Abstract
YZ-50 is an active fraction obtained from the root of Polygala tenuifolia Willd. (Polygalaceae) extract and it has been reported previously to exert beneficial effects on mental health in depressed sufferers, however, its mechanism of action remains unresolved. This study utilized the chronic mild stress (CMS) model of depression in Sprague-Dawley rats to evaluate the effects of YZ-50 on depressive behaviors. Furthermore, we tested the hypothesis that the capacity of YZ-50 to reverse the harmful effects of CMS is relative to the hypothalamo-pituitary-adrenal (HPA) system and brain-derived neurotrophic factor (BDNF) in the hippocampus. Repeated administration of YZ-50 for 28 days at the doses of 140 and 280 mg/kg in CMS, YZ-50 reversed the CMS-induced changes in sucrose consumption, plasma corticosterone levels and open field activity. In addition, CMS significantly decreased hippocampal BDNF mRNA levels. However, YZ-50 counteracted a decrease in hippocampal BDNF mRNA caused by CMS. In conclusion, YZ-50 reversed the harmful effects of CMS on mood and behaviors in rats and it possesses an antidepressant property that is at least in part mediated by the neuroendocrine and neuropropective systems, and it is likely that the HPA system plays an important role in this process.
Introduction
Major depression is a common disorder, and its prevalence is increasing. The World Health Organization estimates that by 2020 unipolar major depression will become the second largest cause of global disease problems in the world, only behind ischemic heart disease (CitationMurray et al., 2001).
It is well known that the abnormality of neuroendocrine and neuroprotective function plays an important role in the occurrence of depression. The most frequently occurring neuroendocrinological abnormality in depressed subjects is hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis characterized by hypersecretion of corticotrophin-releasing factor (CRF), which stimulates adrenocorticotrophic hormone (ACTH) release (CitationTsigos & Chrousos, 2002; CitationBarden, 2004), and thus elevates serum corticosterone (CORT) levels in depressive disorders (CitationO’Brien et al., 2004). Moreover, recent studies have indicated that the brain-derived neurotrophic factor (BDNF), expressed at high levels in the hippocampus (CitationConner et al., 1997), is also involved in the pathogenesis of depression. This decrease in BDNF expression can lead to a reduction of hippocampal volume and vulnerability to subsequent episodes of depression as a result of decreased neurogenesis through the neuroprotective systems (CitationDuman et al., 2000; CitationNestler et al., 2002a, Citation2002b).
Polygalae radix (yuan zhi in Chinese) is the dried root of Polygala tenuifolia Willd. (Polygalaceae), and has been used in traditional medicine as an expectorant, tonic, tranquilizer and antipsychotic agent (CitationChung et al., 1992; CitationHuang, 1993). The dried root of this plant contains polygalitol, tenuigenin, polygalasaponin, oligosaccharides (CitationYukinobu et al., 2004) and xanthone derivatives. Recently our laboratory demonstrated antidepressant actions of the active extracted fraction YZ-50 in animal behavior despair models of mice; however, the mechanisms of its action are still unresolved.
Chronic mild stress is a widely used animal model for antidepressant screening, and this model has made it possible conduct studies investigating the etiology of depression and the mechanisms of action of chronic anti-depressive treatments (CitationGarcia, 2002). More importantly, chronic stress procedure also decreases neurogenesis and neuroendocrinological abnormality in the adult hippocampus (CitationGould et al., 1997; CitationTanapat et al., 1998; CitationCzeh et al., 2001). This study further confirmed the antidepressant effects of YZ-50 in a CMS model of depression in Sprague-Dawley rats, and investigated whether YZ-50 influences the HPA axis function and the hippocampal BDNF mRNA correlated with neuroendocrine and neuroprotective in the stressed animals, in order to evaluate its mechanism of action.
