Abstract
In the present investigation, an ethanol extract of celery [Apium graveolens L. (Apiaceae/Umbelliferae)], at doses of 250 and 500 mg/kg body weight, was evaluated for antigastric ulcer activity using various experimental gastric ulcer models in rats. Ulcers were induced by indomethacin, cytodestructive agents (80% ethanol, 0.2 M NaOH and 25% NaCl) and cold restraint stress. Gastric secretory studies were undertaken by using pylorus ligation (Shay rat model). In addition to gastric wall mucus (GWM), non-protein sulfhydryl (NP-SH) and malondialdehyde (MDA) were also estimated in gastric tissues after 80% ethanol treatment. Pretreatment of celery extract produced dose-dependent reduction in all experimentally induced gastric lesions. Ethanol (80%) decreased the levels of GWM, NP-SH and increase in MDA concentration in gastric tissue. Celery extract showed the ability to significantly replenish the ethanol-induced depleted levels of GWM and gastric mucosal NP-SH. The gastric mucosal MDA level was also significantly lowered in extract pretreated rats. The celery extract showed stomach protection against the models used for ulcerogenesis. Results were further confirmed by using histopathological assessment. The phytochemical screening showed the presence of various chemical constituents such as flavonoids, tannins, volatile oils, alkaloids, sterols and/or triterpenes. Acute toxicity test revealed no deleterious or toxic symptoms or mortality over a period of 14 days. However, the LD50 was found to be 7.55 g/kg, and showed a large margin of safety. The results suggest that Apium graveolens extract significantly protects the gastric mucosa and suppresses the basal gastric secretion in rats, possibly through its antioxidant potential.
Introduction
Celery, Apium graveolens L. (Apiaceae/Umbelliferae), is locally known as “karfas” or “ajmod”. It is an aromatic biennial herb, almost the whole plant is used, including the roots, seeds, leaves, and oil. It is a bitter herb with a pleasant smell that relieves indigestion, reduces inflammation, and acts as a mild diuretic (CitationNewall et al., 1996). CitationKamal (1975) has stated that celery is an aphrodisiac, emmenagogue, and carminative used by ancient Greco-Arab physicians; it is still used by Unani and Ayurvedic medical practitioners for stomach and kidney disorders. The whole plant is gently stimulant, nourishing, and restorative for weak conditions. The leaves and stalk of celery share the same medicinal properties of other parts of the plant. Eating fresh stalks can help stimulate milk flow, while seeds are mainly used as a diuretic, which help clear toxins from the system, so are especially good for gout and other joint diseases (CitationOdy, 1993). Celery is known to possess antiflatulent and antispasmodic properties. It is used in bronchitis, asthma, and to some extent for liver and spleen diseases; it is also used as an antispasmodic and as an ingredient in salad (CitationKapoor, 1990).
Celery seed is generally regarded as safe (GRAS) in the US for human consumption as a spice, natural seasoning and plant extract/essential oil (CitationJellin et al., 2000). In Germany, celery preparations are used to treat loss of appetite, general and nervous exhaustion (CitationWren, 1988). Some pharmacological effects of celery have been reported, such as vasodilatory action in rat thoracic aorta (CitationKo et al., 1991) and mosquito repellent (CitationTuetun et al., 2005). CitationSultana et al. (2005) reported that celery is a potent plant against experimentally induced hepatocarcinogenesis in Wistar rats. The present study assessed the possible antigastric ulcer, cytoprotective, and antisecretory properties of the ethanol extract of Apium graveolens in rats in order to substantiate the traditional Unani, Arab, and Ayurvedic medicine practitioners’ claim of its use in stomach disorders.
Materials and methods
Plant material and extraction
The aerial parts of fresh celery used in this study were purchased from the local vegetable market of Riyadh, and identified by an expert taxonomist, Atiqur Rahman. A voucher specimen (77262) was deposited in the Medicinal, Aromatic and Poisonous Plants Research Center of this college for future reference.
The shade-dried aerial parts (500 g) of celery were coarse powdered and macerated in 3 L of 96% ethanol for 72 h using the percolation method. The solvent was then removed at 40°C under reduced pressure in a Rotavapor (yield 5.2%). The extract was suspended in distilled water before administration.
