Abstract
The uterine stimulatory effect of the ethanol leaf extract of Newbouldia laevis (Beauv.) Seemann ex Bureau (Bignoniaceae) was evaluated in the presence of some antagonists in vitro in an attempt to elucidate the mechanism of action of the extract. The extract was tested in the presence and absence of phentolamine (4.09 and 40.91 nM), diphenhydramine (4.45 and 44.47 nM), atropine (1.18 and 11.91 nM), and verapamil (2.03 and 20.35 nM). The effect of the antagonists on the extract and on oxytocin used as a reference drug in this study was evaluated. The EC50 and Emax were determined and statistically analyzed using one way ANOVA and Dunnett’s post hoc test. There was no significant difference in the EC50 and Emax of the extract and oxytocin in the presence of phentolamine. Diphenhydramine and atropine significantly inhibited (p <0.01) the extract but both drugs had no effect on oxytocin. However, significant differences (p <0.01) were observed in the EC50 and Emax of the extract and oxytocin in the presence of verapamil. These results suggest that the leaf extract of N. laevis contracts the uterus by opening voltage-operated calcium channels and/or by activation of muscarinic receptors.
Introduction
Drugs known to contract the uterus are generally referred to as oxytocics. They are useful clinically in induction or augmentation of labor, controlling postpartum hemorrhage, contraction stress tests and in cases where abortion is imperative (CitationDen Hertog et al., 2001). Currently available oxytocics are not without side effects, it is therefore a worthwhile venture to search for safe and effective uterine stimulatory agents.
Newbouldia laevis (Beauv.) Seeman ex Bureau (Bignoniaceae) is native to tropical Africa and grows from Guinea Savannahs to dense forests, on moist and well-drained soils. It inhabits the secondary forest extending from Senegal to Cameroon, Gabon, Democratic Republic of Congo, Angola (CitationUsman & Osuji, 2007). In Nigeria, the bark is chewed and swallowed for stomach pains, diarrhea and toothache (CitationUsman & Osuji, 2007). The plant has been found to be effective in the treatment of elephantiasis, dysentery, rheumatic swellings, syphilis, constipation, piles, and as a vermifuge for round worms. It has also been found useful for earache, sore feet, chest pain, epilepsy, and convulsion in children (CitationUsman & Osuji, 2007). The leaf, stem, and fruits have been used as a febrifuge, in wound dressing and in stomach ache (CitationUsman & Osuji, 2007). Over the years, traditional midwives in southern Nigeria have used the extract of the leaves of N. laevis to induce or facilitate labor, and traditional birth attendants in Edo state of Nigeria have used this plant for easing childbirth (personal communication). It is also used for early abortion in Edo State, Nigeria (CitationOkoli et al., 2007).
The plant N. laevis is locally known as “fertility tree” or “tree of life” in Nigeria (CitationAkaneme, 2007) due to its use in childbirth. Its ethnic names include: akoko (Yoruba); aduruku (Hausa); kontor (Tiv); ikhimi (Bini); ogirisi (Igbo); ogiriki (Urhobo).
Our unpublished data indicate that the ethanol leaf extract of N. laevis contracted the isolated rat uterus. This present study aims to investigate possible mechanisms by which the plant extract contracts the uterus.
Materials and methods
Preparation of the plant material
The leaves of N. laevis were collected in September 2007 from the premises of the University of Benin, Benin City, Nigeria. The plant was identified by B. Ayinde of the Department of Pharmacognosy where a voucher specimen has been prepared and deposited.
Extraction
The leaves were air-dried for two weeks and reduced to fine powder. The dry powder was extracted with ethanol for 24 h to give a yield of 0.0771% w/w. The ethanol extract of N. laevis (EEN) was then stored in an airtight container in the refrigerator until needed.
Animals
Adult female Sprague-Dawley rats (160-200 g) bred in the animal house of the Department of Pharmacology and Toxicology, University of Benin, Nigeria were used. The animals were maintained under standard conditions and had free access to standard diet (Ladokun Feeds, Ibadan, Nigeria) and water. They were handled according to standard guidelines for use of laboratory animals (National Institutes of Health Public Health Service Policy on Humane Care and Use of Laboratory Animals, 2002).
Drugs
Diethylstilboesterol and phentolamine were obtained from Sigma (UK), atropine from Laborate pharmaceuticals (Panipat, India); diphenhydramine from BDH chemicals (Mumbai, India); and verapamil from May and Baker (Dagenham, UK). The drugs were prepared fresh on the day of the experiment by dissolving in physiological salt solution (composition stated below) with the exception of diethylstilboesterol, which was constituted in ethanol obtained from Sigma.
