann T (1983): Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55–63.
- Neves AC, Neves MTC (1966): Some determinations on the leaves of Phyllanthus reticulatus Poir. of Mozambique. Bol Esc Farm Univ Coimbra 25: 22.
- Omulokoli E, Khan B, Chhabra SC (1997): Antiplasmodial activity of four Kenyan medicinal plants. J Ethnopharmacol 56: 133–137.
- Pangthong A, Kanjanapothi D, Taylor WC (1986): Ethnobotanical review of medicinal plants from Thai traditional books, part I: Plants with antiinflammatory, anti-asthmatic and antihypertensive properties. J Ethnopharmacol 18: 213–228.
- Poopatanapong L, Wongprasert T (1987): Thai Medicinal Plants, Part 5. Bangkok, Chutima Press, pp. 710.
- Rubinstein LV, Shoemaker RH, Paull KD, Simon RM, Tosini S, Skehan P, Scudiero DA, Monks A, Boyd MR (1990): Comparison of in vitro anticancer drug-screening data generated with a tetrazolium assay versus a protein assay against a diverse panel of human tumour cell lines. J Natl Cancer Inst 82: 1113–1117.
- Salehzadeh A, Jabbar A, Jennens L, Ley SV, Annadurai RS, Adams R, Strang RHC (2002): The effects of phytochemical pesticides on the growth of cultured invertebrate and vertebrate cells. Pest Manag Sci 58: 268–276.
- Sangkasila R (1998). Chemical Constituents and Some Bioactivities of Stem of Phyllanthus reticulatus Poir. MS Thesis. Bangkok, Ramkhamhaeng University.
- Sawhney AN, Khan MR, Ndaalio G, Nkunya MH, Wevers H (1978): Studies on the rationale of African traditional medicine. Part II. Preliminary screening of medicinal plants for anti-gonoccoci activity. Pakistan J Sci Ind Res 21: 189–192.
- Selvanyagam ZE, Gnanavendhan SG, Balakrishna K, Rao RB (1994): Antisnake venom botanicals from ethnomedicine. J Herbs Spic Med Plants 2: 45–100.