Animal stock
Wistar albino rats of either sex (home bred), aged 7–8 weeks and weighing 150–200 g, were obtained from the Experimental Animal Care Center, King Saud University, Riyadh, Saudi Arabia. The animals were fed Purina chow diet and water ad libitum and were maintained under standard conditions of humidity (55% ± 5%), temperature (22° ± 2°C) and light (12 h light/12 h dark cycle). The rats were randomly assigned to different control and treatment groups each containing six animals. The conduct of experiments and the procedure of sacrifice (using ether) were approved by the Ethics Committee of the Experimental Animal Care Society, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia.
The ulcerogenic drugs and necrotizing agents were freshly prepared before administration. The stomach was removed, opened along the greater curvature, washed with saline and the inner surface was examined with a 6.4 × binocular magnifier. Lesions were also assessed by two observers unaware of experimental protocol.
Gastric lesions induced by the drugs used in this study were multiple in each stomach. They were evaluated singly according to their dimensions and severity and scored between 0 (no visible ulcers) and 10 (deep lesion with diameter greater than 8 mm) in each stomach. The scores for each single lesion were then totaled (CitationValcavi et al., 1982).
Indomethacin-induced gastric ulcers
Indomethacin was suspended in 1% carboxymethylcellulose in water (6 mg/mL) and administered p.o. at a dose of 30 mg/kg (0.5 mL/100 g) to rats fasted for 36 h (CitationBhargava et al., 1973). Celery extract was administered orally (250 and 500 mg/kg body weight) 30 min before indomethacin. The rats were killed 6 h after indomethacin administration.
Gastric lesions induced by necrotizing agents (cytoprotection studies)
The experiments were done using Wistar male rats fasted for 36 h with access to drinking water ad libitum. The following necrotizing agents were administered orally in a volume of 1 mL: 80% ethanol, 0.2 M NaOH and 25% NaCl (CitationRobert et al., 1983). The celery extract in doses of (250 and 500 mg/kg body weight) was administered orally 30 min before the necrotizing agents treatment.
Hypothermic restraint stress-induced ulcers
The method of CitationLevine (1971) was followed. The animals were fasted for 36 h immobilized in restraint cages and placed inside a ventilated cold room, maintained at a temperature of 3° ± 1°C for 3 h. The rats were taken out of the cold room and sacrificed. Their stomachs were removed and examined for the severity of intraluminal bleeding according to the following arbitrary scale (CitationChiu et al., 1984): 0, no blood detectable; 1, thin blood follows the rugae; 2, thick blood follows the rugae; 3, thick blood follows the rugae with blood clots in certain areas; 4, extensive covering of the whole gastric mucosal surface with thick blood. After wiping off the blood with water, the ulcers in each stomach were scored as described in the protocol.
Antisecretory study
Gastric antisecretory activity was evaluated in rats according to CitationShay et al. (1945). The animals were deprived of food for 36 h with access to water ad libitum. Under light ether anesthesia, a small midline abdominal incision was made and the pylorus ligated. The wound was closed using sterile suture. The plant extract or normal saline was administered intraperitoneally immediately after pylorus ligation. Six h after pylorus ligation the animals were sacrificed by cervical displacement. The stomachs were removed from both groups (treated and control) and the gastric contents collected and centrifuged. The volume of the supernatant was measured and titratable acidity of liquid gastric content was recorded in milliequivalents per liter (mEq/L) and the titratable acidity was calculated. Each stomach was scored for ulcers as described in the protocol.
Determination of gastric wall mucus (GWM)
Gastric wall mucus was determined according to the procedure of CitationCorne et al. (1974). The glandular part of the stomach (0.5 g) was placed in 10 mL 1% Alcian blue solution in 0.16 M sodium acetate (pH 5.8) and left to stain for 2 h. The dye complex was extracted with 0.5 M MgCl2 solution, centrifuged and measured spectrophotometrically at 580 nm using a standard curve of Alcian blue.
Estimation of non-protein sulfhydryl groups (NP–SH)
Gastric mucosal NP-SH was measured according to the method of CitationSedlak and Lindsay (1968). The glandular part of the stomach was homogenized in ice-cold 0.02 M ethylene diamine tetra-acetic acid (EDTA). Aliquots of 5 mL of the homogenates were mixed in 15 mL test tubes with 4 mL distilled water and 1 mL 50% trichloroacetic acid. The tubes were shaken intermittently for 10-15 min and centrifuged at 3000 g. An aliquot of 2 mL supernatant was mixed with 4 mL 0.4 M Tris buffer, pH 8.9 and 0.1 mL 0.4% DTNB [5,5-dithio-bis-(2-nitrobenzoic acid)] was added and the sample was shaken. The absorbance was read within 5 min of addition of DTNB at 412 nm against a reagent blank with no homogenate.
