Abstract
Cladonia clathrata Ahti & L. Xavier-Filho (Cladoniaceae) is a lichen; several Cladonia species extracts have been used for various remedies in folk medicine. In order to evaluate the actions of this lichen, studies were performed on antinociceptive, anti-inflammatory, and antioxidant activities. The hydroalcoholic extract (HE) of C. clathrata stems was used in the following experiments. Oral treatment with the HE of C. clathrata elicited inhibitory activity (p < 0.001) on acetic acid-induced abdominal writhes at 100 (47.2%), 200 (47.2%), and 400 mg/kg (86.4%), and reduced the formalin-induced nociception on both the neurogenic (400 mg/kg, p < 0.01) and inflammatory phases (200 and 400 mg/kg, p < 0.01). It was not associated with non-specific effects, such as muscle relaxation or sedation. The HE reduced the carrageenan-induced edema formation at 100, 200, and 400 mg/kg (p < 0.05) and inhibited neutrophil migration into the peritoneal cavity at 400 mg/kg (p < 0.001). The HE of C. clathrata reacted with the DPPH radical and reduced the same by 50.19%, and exhibited an IC50 value of 69.25 ± 0.65 μg/mL. The HE of C. clathrata stems shows antinociceptive and anti-inflammatory activities, with a moderate antioxidant potential.
Introduction
Lichens are symbiotic organisms composed of fungi (mycobionts) and algae and/or cyanobacteria (photobionts) (CitationIngólfsdóttir, 2002). They produce characteristic secondary metabolites that are unique with respect to those of higher plants. Several lichen extracts have been used for various remedies in folk medicine, and screening tests with lichens have indicated the frequent occurrence of metabolites with antibiotic, antimycobacterial, antiviral, anti-inflammatory, analgesic, antiproliferative, antipyretic, and cytotoxic properties (CitationKumar & Muller, 1999; Muller, 2001; CitationSüleyman et al., 2003; CitationBucar et al., 2004; CitationOmarsdottir et al., 2007). In addition to their reputed effectiveness in the treatment of many diseases, some lichens have also been used traditionally as medicinal plants to treat bronchitis and inflammatory disorders (CitationKumar & Muller, 1999; CitationBucar et al., 2004). Leukotrienes (LT), the products of 5-lipoxygenase (5-LO) metabolism of arachidonic acid, play an important role in a variety of pathophysiological states in humans, particularly those involving inflammation, and it has previously been shown that these mediators might be influenced by lichen-derived compounds (CitationBucar et al., 2004). Other literature reports of biological activities of these naturally occurring compounds are scarce. This may partly be due to the difficulties encountered with collecting substantial amounts of plant material, as most lichen species grow as scattered patches, mainly on stones or on tree trunks.
Of the hundreds of known secondary lichen metabolites, the dibenzofuran derivative usnic acid is without doubt the most extensively studied. Usnic acid is widely distributed in species of Cladonia (Cladoniaceae), Usnea (Usneaceae), Lecanora (Lecanoraceae), Ramalina (Ramalinaceae), Evernia, Parmelia (Parmeliaceae), and other lichen genera (CitationIngólfsdóttir, 2002). Studies have been conducted in which the anti-inflammatory activity of (+)-usnic acid was compared to that of ibuprofen using the rat paw edema assay (acute effects) and the cotton pellet assay (chronic effects) (CitationVijayakumar et al., 2000). Usnic acid and the depside diffractaic acid were identified as active components of an Usnea diffracta Vain. methanol extract, showing analgesic and antipyretic activities in mice (CitationOkuyama et al., 1995; CitationIngólfsdóttir, 2002). Polysaccharides from lichens have shown various biological activities, such as antitumor, anticoagulant, antithrombotic, and immunomodulating activities, and studies were carried out in an attempt to show the importance of polysaccharides as an additional tool in chemotyping (CitationCarbonero et al., 2001; CitationMartinichen-Herrero et al., 2005; CitationOmarsdottir et al., 2007). Many Cladonia species have been used in the treatment of pulmonary tuberculosis (CitationIngólfsdóttir, 2002). Anti-inflammatory, analgesic, and antipyretic effects have been suggested to be linked to the inhibition of prostaglandin synthesis, owing to the uncoupling effects on oxidative phosphorylation (CitationIngólfsdóttir, 2002).
The goal of the present study was to evaluate the antinociceptive, anti-inflammatory, and antioxidant effects of the hydroalcoholic extract (HE) from Cladonia clathrata Ahti & L. Xavier-Filho stems.
Materials and methods
Plant material
Cladonia clathrata was collected in March 2007, in Itabaiana county, Sergipe State, northeastern Brazil (10°44′S, 37°23′W). The lichen was identified by Professor Marcelo Pinto Marcelli (Botanical Institute of São Paulo/SP, Brazil), with voucher number SP 393248. Prior to extraction, stems were dried at 40°C in a forced air oven (Marconi MA 037) for 24 h.
