Abstract
Bauhinia monandra Kurz. (Fabaceae: Caesalpinioideae) is a plant widely employed in Brazilian folk medicine for hypoglycemia. However, little is known about the effect of maternal exposure to this plant on fetal development. The aqueous and ethanol extracts obtained from B. monandra dried leaves were administered to pregnant Wistar rats throughout gestation (day 1 to day 20) at 1,400 or 7,000 mg/kg/day (n = 6/group). Maternal toxicity was not observed in the dams of both groups, and was evaluated by observing body weight, water and food intake during treatment, by measuring serum biochemical levels of creatinine, urea, AST and ALT, and by studying the histopathology of liver, kidney, pancreas, spleen and uterus at the end of treatment (gestation day 20). Both extracts and doses did not impair reproductive performance or delay fetal development, measured by observing implantations and reabsorptions in the uterus, by counting the number of corpora lutea in ovaries, by recording the litter weight and number of live and dead fetuses and by analyzing possible skeleton and viscera malformations in the fetuses. Also, the aqueous extract promoted decreased post-implantation loss when compared to the control group. The aqueous and ethanol extracts from B. monandra dried leaves (1,400 or 7,000 mg/kg/day) did not cause maternal or fetal toxicities and the aqueous extract promoted increased implantation and decreased post-implantation loss in the pregnant rats.
Keywords::
Introduction
Plants are an important source of natural active compounds with a range of pharmacological activities and chemical structures. In recent years, the use of medicinal plants for the treatment of diseases has increased in many countries (CitationMills & Bone, 2000). Approximately 80% of the world’s population employ medicinal plants as first therapeutic source (CitationYamada, 1998). It is known that these plants exert pharmacological actions in the body, promoting curative proprieties and preventing a range of afflictions. In fact, about 25% of medications are obtained from vegetal sources, while about 50% are synthesized in laboratories from natural compounds as prototypes (CitationUgaz, 1994; CitationCechinel et al., 1998). Medicinal plants and phytomedicines are often employed by populations without professional advice under the impression that they are harmless, even when used chronically (CitationElgorashi et al., 2003), justifying the importance of studies directed to the elucidation of the toxicity of medicinal plants.
Native or introduced Bauhinia species (Leguminosae) have been employed in folk medicine for the treatment of diabetes. It is known that its leaves present a lectin that exerts significant hypoglycemic and diuretic effects (CitationCoelho & Silva, 2000; CitationPepato et al., 2002). In Brazil, leaves of the species B. purpurea Linn., B. forficata Link., and B. monandra Kurz. are commonly used as teas for diabetes treatment (CitationAlbuquerque and Silva, 2000). As a consequence of the elevated costs of commercial medicines, the Brazilian population prefers to use infusions obtained from the leaves of Bauhinia sp. as first therapeutic source for the treatment of diabetes, including pregnant women with gestational diabetes (CitationVolpato and Damasceno, 2008). The B. monandra leaves are frequently employed with this purpose in the form of teas, infusions or even as commercial extracts. The uninformed use of these preparations by pregnant women, under the impression that they are harmless, may be a problem. Since no experimental studies about it effects in gestation have been found, the scope of this work was to study whether the aqueous and ethanol extracts obtained from dried B. monandra leaves are able to promote damage to gestation and to fetuses of rats, contributing to the informed use of this medicinal plant in folk medicine.
Material and methods
Plant material
The B. monandra kurz. leaves used in this study were collected in April 2007 in Natal (Rio Grande do Norte, Northeast Brazil; GPS location: latitude 05° 47′ 42″, longitude 35° 12′ 34″). The plant was identified by Iracema Loiola, and a voucher specimen was deposited at the Herbarium “Parque das Dunas” of the Universidade Federal do Rio Grande do Norte (registration number 8,170).
Extract preparation
At the toxicology laboratory of Universidade Federal do Rio Grande do Norte, the fresh leaves were oven dried at 40°C and then milled.