Determination of malondialdehyde
The method reported by CitationUtley et al. (1967) was followed. The animals were killed 1 h after ethanol administration. The stomachs were removed and each tissue was homogenized in 0.15 M KCl (at 4°C, Potter-Elvehjem type C homogenizer) to give a 10% w/v homogenate. Aliquots of homogenate 1 mL in volume were incubated at 37°C for 3 h in a metabolic shaker. Then 1 mL of 10% aqueous trichloroacetic acid was added and mixed. The mixture was then centrifuged at 800 g for 10 min. An aliquot of 1 mL of the supernatant was removed and mixed with 1 mL of 0.67% thiobarbituric acid in water and placed in a boiling water bath for 10 min. The mixture was cooled and diluted with 1 mL of distilled water. The absorbance of the solution was then read at 535 nm. The content of malondialdehyde (nmole/g wet tissue) (index of the magnitude of lipid peroxidation) was then calculated, by reference to a standard curve of malondialdehyde solution.
Histopathological studies
The gastric tissue was fixed in 10% ethanol buffered formalin and processed through graded ethanol, xylene and impregnated with paraffin wax; sections were made by microtome. After staining with hemotoxylin and eosin stain (CitationCulling, 1974), the sections were examined under a research microscope by a person who was not aware of experimental protocols. The different histopathological indices were screened.
Phytochemical screening
A preliminary phytochemical screening of the aerial parts of celery was conducted to determine the presence or absence of alkaloids, cardiac glycosides, flavonoids, tannins, coumarins, anthraquinones, saponins, volatile oil, volatile bases, cyanogenic glycosides, glucosinolates and sugars, according to the methods described by CitationFarnsworth (1966).
Determination of acute toxicity and LD50 in mice
Swiss albino mice were divided into various groups (n = 10 animals per group). Each animal in each group was orally treated with a single dose of celery extract in the dose range 0.25g-12 g per kg. Following treatments, the animals were observed for 6 continuous h and thereafter at intervals of 12 h for up to 72 h. All behavioral changes and mortality during the observation period were recorded. The percentage of death in each group was then calculated. The LD50 was then determined using the methods outlined by CitationPaget (1981) and CitationGhosh (1984).
Statistical analysis
The readings showed are mean ± standard error of mean. The means of treatment and control groups were statistically compared by using ANOVA, followed by Tukey-Kramer post-hoc test.
Results
Effect on indomethacin-induced gastric ulcers
Celery extract produced a dose-dependent significant protection against the ulcerogenic effect induced by indomethacin ().
Table 1. Effect of ethanol extract of celery on gastric lesion induced by indomethacin.
Effect on necrotizing agents-induced gastric lesions
In the ethanol and strong alkali-induced ulcer protocol, it was observed that the treatment with ethanol extract of celery (250 and 500 mg/kg) significantly reduced the lesion index. Although, the ulcer intensity was found to be reduced in the animal groups that received (250 mg/ kg) dose of extract in ethanol- and NaOH-induced mucosal damage, but this reduction of ulceration was statistically insignificant ().
Table 2. Effect of ethanol extract of celery on gastric lesion induced by various necrotizing agents.
Effect on hypothermic restraint stress ulcers
A highly significant reduction of ulceration in rats’ stomachs and intraluminal bleeding was recorded after celery extract pretreatment at the dose of 500 mg/kg orally ().
Table 3. Effect of ethanol extract of celery on hypothermic restraint stress induced intraluminal bleeding and gastric lesions in rats.
Effect on pyloric ligation-induced gastric ulcers
In the gastric secretion determination model, using ligated pylorus for 6 h, the treatment with celery extract (250 and 500 mg/kg, i.p.), reduced the volume of basal gastric secretion, titratable acidity and ulceration significantly in comparison with the control group ().
Table 4. Effect of ethanol extract of celery on the gastric secretion, titratable acidity and lesion in 6 h pylorus ligated Shay rats.