Extraction of Cladonia clathrata
The dried stems of C. clathrata (200 g) were powdered, and extracted by maceration at room temperature with 70% alcohol (1000 mL) for 10 days. The extract of C. clathrata was filtered under vacuum, the solvent was removed using a rotary evaporator, and the resulting semisolid mass was vacuum-dried in a desiccator; the solid residue (hydroalcoholic extract) was stored at 5°C until being re-suspended in saline at different concentrations.
Total phenolics and polysaccharides
The quantitative analysis of total phenolics was based on the method described by CitationBonoli et al. (2004), with minor modifications. C. clathrata hydroalcoholic extract (HE) (10 mg) was dissolved in 10 mL of methanol. An aliquot of 7.5 mL was diluted in 42.5 mL of methanol. One hundred microliters of this solution were added to 500 μL of Folin–Ciocalteu reagent diluted 1:10 with distilled water and mixed during 1 min, then 2 mL of 20% Na2CO3 solution was added and mixed again during 30 s. After 2 h, the absorbance at 750 nm was read. Gallic acid was used to obtain the calibration curve. The measurements were carried out in triplicate. The results are expressed as mg gallic acid/g of the HE ± standard deviation.
The polysaccharide content was measured by the phenol–sulfuric acid colorimetric method (CitationDubois et al., 1956), using a standard curve of glucose solution. C. clathrata HE (10 mg) was dissolved in 1 mL of water and afterward diluted in 1 mL of a phenol solution, then 5 mL of concentrated sulfuric acid was added and the absorbance at 490 nm was read. The measurements were carried out in triplicate. The results are expressed as mg polysaccharide/g of the HE ± standard deviation.
Animals
Swiss mice (20–30 g) and Wistar rats (120–180 g) of both sexes were obtained from the Central Biotery of the Federal University of Sergipe (São Cristóvão, Brazil). Animals were randomly assigned to groups and maintained in plastic boxes at controlled room temperature (25–28°C) with free access to food and water, under a 12:12 h light/dark cycle. All the experimental procedures were carried out during the light period of the day (08:00 a.m. to 05:00 p.m.) and complied with the guidelines on animal care of the Federal University of Sergipe Ethics Committee for Animal Use in Research, conducted in accordance with the internationally accepted principles for laboratory animal use and care. Animals submitted to oral administration of the extract or drugs were fasted for 12 h before the experiments and acclimatized for at least 2 h before the experiments. All efforts were made to minimize the number of animals used and their suffering.
Chemicals and drugs
The following chemicals and drugs were used: aspirin (acetylsalicylic acid, ASA), butylated hydroxytoluene (BHT), carrageenan, dexamethasone, gallic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH), glucose, and morphine hydrochloride from Sigma Chemical Co. (St. Louis, MO, USA); acetic acid from Merck (Damstadt, Germany); formalin from Baker (Santo Amaro, SP, Brazil); solvents from Vetec (Rio de Janeiro, RJ, Brazil). All substances used were dissolved in saline solution, with the exceptions of ASA which was dissolved in 5% Tween 80 in 0.9% NaCl solution, and BHT, gallic acid, and DPPH that were dissolved in methanol. The final concentration of Tween 80 did not exceed 5% and did not cause any effect per se.
Nociceptive tests
Acetic acid-induced abdominal writhes
Abdominal writhes consisted of a contraction of the abdominal muscle together with a stretching of the hind limbs (CitationTonos et al., 1999), induced by intraperitoneal (i.p.) injection in mice of acetic acid (0.6% solution, 0.1 mL/10 g), the nociceptive agent (CitationKoster et al., 1959).
The animals were pre-treated with C. clathrata HE (100, 200, or 400 mg/kg) orally (per os, p.o.) 60 min before initiating algesic stimulation, or with aspirin (ASA, 300 mg/kg, p.o., 60 min before the administration of acetic acid), used as positive control (n = 6/group). The abdominal writhes were observed, in separate individual chambers, for a period of 20 min, starting after the administration of acetic acid.
Formalin test
The formalin test was applied according to the method of CitationDubuisson and Dennis (1977), modified by CitationHunskaar and Hole (1987).
Mice were pre-treated with C. clathrata HE (100, 200, or 400 mg/kg, p.o., 60 min before the administration of formalin), or with morphine hydrochloride (10 mg/kg, i.p., 30 min before the administration of formalin) or ASA (300 mg/kg, p.o., 60 min before the administration of formalin), used as positive controls, before intraplantar injection of 1% formalin solution (20 μL) into the right hindpaw of the animal (n = 6/group). The control group received vehicle, saline (0.1 mL/10 g, p.o., 60 min before the administration of formalin, n = 6).
The time that the animal spent licking or biting its paw was measured during the first phase (0–5 min) and the second phase (20–25 min) of the test.