Materials and methods
Animals
Male Sprague-Dawley rats, weighing 150-200 g, were used. Rats were acclimated to their surroundings for 1 week before experimentation and were individually housed in a temperature (22° ± 2°C), humidity (55% ± 10%), and light (12 h light/12 h dark cycle; lights on at 7 a.m.) controlled environment and were fed food and water ad libitum. All animal experiments were performed in accordance with the local, international and institutional guidelines. The experiments in the present study were designed to minimize the number of animals used and their suffering.
Preparation of the extracts
The voucher specimen of the P. tenuifolia, identified by Ping Liu and registered under the number NU-80617 was preserved at the Herbarium of the Traditional Chinese Medicinal (TCM) Pharmacy, Chinese People’s Liberation Army (PLA) General Hospital. The air-dried rhizomes of P. tenuifolia (965.27 g) were extracted with 8:l(v/v) of 60% EtOH hot water for 2 h, this procedure was repeated twice. The extracts were then filtered and concentrated in vacuum into residues and lyophilized into powder to get the YZE (201.5 g) fraction. This was then suspended, and the liquid material was subjected to a macroporous resin column (1300 Version) and eluted with 50% EtOH, (v/v) to yield the fraction 50 (YZ-50, 48.4 g), the yield of the extract was 5.014% (w/w).
Drug administration
YZ-50 was dissolved in 0.9% (v/v) saline after being dispersed with Tween 80, the final concentration of Tween 80 was less than 0.1%. The selective serotonin and norepinephrine reuptake inhibitor, desipramine (Sigma, St. Louis), was used at the dosage of 20 mg/kg as a reference control. Drugs and vehicle were administered to the rats via gastric intubations at different dosages and twice daily from day 1 to day 28 of the period of chronic mild stress. The doses of these drugs in this study were 140 and 280 mg/kg respectively and had been determined previously ( CitationHuang et al., 2007). The control group received the same volume of the dosing vehicle (saline with Tween 80).
Chronic mild stress procedure
Before CMS procedure was induced, the rats were trained to consume a 1% (w/v) sucrose solution. Training consisted of initial exposed to only sucrose for 72 h continuously without any food or water available. After the period of adaptation, the tests involved a 14-h period of food and water deprivation followed by the offering of a sucrose solution for 1 h. At the end of each test, sucrose intake was measured by the volume of sucrose remaining in the bottles. On the basis of their sucrose intakes in the final baseline test, the animals were randomly divided into five groups (n = 12 in every group) having similar average intake, the control group, the unstressed control group, the CMS with vehicle control group, the CMS with desipramine group and two different dosages of YZ-50 group. Subsequently, sucrose consumption was monitored under similar conditions in 1 h tests (11:00-12:00 h), at 1 and 28 days in the CMS procedure. Some groups were subjected to the chronic mild stress (CMS-treated animals) procedure. The CMS procedure was slightly modified from that previously described by CitationWillner et al. (1987) and CitationPapp et al. (2002). The rats in the experimental groups were subjected to CMS for 28 days. The CMS procedure consisted of a variety of unpredictable mild stressors including one period (2 h) of paired caging, one period (3 h) of tilted cage (45°), one period of food and water deprivation (18 h), one period of (15 min) shaking, one period of (1 h) exposure to an empty bottle, one 21 h period with wet cage (200 mL water in 100 g sawdust bedding) and one period with 36 h of continuous light. Thus, stressors were presented both during the rats’ active (dark) period and during the inactive (light) period. These stressors were randomly scheduled over a one-week period and repeated throughout the 28-day experiment. In contrast to other previous procedures in rats, nociceptive stressors were excluded, and only environmental and social disturbances were applied (CitationPardon et al., 2000). The non-stressed control animals were housed in normal conditions.
Open field test
The open field test (OFT) evaluated the general locomotor and exploratory behavior of rats and the experiments were performed as described previously (CitationKennett et al., 1985). Each rat was placed at the centre of the open field (80 cm square chamber, 40 cm high walls, light of 80 lux with its floor divided into 25 equal squares) for 3 min in a quiet room after being weighed. Parameters assessed were the time in the centre square, the number of crossing squares, and the times of rearing. The next test was performed after the chamber had been cleaned.