Effect on gastric wall mucus
An aliquot of 1 mL 80% ethanol significantly decreased the gastric wall mucus (GWM) secretion of rats from 375.88 ± 51.13 to 194.22 ± 21.92 µg/g (wet glandular tissue). Acute oral pretreatment with celery extract significantly restored the lowered value of gastric wall content of mucus at both doses (250 and 500 mg/kg) in ethanol-induced depletion of gastric wall mucus from 194.22 ± 21.92 to 333.08 ± 38.54 and 354.66 ± 20.38 µg/g wet tissue (P < 0.01; P < 0.001). These results are presented in .
Table 5. Effect of ethanol extract of celery on 80% ethanol-induced GWM, and levels of non-protein sulfhydryls (NP-SH) in rats.
Estimation of NP-SH in gastric tissue
Ethanol 80% induced a significant decrease in gastric mucosal NP-SH level. Prior treatment of animals with ethanol extract of celery significantly replenished the depleted gastric NP-SH contents in both dose groups ().
Determination of MDA in gastric tissue
As tabulated in , MDA levels in the gastric mucosa used as an index of lipid peroxidation were significantly higher in the ethanol only treated group than the control group. On the other hand, celery extract decreased significantly (at 500 mg/kg dose) the MDA content; the lower dose (250 mg/kg), however, decreased the MDA content, but insignificantly.
Table 6. Effect of ethanol extract of celery on the lipid peroxidation (MDA) level in the rat treated with 80% ethanol.
Phytochemical screening
The preliminary qualitative phytochemical screening revealed the presence of volatile oils, alkaloids (positive reaction by spraying Dragendorff’s reagent), flavonoids, saponins, tannins, coumarins, sterols, and/or triterpenes.
Determination of acute toxicity and LD50 in mice
No toxicity symptoms were observed. However, LD50 dose was found to be 7.55 g/kg, and showed a large margin of safety.
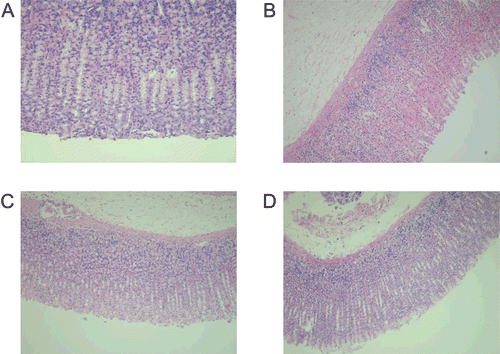
Histopathological studies
The histopathological assessment of gastric tissue substantiates the pharmacological and biochemical findings. shows the normal gastric mucosa. The oral treatment of 80% ethanol alone caused mucosal ulceration and hemorrhage (). Pretreatment of rats with celery extract in both doses (250 and 500 mg/kg) showed absence of mucosal ulceration and hemorrhage, as depicted in and , respectively.
Discussion
This study revealed a significant anti-ulcer effect of ethanol extract of Apium graveolens in experimental models of gastric lesions induced by NSAID indomethacin, various necrotizing agents, hypothermic restraint stress and pylorus ligated Shay rats.
It is well known that ulcers result from an imbalance of the interactive process of aggressive and defensive factors of the stomach (CitationBandyopadhyay et al., 2000). The primary pathology of NSAID-induced acute gastric mucosal damage is likely to be mucosal lesions due to inhibition of cyclooxygenase that prevents prostaglandin biosynthesis which in turn inhibits the release of mucus (CitationKauffman, 1989; CitationHudson et al., 1992), and reduction in gastric mucosal damage is likely to be mucosal lesions due to ischemia. The main mechanism by which NSAIDs reduce gastric mucosal blood flow is thought to be inhibition of COX–1 (CitationFunatsu et al., 2007). This inhibition leads to a deficiency in endogenous prostaglandin E2 and PGI2 which in turn cause microcirculatory disturbances in the gastric mucosa (CitationShorrock & Rees, 1989). The mechanisms involved in prostaglandin action are multiple, including stimulation of mucus and bicarbonate output, enhancement of gastric mucosal blood flow, decreasing gastric motility, and stimulation of cellular growth and repair (CitationGoulart et al., 2005). Our observations that the celery extract prevented gastric lesions induced by indomethacin, may be explained by the ability of celery extract to generate endogenous prostaglandins in stomach tissue.