For preparation of the aqueous extract a portion of the dried and milled leaves (400 g) were boiled in distilled water (1 L) for 10 min. After filtration the aqueous extract (40% m/v) was obtained.
The crude ethanol extract was obtained by macerating another portion of the dried and milled leaves (545 g) with commercial ethyl alcohol 96% (6 L) for seven days. After filtration the solution obtained was concentrated under reduced pressure and reserved. To the same portion of leaves was again added ethyl alcohol 96% for a maceration period of 24 h. The filtrated portion, after concentration at reduced pressure, was added to the first. This methodology with the same vegetal material was repeated twice more, resulting in four extractions. The solutions together after concentration constituted the ethanol extract.
Animals
Male and female Wistar rats (250-270g) about 90 days old were used. The animals were housed under controlled temperature (22° ± 2°C), with a 12:12 h light:dark schedule and free access to food and water. After acclimation for 1 week, two female rats were placed together with one male in the afternoon. The next morning, females showing evidence of mating (vaginal smear with sperm = gestation day 1) were housed in pairs in plastic cages measuring 40 × 50 × 20 cm. The dams were randomly distributed into control and experimental groups (n = 6 per group). The animals used in this study were maintained in accordance with the Ethical Principles in Animal Research adopted by the National Ethics Research Committee (CONEP/MS) and approved by the Onofre Lopes University Hospital Research Ethical Committee (protocol no. 011/06).
Treatment
In folk medicine, approximately 10 g of the leaves in 1 L of water are used to prepare the infusion, and all this volume is commonly consumed in a single day per person. Considering that 70 kg is the adult human median weight, the dose corresponds to 140 mg/kg/day. In this work the experimental animals treated with the infusion received, by gavage, from gestation day (GD) 1 to GD20, doses 10 (1,400 mg/kg/day) or 50 (7,000 mg/kg/day) times higher than the dose employed daily in folk medicine by humans. The ethanol experimental groups received the same doses (1,400 or 7,000 mg/kg/day) of the ethanol extract for the same period. The control groups received tap water by gavage.
Reproductive performance study and tissue and blood collections
On day 20 of gestation the dams were anesthetized with ethyl ether. The blood was collected for evaluation of biochemical parameters (ALT, AST, urea, creatinine) by cardiac puncture. After euthanasia by cervical disjoint, the ovaries and uterus were removed. The ovaries were weighed and the number of corpora lutea was recorded. The fetuses were counted, removed, weighed and examined for any malformations. The number of implantation sites, reabsorptions, dead and live fetuses per dam were recorded. Also, the organ weight was recorded (liver, kidney, spleen, pancreas, uterus, ovaries) and then tissue portions were fixed in formalin 10% for histopathological studies.
Embriofetotoxic study
After fetus euthanasia, they were analyzed for gross external anomalies. Half of each litter was fixed in Bouin’s solution for investigation of viscera variations using Wilson’s slicing technique (Citation1965). The rest of the litter was eviscerated, fixed in 95% ethanol, cleared with potassium chloride and than stained with alizarin red by the technique of CitationTaylor (1986). The extent of ossification was evaluated. The pelvic girdle and the fore limbs and hind limbs were examined for development of the long bones. Cranial bones, vertebrae, ribs, clavicles, sternum, metacarpus, metatarsus, and phalanges were also analyzed.
Statistical analysis
The animals employed for the aqueous extract study were analyzed in different moments, requiring two control groups, one for each of the experimental groups adopted. These data were analyzed by the Student t-test and by the Dunnett’s post hoc test when necessary.
The ethanol extract data were analyzed by the analysis of variance ANOVA and Tukey Kramer post hoc test when necessary.
In all cases, results were considered significant for p < 0.05.
Results
Statistically significant differences were not observed in body weight, body weight gain, water and food intake of the experimental groups when compared to control groups, as presented in .