Anti-inflammatory activity
Measurement of paw edema in rats
The anti-inflammatory activity was studied using the paw edema model induced by 1% carrageenan, administered at a volume of 0.1 mL/animal into the subplantar region of the right hindpaw of the rat (CitationWinter et al., 1962). The volume of the paw was measured by removal of the water column using a hydroplethysmometer (model 7150; Ugo Basile, Varese, Italy), at time 0 and the intervals of 1, 2, 3, and 4 h immediately after the subplantar injection of carrageenan (CitationHarris & Spencer, 1962).
The HE of C. clathrata at the dose of 100, 200, or 400 mg/kg, ASA (300 mg/kg), and vehicle were administrated p.o. 1 h before the edematogenic agent to different groups of animals for each treatment (n = 6/group).
The data obtained for the various groups are reported as mean ± SEM and expressed in milliliters. The percentage inhibition in the edema experiment was calculated based on the area under the curve (AUC) after 4 h.
Neutrophil migration into the peritoneal cavity
Leukocyte migration was induced by injection of carrageenan (1%, 250 μL, i.p.) into the peritoneal cavity of mice 1 h after administration of the HE of C. clathrata (100, 200, and 400 mg/kg, p.o., n = 6) or dexamethasone [2 mg/kg, subcutaneous (s.c.), n = 6] by modification of the technique previously described by CitationMatos et al. (2003). The animals were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and were euthanized by cervical dislocation 4 h after carrageenan injection. Shortly after, saline containing ethylenediaminetetraacetic acid (EDTA; 1 mM, i.p., 3 mL) was injected. Immediately a brief massage was done for further fluid collection, which was centrifuged (2000 rpm, 5 min) at room temperature. The supernatant was disposed of and the precipitate was re-suspended in 300 μL of saline. An aliquot of 10 μL from this suspension was dissolved in 200 μL of Turk solution and the total cells were counted in a Neubauer chamber, under optical microscopy. The results are expressed as the number of neutrophils/mL. The percentage of leukocyte inhibition = (1 – T/C) × 100, where T represents the treated group leukocyte count and C represents the control group leukocyte count.
Measurement of locomotor activity
In order to evaluate a possible non-specific muscle-relaxant or sedative effect of the extract, mice were submitted to the open-field test. The ambulatory behavior was assessed in an open-field test as previously described (CitationRodrigues et al., 2002). The apparatus consisted of a wooden box measuring 40 × 60 × 50 cm. The floor of the arena was divided into 12 equal squares, and the numbers of squares crossed with all paws were counted in a 6-min session. Mice were pre-treated with C. clathrata HE (100, 200, or 400 mg/kg, p.o., n = 6) or vehicle (0.1 mL/10 g, p.o., n = 6) 60 min beforehand.
Quantitative assay of antioxidant activity
The quantitative analysis of antioxidant activity was based on the method described by CitationBrand-Williams et al. (1995) and CitationSánchez-Moreno et al. (1998), with minor modifications. The scavenging of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical was followed by monitoring the decrease in absorbance at 515 nm, which occurred due to reduction by the antioxidant.
The calibration curve was established by preparing dilutions of a DPPH radical stock solution (100 µg/mL) to obtain final concentrations of 5, 10, 15, 20, 25, 30, 60, 65, and 70 μg/mL. The absorbance of each standard concentration was then monitored in a spectrophotometer (UV BEL Photonics 1105) at 515 nm. The measurements were carried out in triplicate with intervals of 1 min. The equation of the concentration vs. absorbance calibration curve for the DPPH radical was C = −0.01923 + 0.0284A, where C is the concentration of the DPPH radical in the medium, and A is the absorbance at 515 nm. The correlation coefficient was R = 0.9918.
A solution containing 500 μg/mL of C. clathrata HE was prepared in methanol, and diluted in concentrations of 30, 60, 90, 120, 150, 180, 360, 390, and 420 µg/mL. The disappearance of the DPPH radical was monitored by the decrease in absorbance at 515 nm, which was recorded after 0, 1, 5, and 10 min, and subsequently every 10 min up to 60 min (CitationSousa et al., 2007). The negative control was pure methanol used for dissolving the samples, while the positive controls were gallic acid and butylated hydroxytoluene (BHT) dissolved in methanol at concentrations of 30, 60, 90, 120, 150, 180, 360, 390, and 420 µg/mL. The mixture of methanol and HE was used as a blank.
The concentration of DPPH radical in the reaction mixture was calculated based on the calibration curve, where [DPPH] is expressed in μg/mL. The percentage of remaining DPPH (%DPPHREM) was calculated according to CitationBrand-Williams et al. (1995), as follows: %DPPHREM = [DPPH]T/[DPPH]T0 × 100, where T is the time when absorbance was determined (1–60 min) and T0 is time zero. The amount of antioxidant necessary to decrease the initial concentration of DPPH radical by 50% (IC50) was calculated by plotting %DPPHREM at time 60 min with 420 μg/mL of extract. The results are expressed as μg antioxidant/mL DPPH ± standard deviation.