In the present study, oral administration of celery extract inhibited the gastric damage caused by ethanol and strong alkali, the most commonly employed tests in the evaluation of anti-ulcer/cytoprotective activity (CitationOliveira et al., 2004). It is suggested that oxygen radicals may contribute to the induction of ethanol and other necrotizing agents-induced gastric mucosal lesions (CitationTrier et al., 1987; CitationMatsumoto et al., 1992) and anti-oxidants are protective against the damage caused by oxidants (CitationMizui et al., 1987; CitationFarina et al., 1998). Ethanol has been shown to deplete the level of non-protein sulfhydryl content in stomach tissues and its restoration appears to be important in gastroprotection since they provide a substrate for free radicals to replenish GSH stores (CitationTrier et al., 1987). The celery extract in the present investigation significantly restored the NP-SH contents of rat stomach. Therefore, it is suggested that celery extract prevention of the ethanol-induced gastric damage may at least, in part, be due to its anti-oxidant mechanism. The chemical constituents of celery responsible for antiulcer activity are not known. However, many naturally occurring flavonoids and tannins have been shown to possess antigastric ulcer activity (CitationIslam et al., 2002). The preliminary qualitative phytochemical evaluation of celery showed the presence of flavonoids, tannins, volatile oils, saponins, alkaloids, sterols and/or triterpenes (CitationAl-Howiriny et al., 2003). The antioxidant properties of flavonoids and tannins have been related to antiulcer activity (CitationHodek et al., 2002) since free radicals are developed in gastric mucosal lesions (CitationPihan et al., 1987). Flavonoids and tannins have shown cytoprotective activity in various models (CitationAl-Rehaily et al., 2002; CitationAl-Howiriny et al., 2003). Some authors (CitationRao et al., 1976; CitationSairam et al., 2001) have reported an antiulcer effect for saponins.
Celery extract significantly reduced the intensity of gastric ulceration induced by hypothermic restraint-stress. Gastric ulceration induced by stress is probably mediated by histamine release and vagal over-activity (CitationGrijalva & Novin, 1990) with enhancement in acid secretion and a reduction in mucus production (CitationSenay & Levine, 1967).
Moreover, gastric acid is an important factor for the genesis of ulceration in pylorus-ligated rats (CitationShay et al., 1945). Vagal activation by stimulation of pressure receptors in the antral gastric mucosa in the hypersecretion model of pylorus ligature is believed to increase gastric acid secretion (CitationBaggio et al., 2003). The current data clearly demonstrated that celery extract not only reduced basal gastric acid secretory volume but also the titratable acidity and ulceration. Gastric wall mucus is thought to play an important role as a defensive factor against gastrointestinal damage (CitationMarhuenda et al., 1993). Gastric wall mucus was used as an indicator for gastric wall mucus secretion (CitationMersereau & Hinchey, 1982). It was observed in the present investigation that celery extract has caused a significant elevation of gastric wall mucus that has been depleted by 80% ethanol in rats. This further confirms the capacity of celery to prevent and/or ameliorate the effects of damaging agents. This finding indicates that celery extract preserves gastric mucus secretion and strengthens the defense factors of gastric mucosa in experimental rats (CitationDavenport, 1968; CitationGuth, 1972).
Our results showed a significant reduction in NP-SH content of gastric mucosa after ethanol administration. Sulfhydryl compounds have been significantly implicated in maintenance of gastric integrity, particularly when reactive oxygen species are involved in the pathophysiology of tissue damage (CitationKimura et al., 2001; CitationNatale et al., 2004). Pretreatment of rats with celery extract significantly prevented NP-SH depletion. Non-protein sulfhydryls are known to be involved in protecting gastric mucosa against various noxious chemicals (CitationSzabo et al., 1981). On the other hand, celery extract exhibited a marked reduction of lipid peroxidation induced by ethanol administration. It significantly decreased malondialdehyde concentration in the rat stomach tissue. MDA is one of the end products resulting from peroxidation of polyunsaturated fatty acids and related esters within cell membranes, and measurement of this substance represents a suitable index of lipid peroxidation (CitationKwicien et al., 2002). Recent reports indicate that lipid peroxidation was prevented by flavonoids (CitationKahraman et al., 2003; CitationAlqasoumi et al., 2008).
In order to confirm the results of the in vivo experiment, the stomachs were also evaluated by histopathological means. In histological examination, the gastric mucosa of rats revealed that the ethanol treatment caused hemorrhagic necrosis. Celery extract pretreatment exerted no ulceration or hemorrhage in the gastric antrum. These findings are supportive to the results obtained in pharmacological and biochemical parameters.