Table 1. Body weight, weight gain, food and water intake of the control and experimental dams treated during gestation with the aqueous or the ethanol extract of B. monandra leaves.
No significant dam organ weight alterations were recorded (). The histopathological study revealed discrete congestion in pancreas, spleen, liver and kidney of the animals treated with the higher aqueous extract dose, suggesting that if the treatment be extended, inflammation could possibly occur. The islets of Langerhans also showed increased size in these animals when compared with the respective control group. Also, mild tumefaction of the renal ductile cells and moderate poliviscera and splenic congestion in these experimental groups were detected (). The tissue portions of the rats treated with the ethanol extract showed absence of important alterations. Only mild tumefaction of the kidney and liver cells were observed. However, the statistical analysis revealed normal biochemical parameters, except for reduced AST levels (F(2/19) = 3.045; p <0.05) in the experimental dams treated with the minor aqueous extract dose (1,400 mg/kg/day), as observed in .
Figure 1. Liver tissue portions (100 ×) of control (A) and experimental (B, C, D and E) dams. Observe the mild congestion in the hepatocytes of the 7,000 mg/kg aqueous extract dose treated dams (C arrows). (B) and (C) 1,400 and 7,000 mg/kg/day aqueous extract, respectively. (D) and (E) 1,400 and 7,000 mg/kg/day ethanol extract, respectively.
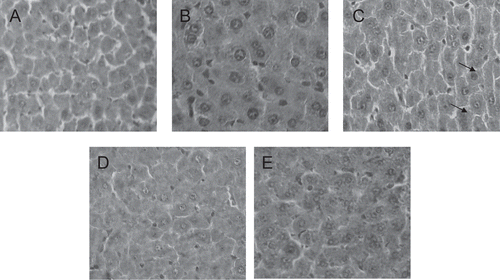
Figure 2. Kidney tissue portions (100 ×) of control (A) and experimental (B, C, D and E) dams. Observe the mild congestion in the renal ductile cells of the 7,000 mg/kg/day aqueous dose treated dams (C arrows). (B) and (C) 1,400 and 7,000 mg/kg/day aqueous extract, respectively. (D) and (E) 1,400 and 7,000 mg/kg/day ethanol extract, respectively.
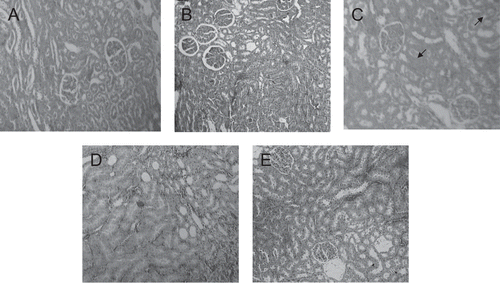
Figure 3. Pancreas tissue portions (100 ×) of control (A) and experimental (B, C, D and E) dams. Observe the mild congestion and increased islets of Langerhans size of the 7,000 mg/kg/day aqueous dose treated dams (C arrows). (B) and (C) 1,400 and 7,000 mg/kg/day aqueous extract, respectively. (D) and (E) 1,400 and 7,000 mg/kg/day ethanol extract, respectively.
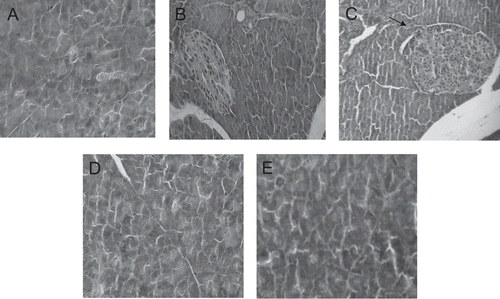
Figure 4. Spleen tissue portions (100 ×) of control (A) and experimental (B, C, D and E) dams. Observe the moderate poliviscera and splenic congestion at the 7,000mg/kg/day aqueous dose treated dams (C-arrows). (B) and (C) 1,400 and 7,000 mg/kg/day aqueous extract, respectively. (D) and (E) 1,400 and 7,000 mg/kg/day ethanol extract, respectively.