Blood and organ collection
On the day following the end of the CMS period, 12 rats per group were anaesthesia (between 9 and 11 a.m.) and trunk blood was collected and then processed with the serum being collected and stored at -20°C for CORT and ACTH measurement. Brains were also removed rapidly and the hippocampus was then micro-dissected and frozen on dry ice and subsequently stored at -80°C for RT-PCR analysis. Tissues (pituitary area and adrenal gland, about 30 mg) were boiled for 3 min in 1 mL saline solution, and then homogenized in 0.5 mL of 1 M acetic acid at 4°C using an ultrasonic cell disrupter, set at 4°C for 1 h. At the end of this period, 0.5 mL of 1 M NaOH was added and centrifuged at 10,000 rpm for 10 min at 4°C (CitationVale et al., 1983; CitationTang et al., 1995). The clear supernatants were collected and stored at -80°C until assayed for CORT or ACTH and the CORT and ACTH levels were measured by a radioimmunoassay kit (ICN Biomedicals, Costa Mesa, CA).
BDNF mRNA in hippocampus isolation and RT-PCR analysis
Total RNA from the hippocampus was extracted using TRIzol® reagent in accordance with the manufacturer’s instructions. The RNA product was resuspended in 20 μL diethyl pyrocarbonate (DEPC)-treated water. The quality of RNA was judged from the pattern of ribosomal RNA after electrophoresis of RNA through 1.5% agarose gel containing ethidium bromide (EB), and visualization was performed by UV illumination with the RNA being stored at -80°C until use. Total RNA was reverse transcribed to cDNA with the use of the Rever Tra Ace-α-R kit (Toyobo Biotech, Osaka), according to the manufacturer’s protocol. Following the RT reaction, the cDNA was amplified using adequate primers and the BDNF primers were: forward, 5′-TCCCTGGCTGACACTTTTGAG-3′; reverse, 5′-ATTGGGTAGTTCGGCATTGCG-3′.
As a control to eliminate variations for sample-to-sample differences in RNA extraction and conversion to cDNA, the housekeeping gene β-actin was amplified. β-actin primers were: forward, 5′-CGTCTGGACCTGGCTGGCCGGGACC-3′; reverse, 5′-CTAGAAGCATTTGCGGTGGACGATG-3′. The PCR reaction was carried out with the following cycle parameters: BDNF: 94°C, 1 min; 94°C, 30 s; 55°C, 30 s; 72°C, 30 s, 25 cycles; 72°C, 2 min extension. β-actin: 94°C, 1 min; 94°C, 30 s; 55°C, 30 s; 72°C, 30 s, 25 cycles; 72°C, 2 min extension. Amplified products were separated on 1.5% agarose gels, stained with ethidium bromide and photographed under UV illumination with gel-documentation system. Results were evaluated as a relative unit determined by normalization of the optical density (OD) of BDNF to that of the β-actin band. The density of the products was quantitated using Quantity One, version 4.2.2 software (Bio-Rad, Hercules, CA).
Data analysis
The data was analyzed by two-way ANOVA and tests of significant differences were determined by Tukey’s at *P <0.05 and **P <0.01. All data are expressed as means ± standard deviation (SD) and are given in the symbols and columns in the figures.
Results
Body weight
As shown in , the CMS used significantly affected the body weight of the animals. Stressed animals gained significantly less weight than non-stressed objects, and a more pronounced effect was seen in vehicle-treated stressed groups. As compared to vehicle-treated stressed groups, body weights of stressed animals were significantly affected by YZ-50 at the dosage of 280 mg/kg at the end of the treatment period.