In conclusion, the results of the present study show that the ethanol extract of Apium graveolens displays gastroprotective activity, as demonstrated by its significant inhibition of the formation of ulcers induced by different experimental models, and its ability to decrease basal gastric acid secretion. This gastric antiulcer capacity of celery extract could be related to its antioxidant properties, resulting in reduction of the lipid peroxidation and elevation of the NP-SH contents, in addition to improving the mucus coat of the stomach. Therefore, we suggest that due to its antioxidative effects, it may be useful in the prevention of gastric disorders.
Acknowledgements
Nazam Ansari and Malik Sawood Ahmed for his technical assistance.
Declaration of interest
The authors appreciate a grant awarded by the College of Pharmacy, Research Center (grant CPRC 201), Mohd.
References
- Al-Howiriny T, Al-Sohaibani M, ElTahir KEH, Rafatullah S (2003): Prevention of experimentally induced gastric ulcers in rats by an ethanolic extract of Parsley Petroselinum crispum. Am J Chinese Med 31: 699–711.
- Alqasoumi S, Howiriny T, Al-Yahya MA, Rafatullah S (2008): Gastroprotective effects of radish Raphanus sativus L. on experimental gastric ulcer models in rats. Farmacia 56: 204–214.
- Al-Rehaily AJ, Al-Said MS, Al-Yahya MA, Mossa JS, Rafatullah S (2002): Ethnopharmacological studies on allspice (Pimenta dioica) in laboratory animals. Pharm Biol 40: 200–205.
- Baggio CH, Freitas CS, Rieck L, Marques MCA (2003): Gastroprotective effects of a crude extract of Baccharis illinita DC in rats. Pharmacol Res 47: 93–98.
- Bandyopadhyay D, Biswas K, Bandyopadhyay U, Reiter RJ, Banerjee RK(2000): Melatonin protects against stress-induced gastric lesions by scavenging the hydoxy radicals. J Pineal Res 29: 143–151.
- Bandyopadhyay SK, Pakrashi SC, Pakrashi A (2007): The role of antioxidant activity of Phyllanthus emblica fruits on prevention from indomethacin induced gastric ulcer. J Ethnopharmacol 70: 171–176.
- Beil W, Birkholz C, Sewing KFR (1995) Effects of flavonoids on parietal cell acid secretion, gastric mucosal prostaglandin production and Helicobacter pylori growth. Arzneim Forsch (Drug Res) 45: 697–700.
- Bhargava KP, Gupta MB, Tangri KK (1973): Mechanism of ulcerogenic activity of indomethacin and oxyphenbutazone. Eur J Pharmacol 22: 191–195.
- Brodie DA, Marshall RW, Warrington PJ, Ashwood-Smith MJ, Poulton GA (1986): Oxypeucedarin a major furocoumarin in parsley, Petroselinum crispum. Plant Med J Med Plant Res 6: 462–464.
- Chiu PJS, Gerhart C, Brown AD, Barnett A (1984): Effects of a gastric antisecretory cytoprotectant 2-methyl-8(phenylmethoxy)imidazo (1,2-a)-pyridine-3-acetonitrile (Sch 28080) on cysteamine, reserpine and stress ulcers in rats. Arzneim Forsch 34: 783–786.
- Corne SJ, Morrissey SM, Woods RJ (1974): A method for the quantitative estimation of gastric barrier mucus. J Physiol 242: 116–117.
- Culling CFA (1974): Handbook of Histopathological and Histochemical Techniques, third edition. London, Butterworth, pp. 73, 126 and 159.
- Davenport HW (1968): Destruction of gastric mucosal barrier by detergents and urea. Gastroenterology 54: 175–180.
- Farina C, Pinza M, Pifferi G (1998): Synthesis and anti-ulcer activity of new derivatives of glycyrrhetic, oleanolic and ursolic acids. I Farmac 53: 22–32.
- Farnsworth NR (1966): Biological and phytochemical screening of plants. J Pharm Sci 55: 225–276.
- Funatsu T, Chono K, Hirata T, Keto Y, Kimoto A, Sasamata M (2007): Mucosal acid causes gastric mucosal microcirculatory disturbance in nonsteroidal anti-inflammatory drug-treated rats. Eur J Pharmacol 544: 53–59.