The absorbance values observed in all samples at 60 min (420 μg/mL) were transformed into percentage of inhibition (IP), determined by the equation: IP = {[Abscontrol – (Abssample – Absblank)] × 100}/Abscontrol, where Abscontrol is the initial absorbance of the methanol solution of the DPPH radical and Abssample is the absorbance of the reaction mixture (DPPH + sample). The results are expressed as % of inhibition.
Statistical analysis
The results for antinociceptive, anti-inflammatory, and locomotor activities are presented as the mean ± SEM of n animals per group. The antioxidant activity was determined using Origin version 7.5 (Microcal, Northampton, MA, USA), and values are presented as the mean ± standard deviation of three assays. Statistical evaluation of the data was performed using one-way analysis of variance (ANOVA) followed by Bonferroni’s or Tukey’s test; p values less than 0.05 were considered significant.
Results
Total phenolics and polysaccharides
The concentration of total phenolics expressed as mg gallic acid/g of HE was 371.8 ± 57.3, and the concentration of total polysaccharides was 198.0 ± 5.5 mg/g of HE.
Acetic acid-induced writhing in mice
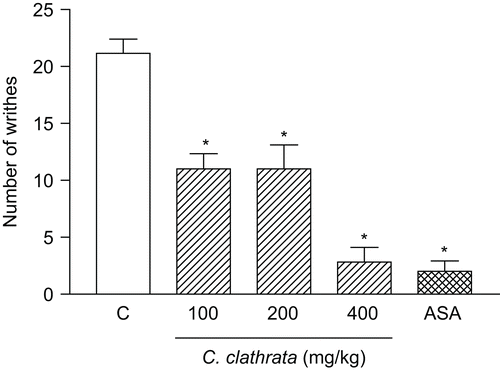
The results in show that C. clathrata stem HE given p.o. (100–400 mg/kg, n = 6/group) 1 h before the administration of acetic acid caused an inhibition of 47.2, 47.2, and 86.4% (p < 0.001) on acetic acid-induced writhes at the doses of 100, 200, and 400 mg/kg, respectively. ASA (300 mg/kg, n = 6) exhibited significant (90.4%, p < 0.001) inhibition of the control writhes in acetic acid-induced writhing.
Figure 1. Influence of C. clathrata hydroalcoholic extract (HE) in nociceptive behavior of mice evaluated in acetic acid-induced abdominal writhing model. Nociception was registered by the number of writhes that the animal presented 20 min following i.p. acetic acid (0.6%) injection. Groups of animals were pre-treated with vehicle (C, control group, n = 6, open column), aspirin (acetylsalicylic acid, ASA, 300 mg/kg, n = 6, cross-hatched column), or HE (100–400 mg/kg, n = 6/group, right-hatched columns), p.o., 60 min before acetic acid. Each column represents the mean ± SEM. Asterisks denote statistical significance, *p < 0.001, in relation to control group. ANOVA followed by Bonferroni’s test.
Formalin reaction time in mice
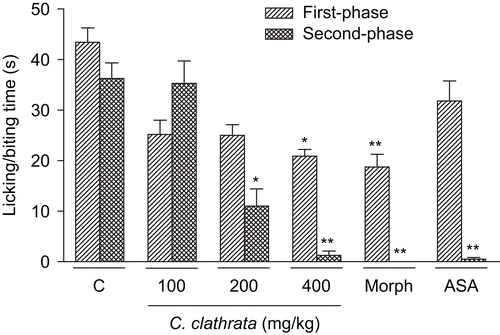
As shown in , C. clathrata HE administered orally (100–400 mg/kg, 1 h before the administration of formalin, n = 6/group) produced marked inhibition on intraplantar injection of formalin in mice both against neurogenic pain (first phase) at the dose of 400 mg/kg (p < 0.01) and against inflammatory pain (second phase) at the doses of 200 (p < 0.01) and 400 mg/kg (p < 0.001). ASA (300 mg/kg, p.o., 60 min before the administration of formalin) caused inhibition of the second phase of formalin-induced nociception (p < 0.001, n = 6, ). Morphine (10 mg/kg, i.p., 30 min before the administration of formalin) caused significant inhibition of both phases of formalin-induced nociception (p < 0.001, n = 6, ).
Figure 2. Effect of C. clathrata HE in nociceptive behavior of mice evaluated in formalin-induced nociception model. Groups of mice were pre-treated with vehicle (column C, control group, 10 mL/kg, p.o., 60 min before the administration of formalin, n = 6), aspirin (acetylsalicylic acid, ASA, 300 mg/kg, p.o., 60 min before the administration of formalin, n = 6), morphine (Morph, 10 mg/kg, i.p., 30 min before the administration of formalin, n = 6), or C. clathrata HE (100–400 mg/kg, p.o., 60 min before the administration of formalin, n = 6/group) against the early phase (0–5 min, right-hatched columns) or late phase (20–25 min, cross-hatched columns) of formalin-induced nociception in mice. Each column represents the mean ± SEM. Asterisks denote statistical significance, *p < 0.01 and **p < 0.001, in relation to control group. ANOVA followed by Bonferroni’s test.