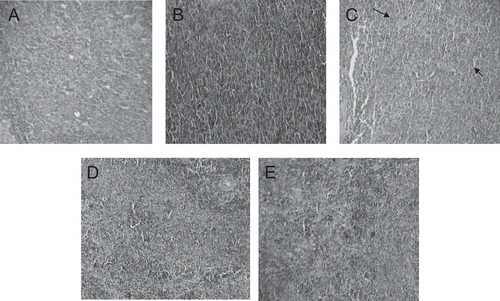
Table 2. Organ weight/body weight ratio and serum biochemical parameters of control and experimental dams treated during gestation with the aqueous or the ethanol extract of B. monandra leaves (data × 10−2).
Table 3. Biochemical serum parameters of the rats treated with the aqueous extract during pregnancy.
The reproductive performance study, evaluated by observation of the parameters pregnancy duration, litter weight, number of male and female pups, number of dead pups (), showed that the aqueous and ethanol extracts, at these concentrations and administered during gestation, were not able to promote maternal toxicity nor to impair their reproductive performance. In addiction, the statistical analyses showed reduced pre-implantation loss [(1,400 mg/kg/day): F(1/14) = 6.351, p <0.05; (7,000 mg/kg/day): F(1/11) = 5.198, p <0.05] and increased implantation percentage [(1,400 mg/kg/day): F(1/14) = 6.348; p <0.05; (7,000 mg/kg/day): F(1/14) = 5.197; p <0.05] for the experimental dams treated with both doses of the aqueous extract ().
Table 4. Reproductive performance of the control and experimental dams treated during gestation with the aqueous or the ethanol extract of B. monandra leaves.
No viscera or skeletal malformations, understood as permanent structural changes due to adverse effects of the extracts, were observed in the experimental fetuses.
Discussion
The parameters body weight, body weight gain, food and water ingestion employed for evaluation of maternal toxicity were monitored during the treatment period. The statistical analyses did not reveal alterations in these parameters, suggesting that both extracts, at the doses administered during rat gestation, did not promote maternal toxicity.
The histopathological analysis showed modest tissue changes in liver, kidneys, spleen, and pancreas of the experimental dams treated with the higher dose of the aqueous extract. All these experimental tissue portions presented discreet congestion without leukocyte overflow. The experimental pancreas also presented increased islet of Langerhans size. However, these findings were not sufficient enough to suggest toxicity. They could suggest that an inflammation condition could start at 7,000 mg/kg dose. However, only studies performed with this dose at prolonged periods will help answer this question. The ethanol extract was not able to promote histopathological alterations in the organs analyzed.
A significant (p <0.05) AST reduced level was detected in the blood of the rats treated with the lower aqueous extract dose when compared to the control group values. This data suggests absence of hepatic lesion, corroborating and supporting the histopathological findings. The serum biochemical parameters were not measured in the ethanol extract groups.
Progesterone, the main hormone involved in pregnancy sustenance, is produced in the corpora lutea, the most notable ovary element and major progesterone synthesis site during early pregnancy. The corpora lutea area increases as pregnancy advances (CitationWaynforth, 1971), and the area is correlated with the increase of progesterone levels (CitationUchida et al., 1970). The ovary weight and number of corpora lutea did not seem different between the experimental and control groups, suggesting normal volume of corpora lutea and consequently, normal progesterone production.
It is known that progesterone is an important factor in the implantation process during pregnancy and that the implantation rate that correlates with the number of corpora lutea and implantations, indicates the reproductive capability success. Also, the presence of reabsorptions means progress failure on embryo development. The data collected in this study show that the number of implantations in uterus was increased in the low and high experimental groups treated with the aqueous extract. No alterations were observed in the ethanol extract treated groups. We suggest that the aqueous extract facilitated embryo implantation in uterus, increasing fertility capability, while the ethanol extract did not promote change. Finally, the control and experimental groups presented analogue failures on embryo development progress because the reabsorption rate was similar in all groups.