- Ghosh MN (1984): Fundamentals of Experimental Pharmacology. Calcutta, Scientific Book Agency, pp. 153-158 and 187-189.
- Goulart YCF, Sela VR, Obici S, Vanessa J, Martin C, Otobone F, Cortez DA, Audi EA (2005): Evaluation of gastric antiulcer activity in a hydro-ethanolic extract from Kielmeyera coriacea. Brazilian Arch Biol Tech 48: 211–216.
- Grijalva CV, Novin D (1990): The role of hypothalamus and dorsal vagal complex in gastrointestinal function and pathophysiology. Ann New York Acad Sci 597: 227–243.
- Guth PH (1972): Gastric blood flow in restraint stress. Dig Dis 17: 807–813.
- Halliwell B (1991): Introduction to free radicals in human disease. Saudi Med J 12: 13–19.
- Hodek P, Trefil P, Stiborova M (2002): Flavonoids – potent and versatile biologically active compounds interacting with cytochromes P450. Chem Biol Interact 139: 1–21.
- Hudson N, Hawthorne AB, Cole AT, Jones PD, Howley CJ (1992): Mechanism of gastric and duodenal damage and protection. Hepatogastroenterology 39: S31–36.
- Islam MW, Zakaria MNM, Radhakrishnan R, Kamil M, Chan KC, Al-Attas A (2002): Effect of Teucrium stocksianum on gastric ulceration and secretion in rats. Pharm Biol 40: 216–220.
- Jellin JM, Gregory P, Batz F, Hitchens K (2000): Pharmacist’s Letter/Prescriber’s Letter Natural Medicines Comprehensive Database, third edition. Stockton, CA, Therapeutic Research Faculty, pp. 249–250.
- Kahraman A, Erkasap N, Koken T, Serteser M, Aktepe F, Erkasap S (2003): The antioxidative and antihistaminic properties of quercetin in ethanol-induced gastric lesions. Toxicology 183: 133–142.
- Kamal H (1975): Encyclopaedia of Islamic Medicine. Cairo: General Egyptian Book Organization, p. 64.
- Kapoor LD (1990): CRC Handbook of Ayurvedic Medicinal Plants, Boca Raton, FL, CRC Press, p. 44.
- Kauffman G (1989): Aspirin induced gastric mucosal injury: Lesson learned from animal model. Gastroenterology 96: 606–614.
- Kimura M, Goto S, Ihara Y, Wada A, Yahiro K, Niidome T, Aoyagi H, Hirayama T, Kondo T (2001): Impairment of glutathione metabolism in human gastric epithelial cells treated with vacuolating cytotoxin from Helicobacter pylori. Microb Pathol 31: 29–36.
- Kitagawa H, Fujwara M, Osumi Y (1979): Effect of water immersion stress on blood flow in rats. Gastroenterology 77: 298–302.
- Ko FN, Huang TF, Teng CM (1991): Vasodilatory action mechanism of apigenin isolated from Apium graveolens in rat thoracic aorta. Biochim Biophys Acta 1115: 69–74.
- Koo MWL, Ogle CW, Cho CH (1986): Effect of verapamil, carbonexolone and N-acetylcysteine on gastric wall mucus and ulceration in stress rats. Pharmacology 32: 326–334.
- Kwicien S, Brzozowski T, Konturek SJ (2002): Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J Physiol Pharmacol 53: 39–50.
- Levine RJ (1971): A method for rapid production of stress ulcers in rats, in:Pfeiffer CJ ed., Peptic Ulcer. Munksgaard, Copenhagen, pp. 92–97.
- Marhuenda E, Martin MI, Alarcon de la Lastra C (1993): Antiulcerogenic activity of aescine in different experimental models. Phytother Res 7: 13–16.
- Matsumoto T, Moriguchi R, Yamada H (1992): Role of polymorphonuclearleucocytes and oxygen-derived free radicals in the formation of gastric lesions induced by HCl/ethanol, and a possible mechanism of protection by antiulcer polysaccharide. J Pharm Pharmacol 45: 535–539.
- Mersereau WA, Hinchey EJ (1982): Role of gastric mucosal folds in formation of focal ulcers in rats. Surgery 91: 150–155.
- Mizui T, Shimono N, Doteuchi M (1987): A possible mechanism of protection by polyamines against gastric damage induced by acidified ethanol in rats: Polyamine protection may depend on its antioxidative properties. Jpn J Pharmacol 44: 43–50.