Carrageenan-induced paw edema in rats
As observed in , the single oral treatment of rats with the HE of C. clathrata (100–400 mg/kg, 1 h before the administration of carrageenan, n = 6/group) was capable of reducing (p < 0.05) the edema formation induced by carrageenan (1%, 100 µL/paw), an effect observed at 3 and 4 h after the administration of this phlogistic agent. Likewise, ASA (300 mg/kg, p.o., 1 h before the administration of carrageenan, n = 6) significantly inhibited (p < 0.001) the edematogenic response evoked by carrageenan in rats, at 1, 2, 3, and 4 h ().
Figure 3. Effect of C. clathrata HE on rat paw edema induced by carrageenan. Groups of rats were pre-treated with vehicle (control group, 10 mL/kg, p.o., n = 6), aspirin (acetylsalicylic acid, ASA, 300 mg/kg, p.o., n = 6), or C. clathrata HE (100–400 mg/kg, p.o., n = 6/group) 60 min before carrageenan-induced paw edema. Measurements were performed at times 0, 1, 2, 3, and 4 h after the subplantar injection of carrageenan (1%, 100 µL). Each value represents the mean ± SEM. Asterisks denote statistical significance, *p < 0.05, **p < 0.01, and ***p < 0.001, in relation to control group. ANOVA followed by Bonferroni’s test.
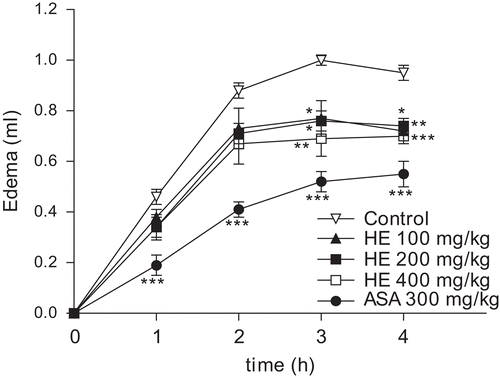
The mean AUC found in carrageenan-treated rats was 1.67 ± 0.04 mL × h. (n = 6). Based on AUC values, the HE at 100, 200, and 400 mg/kg caused 21.6, 22.2, and 28.1% (p < 0.05) of inhibition on the edema response, respectively (n = 6/group). ASA at 300 mg/kg (n = 6) caused an inhibition of 47.9% (p < 0.001).
Carrageenan-induced peritonitis in mice
Carrageenan (1%, 250 μL) induced neutrophil migration into the peritoneal cavity 4 h after stimulus (). shows the inhibitory effect of the HE of C. clathrata (41.5% at the dose of 400 mg/kg, p < 0.001, n = 6) on the carrageenan-induced response.
Figure 4. Effect of C. clathrata HE on leukocyte migration into the peritoneal cavity induced by carrageenan in mice. Groups of animals were pre-treated with vehicle (C, control group, 10 mL/kg, p.o., open column), dexamethasone (Dexa, 2 mg/kg, s.c., cross-hatched column), or HE at the doses of 100, 200, and 400 mg/kg (p.o., right-hatched columns) 60 min before carrageenan (1%, 250 μL, i.p.)-induced peritonitis. Cell counts were performed at time 4 h after the injection of carrageenan. Each value represents the mean ± SEM (n = 6/group). Asterisks denote statistical significance, *p < 0.001, in relation to control group. ANOVA followed by Bonferroni’s test.
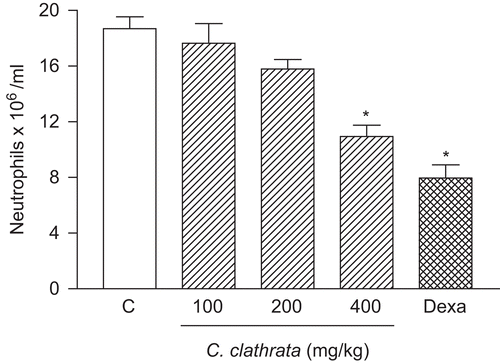
The results obtained with the control group support the effect of the HE of C. clathrata stems, since the vehicle presented no activity, and the control drug dexamethasone (2 mg/kg, s.c., 1 h before the administration of carrageenan, n = 6) inhibited (57.4%, p < 0.001) carrageenan-induced neutrophil migration into the peritoneal cavity ().
Evaluation of locomotor activity
The HE of C. clathrata (100–400 mg/kg, p.o.) did not affect the locomotor activity in the open-field test when compared with animals that received vehicle (control group). The mean numbers ± SEM of crossed squares were 121.0 ± 3.1, 120.2 ± 2.7, 121.7 ± 3.3, and 126.2 ± 2.8 for the control, 100, 200, and 400 mg/kg groups, respectively.