Sucrose test
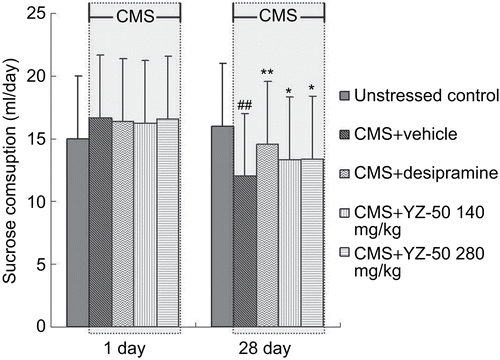
In the sucrose solution training phase (baseline phase), sucrose consumption did not differ significantly among the groups. CMS gradually reduced the consumption of the sucrose solution. As compared to the 16.9 mL/day intake in the baseline test, 28 days later the sucrose intake was reduced to 12.1 mL/day in the CMS-stressed animals, while treatment with YZ-50 and desipramine caused a gradual recovery of the sucrose intake (). At the end of 28 days and thereafter the 1 h of sucrose solution taken by the stressed animals receiving YZ-50 (280 mg/kg) was significantly higher than that of the vehicle-treated stressed animals. It appears that YZ-50 might be more efficacious in restoring the sucrose intake in CMS-stressed animals.
Figure 2. Effects of YZ-50 on the sucrose consumption of rats exposed to chronic mild stress (mean ± SD, n = 12). Chronic treatment with YZ-50 (140 or 280 mg/kg) was given during 28 days of chronic mild stress procedure. Data are expressed as means ± SD (n = 12). ##P <0.01 compared with unstressed control group; *P <0.05, **P <0.01 compared with vehicle + CMS group.
Open field test
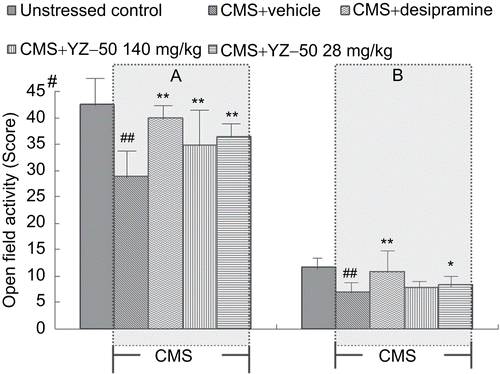
The CMS-stressed animals showed differences in activity compared with non-stressed control animals in the 3-min open field test (). After 28 days of treatment with YZ-50 or desipramine, behavioral changes in locomotor activity showed a greater number of crossing squares and more rearing on open field performance of the animals at the dose range used in the present study.
Figure 3. Effects of YZ-50 on open field behavior during the 28-day study period in the chronic mild stressed rats. (A) The number of crossings during the test session and (B) the times of rearing during the test session. Chronic treatment with YZ-50 (140 and 280 mg/kg) was given during 28 days chronic mild stress procedure. Data are expressed as means ± SD (n = 12). ##P <0.01 compared with unstressed control group; *P <0.05, **P <0.01 compared with vehicle + CMS group.
Plasma CORT and ACTH level
As shown in , the plasma CORT and ACTH level of the CMS-treated rats was significantly higher than that of the unstressed control (170 versus156 ng/mL F1, 22 = 6.78, p <0.01; 34.75 versus 23.62 pg/mL, F1, 22 = 12.52, p <0.01). Repeated treatment with desipramine (20 mg/kg) or YZ-50 (280 mg/kg) for 28 days significantly decreased the elevated CORT and ACTH level. Meanwhile, the pituitary area ACTH and adrenal gland CORT levels also increased in CMS-treated rats and YZ-50 (140 and 280 mg/kg) produced a significant decrease in ACTH and CORT in these tissues.
Table 1. Effects of YZ-50 on the plasma and tissue corticosterone (CORT) or adrenocorticotrophic hormone level (ACTH) of chronic mild stress (CMS) rats (mean ± S.D., n = 12).