- Natale G, Lazzeri G, Lubrano V, Colucci R, Vassalle C, Fornai M, Blandizzi C, del Tacca M (2004): Mechanisms of gastroprotection by lansoprazole pretreatment against experimentally induced injury in rats: Role of mucosal oxidative damage and sulfhydryl compounds. Toxicol Appl Pharmacol 195: 62–72.
- Newall CA, Anderson LA, Phillipson JD (1996): Herbal Medicines: A Guide for Health-Care Professionals. London, Pharmaceutical Press, pp. 65–66.
- Ody P (1993): The Herb Society’s Complete Medicinal Herbal, London, Dorling Kindersley, p. 57.
- Oliveira FA, Vieira-Junior G, Chaves MH, Almeida FRC, Florencio MG, Lima RCP, Silva RM, Santos FA, Rao VSN (2004): Gastroprotective and anti-inflammatory effects of resin from Protium heptaphyllum in mice and rats. Pharmacol Res 49: 105–111.
- Paget E (1981): The LD50 test. Acta Pharm Toxicol 52: S1–14.
- Pihan G, Regill C, Szabo S (1987): Free radical and lipid peroxidation in ethanol or aspirin-induced gastric mucosal injury. Dig Dis Sci 32: 1395–1401.
- Rao CB, Rao TN, Suryaprakasam S (1976): Cardamonin and alpinetin from seeds of Amomum subulatum. Planta Med 29: 391–392.
- Robert A, Nezamis C, Lancaster JP, Davis SO, Hanchar AJ (1983): Mild irritants prevent gastric necrosis through adaptive cytoprotection mediated by prostaglandins. Am J Physiol 245: G113.
- Sairam K, Rao VC, Babu DM, Goel RK (2001): Prophylactic and curative effects of Bacopa monniera in gastric ulcer models. Phytomedicine 8: 423–430.
- Sedlak J, Lindsay RH (1968): Estimation of total protein bound and nonprotein sulfhydryl group in tissue with Ellman’s reagents. Analyst Biochem 25: 192–205.
- Senay EC, Levine RL (1967): Synergism between cold and restraint for rapid production of stress ulcers in rats. Proc Soc Exp Biol Med 124: 1221–1231.
- Shay H, Komarov SA, Fels SE, Mcraze D, Gruenstin M, Siplet H (1945): A simple method for the uniform production of gastric ulceration in the rat. Gastroenterology 5: 43–61.
- Shorrock CJ, Rees WD (1989): Mechanisms of gastric damage by nonsteroidal anti-inflammatory drugs. Scand J Rheumatol 78: 5–11.
- Sultana S, Ahmed S, Jahangir T, Sharma S (2005): Inhibitory effect of celery seeds extract on chemically induced hepatocarcinogenesis: Modulation of cell proliferation, metabolism and altered hepatic foci development. Cancer Lett 221: 11–20.
- Szabo S, Trier JS, Frankel PW (1981): Sulfhydryl compounds may mediate gastric cytoprotection. Science 214: 200–202.
- Trier JS, Szabo S, Allen CH (1987): Ethanol-induced damage to mucosal capillaries of rat stomach. Ultra-structural features and effects of prostaglandin E2 and cystamine. Gastroenterology 92: 13–22.
- Tuetun B, Choochote W, Kanjanapothi D, Rattanachanpichai E, Chaithong U, Chiwong P, Jitpakdi A, Tippawangkosol P, Riyong D, Pitasawat B (2005): Repellent properties of celery. Apium graveolens L., compared with commercial repellents, against mosquitoes under laboratory and field conditions. Trop Med Intl Health 10: 1190–1198.
- Utley HG, Bernheim F, Hochstein P (1967): Effect of sulfhydryl reagents on peroxidation in microsomes. Arch Biochem Biophys 118: 29–32.
- Valcavi U, Caponi R, Brambilla A, Palmira F, Fuwagali R (1982): Gastric anti-secretory, anti-ulcer and cytoprotective properties 9-hydroxy-19,20-bis-nor-prostanoic acid in experimental animals. Arzneim Forsch 32: 657–663.
- Wren RC (1988): Potter’s New Cyclopaedia of Botanical Drugs and Preparations. Saffron Walden, C.W. Daniel, pp. 1–384.