Antioxidant activity
The amount of DPPH radical that reacted with the stem HE (420 μg/mL, 60 min, n = 3) was 50.19%, while for the positive controls BHT and gallic acid (420 μg/mL, 60 min, n = 3) the amount of DPPH radical used was higher at 96% (96.91 and 96.24%, respectively).
The IC50 value for the HE of stems was higher (69.25 ± 0.65 μg/mL DPPH) as compared with the IC50 values for the reference compounds BHT and gallic acid (12.33 ± 3.25 and 9.33 ± 0.22 μg/mL DPPH, respectively, n = 3).
Discussion
The present study demonstrates that C. clathrata HE displays antinociceptive and anti-inflammatory properties with a moderate antioxidant potential, and provides some evidence on the mechanisms implicated in these effects.
For the first time, this work shows that C. clathrata HE p.o. produces significant antinociception according to assessment of the abdominal writhes elicited by acetic acid and of formalin-induced pain. In addition, the doses of the extract that caused significant antinociception did not produce any statistically significant motor dysfunction.
It has been suggested that acetic acid acts by releasing endogenous mediators that stimulate the nociceptive neurons (CitationCollier et al., 1968). This method is sensitive to non-steroidal anti-inflammatory drugs (NSAIDs) and to narcotics and other centrally acting drugs (CitationCollier et al., 1968; CitationSantos et al., 1998; CitationReichert et al., 2001).
CitationRibeiro et al. (2000) have demonstrated that the nociceptive activity of acetic acid may be due to the release of cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-8, by resident peritoneal macrophages and mast cells. Thus, the previous findings and the results presented herein might indicate that the antinociceptive action of the HE of C. clathrata in the acetic acid-induced writhing test could be due to inhibition of the release of TNF-α, IL-1β, and IL-8 by resident peritoneal cells. However, this possibility remains to be tested in future studies.
The results of the present study have also shown that morphine is largely effective in preventing both the first and second phases of formalin-induced pain. Morphine and opioid drugs exert their antinociceptive activities by performing in receptors (μ, κ, and δ) and thereby modulating the stimulus transmission without interfering in the cause. It is well known that NSAIDs (such as aspirin, acetaminophen, and diclofenac), known to inhibit cyclo-oxygenase (COX) activity, are largely ineffective or cause very weak inhibition at the first phase of the formalin test (CitationHunskaar & Hole, 1987; CitationMalmberg & Yaksh, 1992; CitationSantos et al., 1998). In addition, NSAIDs can attenuate, in a dose-related manner, the second phase of formalin-induced licking (CitationHunskaar & Hole, 1987; CitationMalmberg & Yaksh, 1992; CitationSantos et al., 1998). Previous studies have shown that formalin releases various inflammatory mediators such as substance P, neurokinins A and B, and prostaglandins (CitationHunskaar et al., 1986; CitationHunskaar & Hole, 1987; CitationSantos & Calixto, 1997). Our results show, however, that C. clathrata HE p.o. produced inhibition on both the first (neurogenic nociception) and the second phase (inflammatory nociception) of the formalin test in mice, similar to the morphine effect. These data suggest that the HE of stems can produce antinociceptive action through a contribution of opioid receptor-mediated mechanisms and/or inhibition of COX, and consequently prostaglandin synthesis. However, the possibility that C. clathrata acts on COX remains to be tested in future studies.
The anti-inflammatory effect of C. clathrata HE was evaluated in carrageenan-induced paw edema and peritonitis models. In rats, the inflammatory response induced by carrageenan in the paw edema model is characterized by a biphasic response with marked edema formation resulting from the rapid production of several inflammatory mediators such as histamine, serotonin, and bradykinin, which is subsequently sustained by the release of prostaglandins and nitric oxide (NO) with a peak at 3 h, produced by inducible isoforms of COX (COX-2) and nitric oxide synthase (iNOS), respectively (CitationSeibert et al., 1994; CitationNantel et al., 1999). In the present work, previous oral treatment with the HE of C. clathrata was effective in reducing the edematogenic response evoked by carrageenan in rats between the third and fourth hours after the injection. This evidence allows us to suggest that the anti-inflammatory action of the HE of C. clathrata is related to the inhibition of one or more intracellular signaling pathways involved in the effects of several inflammatory mediators.
Cell recruitment during inflammation depends on the orchestrated release of local mediators which are responsible for local vascular and tissue changes as well as for the recruitment of host defense cells (CitationLuster et al., 2005). The inflammation induced by carrageenan involves cell migration, plasma exudation, and production of mediators, such as NO, prostaglandin E2, IL-1β, IL-6, and TNF-α (CitationSalvemini et al., 1996; CitationLoram et al., 2007). These mediators are able to recruit leukocytes, such as neutrophils, in several experimental models. The HE inhibited leukocyte migration induced by i.p. injection of carrageenan (in a peritonitis model). A putative mechanism associated with this activity may be inhibition of the synthesis of many inflammatory mediators whose involvement in cell migration is well established.