BDNF level in hippocampus
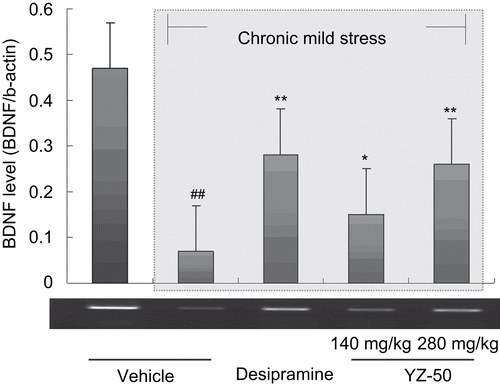
BDNF mRNA level in the hippocampus was determined by RT-PCR and the corresponding bands in electrophoresis were semi-quantitatively calculated. RT-PCR of BDNF with control template β-actin in the hippocampus yielded the products of expected length: 456 base pair (bp) for brain-derived neurotrophic factor, 600 bp for β-actin. In the present study, the average BDNF level decreased in the hippocampus of rats exposed to chronic mild stress (F1, 18 = 23.61 P <0.01) compared with unstressed control animals, which is shown as the decreasing gray value (). After 28 days repeated administration of YZ-50 or desiprimine (20 mg/kg), the reduction of BDNF expression was reversed, and the effect of YZ-50 (280mg/kg) was significant and is comparable to the positive control (F1, 18 = 3.61 P >0.05).
Figure 4. Effects of YZ-50 on BDNF level of hippocampus of chronic mild stressed rats. Chronic treatment with YZ-50 (140 or 280 mg/kg) was given during 28 days chronic mild stress procedure. Data are expressed as means ± SD (n = 7). ##P <0.01 compared with unstressed control group; *P <0.05, **P <0.01 compared with vehicle + CMS group.
Discussion
The CMS model of depression involves the presentation of a series of varied and unpredictable environmental stressors, such as food and water deprivation, wet cages and light-dark reversal. Following such exposure, animals have been reported to exhibit a persistent reduction in responsiveness to pleasurable stimuli, measured by a decrease in their consumption of 1% sucrose solution (CitationD’Aquila et al., 1994). Reductions in sucrose consumption produced by CMS procedure have been shown to be reversed by chronic treatment with either tricyclic antidepressants or Selective Serotomin Reuptake Inhibitors (SSRIs) (CitationWillner, 1997). In the present study, 28 days chronic treatment with YZ-50, at the dose of 140 and 280 mg/kg, reversed CMS-induced reduction of sucrose intake indicating that administration of YZ-50 has an antidepressant-like activity in the CMS model of depression in Sprague-Dawley rats. Meanwhile, the CMS-stressed animals showed an interesting variation in locomotor activity performance in the open field test. Decreased locomotor activity has been used as an index of high emotionality in rats (CitationRoyce, 1977; CitationKatz et al., 1981), while 28 days YZ-50 administration led to the open field activity of the CMS rats to improve significantly.
In the present study, the CMS procedure we used increased CORT and ACTH levels significantly, suggesting an activation of hypothalamo-pituitary-adrenal (HPA) axis under CMS conditions. This was consistent with previous reports (CitationAyensu et al., 1995; CitationHarris et al., 1998). These results indicate that the stressed animals might show an impaired feedback regulation in the HPA axis after exposure to CMS procedure. It has been well demonstrated that chronic stress produces atrophy and dendritic arborization of CA3 pyramidal neurons (CitationMagarinos et al., 1999). This dendritic remodeling is hypothesized to be related to the prolonged HPA axis activation and the resulting elevation of excitatory amino acids and corticosteroid activation during stress (CitationMcEwen, 1992; CitationSapolsky, 1996), and can be reversed by antidepressant treatment (CitationWatanabe et al., 1992).
Depressed patients often exhibit hyperactivity in the HPA axis such as hypersecretion of basal CORT (CitationCarroll et al., 1976). The increase of CORT levels might be relative to the decrease of hippocampal BDNF mRNA as it is known that BDNF has well-established effects on neurotrophic procedure and neurogenesis (CitationBarde, 1989; CitationFossati et al., 2004). BDNF might provide these effects by inhibiting cell death cascades. Decrease of BDNF gene expression might result in hippocampus atrophy by an excess of neural loss (apoptosis) and an altered regulation of the neurotrophic processes (CitationYuan & Yankner, 2000) and be responsible for the harmful effects of CMS on spatial performance and emotion (CitationSmith et al., 1995; CitationUeyama et al., 1997; CitationSchaaf et al., 1999; CitationVellucci et al., 2001).