Neither the low nor the high dose of the extracts increased the incidence of anatomical and visceral damage or delayed the skeleton ossification in the experimental fetuses. The extracts did not harm the structural development of the offspring, evidencing absence of embriofetotoxicity.
The controls have different weights because the experiments were performed in different periods of the year. As this kind of variation is frequent in our colony, we decided to use two control groups. Each experimental group was compared only with its respective control group. The experimental and its respective control group were maintained in the laboratory and the experiments were performed at the same time.
The data obtained suggest that the two extracts and two doses employed did not promote maternal toxicity, did not impair gestation and reproductive performance or fetal development. Also, the aqueous extract facilitated, contrary to expectation, the embryo implantation in the uterus, probably by protecting the embryo before implantation, since decreased pre-implantation loss was observed.
Acknowledgements
Special thanks to Luiz Reginaldo Menezes da Rocha of the Pathology Department of Centro de Ciências da Saúde from Universidade Federal do Rio Grande do Norte for the confection of the tissue portions enabling the histopathological study, and to the Health Science Centre Bioterium of the Universidade Federal do Rio Grande do Norte, for providing the animals, the chow and the standardized place to maintain the animals in this study.
Declaration of interest
This study was supported by CNPq and is referent to the project developed by the first author at Universidade Federal do Rio Grande do Norte.
References
- Albuquerque ABP, Silva AV (2000): Pharmacobotanical study of species in the treatment of diabetes. Acta Pharm Bonaer 19: 7–12.
- Cechinel Filho V, Yunes RA (1998): Strategies for obtaining pharmacologically active compounds from medicinal plants: concepts about structural modification for improve the activity. Quim Nova 21: 99–105.
- Coelho LCBB, Silva MBR (2000): Simple method to purify milligram quantities of the galactose-specific lectin from the leaves of Bauhinia monandra. Phytochem Anal 11: 295–300.
- Elgorashi EE, Taylor JLS, Maes A, van Staden J, De Kimpe N, Verschaeve L (2003): Screening of medicinal plants used in South African traditional medicine for genotoxic effects. Toxicol Letters 143: 195–207.
- Mills S, Bone K (2000): Principles and Practice of Phytotherapy. Edinburgh, Churchill Livingstone, pp. 1-3.
- Pepato MT, Keller EH, Baviera AM, Kettelhut IC, Vendramini RC, Brunetti IL (2002): Anti-diabetic activity of Bauhinia forficata decoction in streptozotocin-diabetic rats. J Ethnopharmacol 81: 191–197.
- Taylor P (1986): Skeletal examination. Practical Teratology. London, Academic Press, Chapter 10, pp. 77-100.
- Uchida K, Kadowaki M, Nomura Y, Miyata K, Miyake T (1970): Relationship between ovarian progestin secretion and corpora lutea function in pregnant rat. Endocrinol Japonica 17: 499–507.
- Ugaz OL (1994): Investigación Fotoquímica (Photochemical investigation). Fondo Editorial. Lima: Pontificia Universidad Católica del Peru, Lima: Peru.
- Volpato GT, Damasceno DC (2008): Effect of Bauhinia forficata aqueous extract on the maternal-fetal outcome and oxidative stress biomarkers of streptozotocin-induced diabetic rats. J Ethnopharmacol 116: 131–137.
- Waynforth HB (1971): Changes in the volume of rat corpus luteum during pregnancy and after surgical interference with the uterus and placenta. Acta Endocrinologica 66: 296–302.
- Wilson JC (1965): Methods for administering agents and detecting malformations in experimental animals, in:Wilson JC, Warkany J, Teratology: Principles and Techniques. Chicago: University of Chicago Press. pp. 262–277.
- Yamada CSB (1998): Fitoterapia sua história e importância. Racine. 50–51.