The DPPH test is a very convenient method for screening small antioxidant molecules because the intensity of the reaction may be analyzed by a simple spectrophotometric assay (CitationSánchez-Moreno et al., 1998; CitationSoler-Rivas et al., 2000). The DPPH radical is scavenged by antioxidants through the donation of hydrogen to form the stable reduced DPPH molecule. The radicals are stabilized through the formation of non-radical products by reaction with antioxidant agents (CitationArgolo et al., 2004).
The capacity for scavenging free radicals was evaluated for the HE of lichen C. clathrata. IP and IC50 values are considered to be good measures of the antioxidant efficiency of extracts and pure compounds. Typical IP values for plant materials with acknowledged potent antioxidant activities are around 70–90%, as observed for the stem bark (93%) and leaves (75%) of Cassia fistula, a traditional Indian medicine (CitationSiddhuraju et al., 2002), and for the edible seeds (52–80%) of Rosa rubiginosa (CitationMoure et al., 2001). Less potent materials show IP values in the range of 9–14%, as for example the seeds of Gevuina avellana (CitationMoure et al., 2001). Our results showed that the HE of C. clathrata stems has a moderate antioxidant potential. It is thus suggested that this antioxidant effect is unrelated to the functional (antinociceptive and anti-inflammatory) activities of the HE.
Polysaccharides stimulate a wide range of immune responses, such as cytokine release, generation of reactive oxygen species, generation of NO, release of arachidonic acid metabolites, and activation of NF-κB by macrophages (CitationSchepetkin & Quinn, 2006). Thus, it is clear that polysaccharides from lichen have significant therapeutic potential that can be related to the antinociceptive and anti-inflammatory effects.
In conclusion, our study revealed that the HE of C. clathrata stems shows antinociceptive and anti-inflammatory activities that do not depend on its antioxidant activity. The identification and the isolation of such bioactive components are in progress, which could elucidate the antinociceptive and anti-inflammatory properties of C. clathrata.
Declaration of interest
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq – PIBIC/CNPq/UFS) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
References
- Argolo ACC, Sant’Ana AEG, Pletsch M, Coelho LCBB (2004): Antioxidant activity of leaf extracts from Bauhinia monandra. Bioresour Technol 95: 229–233.
- Bonoli M, Verardo V, Marconi E, Caboni MF (2004): Antioxidant phenols in barley (Hordeum vulgare L.) flour: Comparative spectrophotometric study among extraction methods of free and bound phenolic compounds. J Agric Food Chem 52: 5195–5200.
- Brand-Williams W, Cuvelier ME, Berset C (1995): Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol 28: 25–30.
- Bucar F, Schneider I, Ogmundsdóttir H, Ingólfsdóttir K (2004): Anti-proliferative lichen compounds with inhibitory activity on 12(S)-HETE production in human platelets. Phytomedicine 11: 602–606.
- Carbonero ER, Sassaki GL, Stuelp PM, Gorin PA, Woranovicz-Barreira SM, Iacomini M (2001): Comparative studies of the polysaccharides isolated from lichenized fungi of the genus Cladonia: Significance as chemotypes. FEMS Microbiol Lett 194: 65–69.
- Collier HO, Kinneen LC, Johnson CA, Schneider C (1968): The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol 32: 295–310.
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956): Colorimetric method for determination of sugars and related substances. Nature 28: 350–356.
- Dubuisson D, Dennis SG (1977): The formalin test: A quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 4: 161–174.
- Harris JM, Spencer PSJ (1962): A modified plethysmographic apparatus for recording volume changes in rat paw. J Pharm Pharmacol 14: 464–466.
- Hunskaar S, Berge OG, Hole K (1986): Dissociation between antinociceptive and anti-inflammatory effects of acetylsalicylic acid and indomethacin in the formalin test. Pain 25: 125–132.
- Hunskaar S, Hole K (1987): The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 30: 103–114.
- Ingólfsdóttir K (2002): Usnic acid. Phytochemistry 61: 729–736.
- Koster R, Anderson N, Debber EJ (1959): Acetic acid for analgesic screening. Fed Proc 18: 418–420.
- Kumar KC, Müller K (1999): Lichen metabolites. 1. Inhibitory action against leukotriene B4 biosynthesis by a non-redox mechanism. J Nat Prod 62: 817–820.
- Loram LC, Fuller A, Fick LG, Cartmell T, Poole S, Mitchell D (2007): Cytokine profiles during carrageenan-induced inflammatory hyperalgesia in rat muscle and hind paw. J Pain 8: 127–136.
- Luster AD, Alon R, von Andrian UH (2005): Immune cell migration in inflammation: Present and future therapeutic targets. Nat Immunol 6: 1182–1190.
- Malmberg AB, Yaksh TL (1992): Antinociceptive actions of spinal nonsteroidal anti-inflammatory agents on the formalin test in the rat. J Pharmacol Exp Ther 263: 136–146.