In the present study, the CMS-induced elevation of plasma CORT and ACTH level was reversed by chronic YZ-50 administration. Therefore, these results show that YZ-50 possesses a certain antidepressant property and its mechanism of action may be related to HPA axis, which might be associated with circulating CORT and ACTH levels. Moreover, the decrease of BDNF in stressed rats treated with YZ-50 was much less pronounced compared with rats without stress following the end of CMS, this indicates that YZ-50 can up-regulate the BDNF levels of stressed rats.
In conclusion, YZ-50 might be exerting its effects at least in part by up-regulating the level of BDNF, inhibiting hyperactivity in the HPA axis such as hypersecretion of basal CORT, and affecting the neuroendocrine and neuroprotective systems. Further studies are necessary to elucidate the active components, and their synergistic effects responsible for the antidepressant activity present in the herbal medicine extract YZ-50.
Declaration of interest
The authors are grateful to three National Natural Science Foundations of China for financial support of this study (No. 30572354, No. 90209036 and No. 30801524).
References
- Ayensu WK, Pucilowski O, Mason GA, Overstreet D, Rezvani AH, Janowsky DS (1995): Effects of chronic mild stress on serum complement activity, saccharin preference and corticosterone levels in Flinders lines of rats. Physiol Behav 57: 165–169.
- Barde YA (1989): Trophic factors and neuronal survival. Neuron 2: 1525–1534.
- Barden N (2004): Implication of the hypothalamic-pituitary-adrenal axis in the physiopathology of depression. J Psych Neurosci 29: 185–193.
- Carroll BJ, Curtis GC, Mendels J (1976): Cerebrospinal fluid and plasma free corticosteroneconcentrations in depression. Psychol Med 6: 235–244.
- Chung IW, Kim SR, Kim EG (1992): Dopamine-2 and serotonin-2 receptor bindings in antipsychotic medicines from natural products. J Korean Neuropsych Assoc 31: 856–867.
- Conner J, Lauterborn J, Yan Q, Gall C, Varon S (1997): Distribution of brain derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci 17: 2295–22313.
- Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E (2001): Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Nat Acad Sci USA 98: 12796–12801.
- D’Aquila P, Brain PF, Willner P (1994): Effects of chronic mild stress in behavioural tests relevant to anxiety and depression. Physiol Behav 56: 861–867.
- Duman RS, Malberg J, Nakagawa S, D’Sa C. (2000): Neuronal plasticity and survival in mood disorders. Biol Psych 48: 732–739.
- Fossati P, Radtchenko A, Boyer P (2004): Neuroplasticity: From MRK to depressive symptoms. Eur Neuropsychopharmacol 14: 503–510.
- Garcia R (2002): Stress, metaplasticity, and antidepressants. Curr Molec Med 2: 629–638.
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E (1997): Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci 17: 2492–2498.
- Harris RBS, Zhou J, Youngblood BD, Smagin GN, Ryan DH (1998): Failure to change exploration or saccharin preference in rats exposed to chronic mild stress. Physiol Behav 63: 91–100.
- Huang KC (1993): The Pharmacology of Chinese Herbs. Boca Raton, FL, CRC Press, p. 343.
- Huang XW, Xie TT, Wang DX (2007): Antidepr ession effect of polygala tenuifolia Willd. ZhongGuoYaoWuYingYongYuJianCe 4: 22–25 (In Chinese).
- Katz RJ, Roth KA, Carroll BJ (1981): Acute and chronic stress effects on open field activity in the rat: Implications for a model of depression. Neurosci Biobehav Rev 5: 247–251.
- Kennett GA, Dickinson SL, Curzon G (1985): Enhancement of some 5-HT dependent behavioural responses following repeated immobilization in rats. Brain Res 330: 253–263.
- Magarinos AM, Deslandes A, McEwen BS (1999): Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur J Pharmacol 371: 113–122.
- McEwen BS (1992): Re-examination of the glucocorticoid hypothesis of stress and aging. Prog Brain Res 93: 365–381.
- Murray CJL, Lopez AD, Mathers CD, Stein C (2001): The Global Burden of Disease 2000 project: Aims, methods and data sources. Geneva, World Health Organization, pp. 131–135.
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM (2002a): Neurobiology of depression. Neuron, 34: 13–25.
- Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld H (2002b): Preclinical models status of basic research in depression. Biol Psych 52: 503–528.
- O’Brien SM,Scott LV,Dinan TG (2004): Cytokines: Abnormalities in major depression and implications for pharmacological treatment. Human Psychopharm: Clin Exp 19: 397–403.
- Papp M, Nalepa I, Antkiewicz-Michaluk L, Sanchez C (2002): Behavioural and biochemical studies of citalopram and WAY 100635 in rat chronic mild stress model. Pharmacol Biochem Behav 72: 465–474.
- Pardon MC, Pérez-Diaz F, Joubert C, Cohen-Salmon C (2000): Influence of chronic ultramild stress procedure on decision-making in mice. J Psychiatry Neurosci 25: 167–177.
- Royce J (1977): On the construct validity of open field measures. Psychol Bull 84: 1098–1106.
- Sapolsky RM (1996): Stress, glucocorticoids, and damage to the nervous system: The current state of confusion. Stress 1: 1–19.
- Schaaf M, Siburg R, Duurland R, Fluttert M, Oitzl M, de Kloet E (1999): Corticosterone effects on BDNF mRNA expression in the rat hippocampus during Morris water maze training. Stress 3: 173–183.
- Smith M, Makino S, Kvetnansky R, Post R (1995): Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosc 15: 1768–1777.
- Tanapat P, Galea LA, Gould E (1998): Stress inhibits the proliferation of granule cell precursors in the developing dentate gyrus. Int J Dev Neurosci 16: 235–239.
- Tang MY, Shen WB, Zhang CL (1995): Radioimmunoassay assay of corticotrophin releasing hormone. Clin J Appl Physiol 11: 284–287.
- Tsigos C, Chrousos GP (2002): Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res 53: 865–871.
- Ueyama T, Kawai Y, Nemoto K, Sekimoto M, Tone S, Senba E (1997): Immobilization stress reduced the expression of neurotrophins and their receptors in the rat brain. Neurosci Res 28: 103–110.
- Vale W, Vaughan J, Yamamoto G, Bruhn T, Douglas C, Dalton D, Rivier C, Rivier J (1983): Assay of corticotropin releasing factor. Methods Enzymol 103: 565–577.
- Vellucci S, Parrott R, Mimmack M (2001): Down-regulation of BDNF mRNA, with no effect on TrkB or glucocorticoid receptor mRNAs, in the porcine hippocampus after acute dexamethosone treatment. Res Vet Sci 70: 157–162.
- Watanabe Y, Gould E, McEwen BS (1992): Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res 588: 341–345.
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R (1987): Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacol (Berlin) 93: 358–364.
- Willner P (1997): Validity, reliability and utility of the chronic mild stress model of depression: A 10-year review and evaluation. Psychopharmacol 134: 319–329.
- Yuan J, Yankner BA (2000): Apoptosis in the nervous system. Nature 407: 802–809.
- Yukinobu Ikeya, Shigefumi Takeda, Mitsuo Tunakawa, Humito Karakida, Kouin Toda, Takuji Yamaguchi, Masaki Aburada (2004): Cognitive improving and cerebral protective effects of acylated oligosaccharides in Polygala tenuifolia. Biol Pharm Bull 27: 1081–1085.