- Martinichen-Herrero JC, Carbonero ER, Sassaki GL, Gorin PA, Iacomini M (2005): Anticoagulant and antithrombotic activities of a chemically sulfated galactoglucomannan obtained from the lichen Cladonia ibitipocae. Int J Biol Macromol 35: 97–102.
- Matos LG, Santos LDAR, Vilela CF, Pontes IS, Tresvenzol LMF, Paula JR, Costa EA (2003): Atividades analgésica e/ou antiinflamatória da fração aquosa do extrato etanólico das folhas da Spiranthera odoratissima A. St. Hillaire (manacá). Rev Bras Farmacogn 13(Suppl.): 15–16.
- Moure A, Franco D, Sineiro J, Dominguez H, Nunes MJ, Lema JM (2001): Antioxidant activity of extracts from Gevuina avellana and Rosa rubiginosa defatted seeds. Food Res Intern 34: 103–109.
- Müller K (2001): Pharmaceutically relevant metabolites from lichens. Appl Microbiol Biotechnol 56: 9–16.
- Nantel F, Denis D, Gordon R, Northey A, Cirino M, Metters KM, Chan CC (1999): Distribution and regulation of cyclooxygenase-2 in carrageenan induced inflammation. Br J Pharmacol 128: 853–859.
- Okuyama E, Umeyama K, Yamazaki M, Kinoshita Y, Yamamoto Y (1995): Usnic acid and diffractaic acid as analgesic and antipyretic components of Usnea diffracta. Planta Med 61: 113–115.
- Omarsdottir S, Freysdottir J, Olafsdottir ES (2007): Immunomodulating polysaccharides from the lichen Thamnolia vermicularis var. subuliformis. Phytomedicine 14: 179–184.
- Reichert JA, Daughters RS, Rivard R, Simone DA (2001): Peripheral and preemptive opioid antinociception in a mouse visceral pain model. Pain 89: 221–227.
- Ribeiro RA, Vale ML, Thomazzi SM, Paschoalato ABP, Poole S, Ferreira SH, Cunha FQ (2000): Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur J Pharmacol 387: 111–118.
- Rodrigues AL, da Silva GL, Mateussi AS, Fernandes ES, Miguel OG, Yunes RA, Calixto JB, Santos AR (2002): Involvement of monoaminergic system in the antidepressant-like effect of the hydroalcoholic extract of Siphocampylus verticillatus. Life Sci 70: 1347–1358.
- Salvemini D, Wang ZQ, Wyatt PS, Bourdon DM, Marino MH, Manning PT, Currie MG (1996): Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br J Pharmacol 118: 829–838.
- Sánchez-Moreno C, Larrauri JA, Saura-Calixto F (1998): A procedure to measure the antiradical efficiency of polyphenols. J Sci Food Agric 76: 270–276.
- Santos ARS, Calixto JB (1997): Further evidence for the involvement of tachykinin receptor subtypes in formalin and capsaicin models of pain in mice. Neuropeptides 31: 381–389.
- Santos ARS, Vedana EMA, Freitas GAG (1998): Antinociceptive effect of meloxicam, in neurogenic and inflammatory nociceptive models in mice. Inflamm Res 47: 302–307.
- Schepetkin IA, Quinn MT (2006): Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int Immunopharmacol 6: 317–333.
- Seibert K, Zhang Y, Leahy K, Hauser S, Masferrer J, Perkins W, Lee L, Isakson P (1994): Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci USA 91: 12013–12017.
- Siddhuraju P, Mohan PS, Becker K (2002): Studies on the antioxidant activity of Indian Laburnum (Cassia fistula L.): A preliminary assessment of crude extracts from stem bark, leaves, flowers and fruit pulp. Food Chem 79: 61–67.
- Soler-Rivas C, Espín JC, Wichers HJ (2000): An easy and fast test to compare total free radical scavenger capacity of foodstuffs. Phytochem Anal 11: 1–9.
- Sousa CMM, Silva HR, Vieira GM Jr, Ayres MCC, Costa CLS, Araújo DS, Cavalcante LCD, Barros EDS, Araújo PBM, Brandão MS, Chaves MH (2007): Fenóis totais e atividade antioxidante de cinco plantas medicinais. Quim Nova 30: 351–355.
- Süleyman H, Odabasoglu F, Aslan A, Cakir A, Karagoz Y, Gocer F, Halici M, Bayir Y (2003): Anti-inflammatory and antiulcerogenic effects of the aqueous extract of Lobaria pulmonaria (L.) Hoffm. Phytomedicine 10: 552–557.
- Tonos MP, Sáenz MT, Garcia MD, Fernández MA (1999): Antinociceptive effects of the tubercles of Anredera leptostachy. J Ethnopharmacol 68: 229–234.
- Vijayakumar CS, Viswanathan S, Kannappa-Reddy M, Parvathavarthini S, Kundu AB, Sukumar E (2000): Anti-inflammatory activity of (+)-usnic acid. Fitoterapia 71: 564–566.
- Winter CA, Risley EA, Nuss GW (1962): Carrageenin-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med 111: 544–547.