Preparation of uterine tissues
The animals were administered diethylstilboesterol (0.2 mg/kg i.p.) 24 h prior to the commencement of the experiment to induce estrus. Estrus was confirmed by microscopic observation of vaginal smears and macroscopic observation of the vulva. The rats were sacrificed under diethyl ether anesthesia and uterine segments, 2 cm in length, were rapidly dissected out and freed of adhering connective tissues and fat. The segments were mounted in 40 mL organ baths containing physiological salt solution of the following composition in g per 5 L: NaCl,45; NaHCO3, 2.5; D-glucose, 2.5;KCl, 2.1; CaCl22H2O, 1.32. The lower ends of the tissues were attached to a tissue holder by means of silk suture and the upper ends to a Ugo Basile isometric force-displacement transducer (model no. 82145) (Comerio VA, Italy) connected to a Ugo Basile unirecorder (model no.7050). The solution was maintained at 37°C and continuously aerated (CitationEferekeya & Nworgu, 1985). The preparations were equilibrated for 45 min at resting tension of 0.75 g before the start of the experiment.
Effect of antagonists on the responses of the extract and oxytocin on the uterus
Concentration-response curves for oxytocin and EEN were obtained on the rat isolated uterus as described above in the presence and absence of the following antagonists phentolamine (4.09 and 40.91 nM), diphenhydramine (4.45 and 44.47 nM), atropine (1.18 and 11.91 nM), and verapamil (2.03 and 20.35 nM). The antagonists were allowed to bath the tissue for 15 min before addition of the agonists.
Statistical analysis
All values were expressed as mean ± standard error of mean (SEM) and n represents the number of rats from which uterine segments were obtained. The EC50 (concentration which produced 50% of maximum response) and Emax (maximum achievable response) were computed for each concentration-response experiment. Comparisons were made using one-way ANOVA with Dunnett’s multiple comparison tests. Statistical significance of p <0.05 was used in all cases.
Results
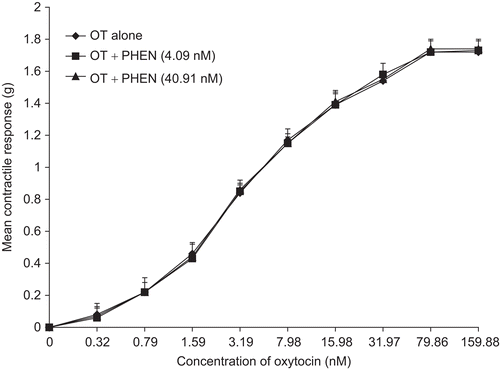
Effect of phentolamine (PHEN) on EEN- and oxytocin-induced uterine contractions
No significant inhibition of the extract or oxytocin was observed in the presence of PHEN, this was evident by the lack of significant increase in the EC50 ( and ) and Emax ( and ).
Table 1. Effect of antagonists on the EC50 of EEN.
Table 2. Effect of antagonists on the EC50 of oxytocin.
Figure 1. Concentration-response curves showing the uterine contractile response of the ethanol extract of N. laevis (EEN) in the absence and presence of phentolamine (PHEN). There was no significant difference in the Emax of EEN (n = 6 rats).
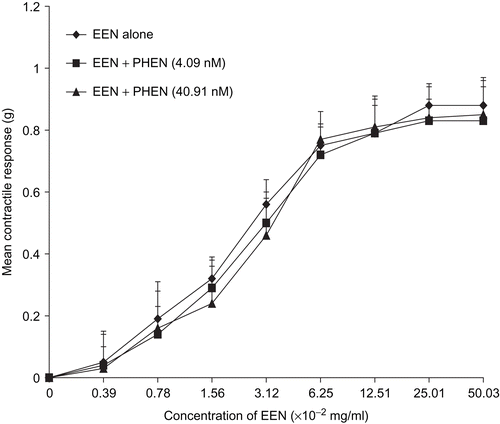
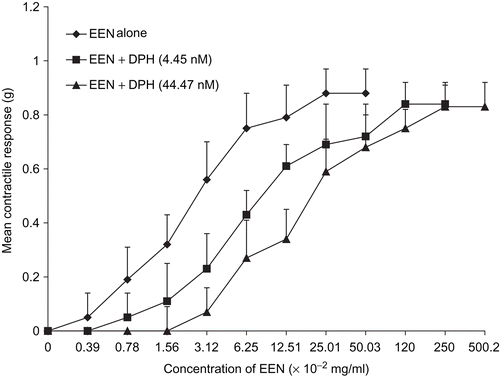
Effect of diphenhydramine and atropine on EEN- and oxytocin-induced uterine contractions
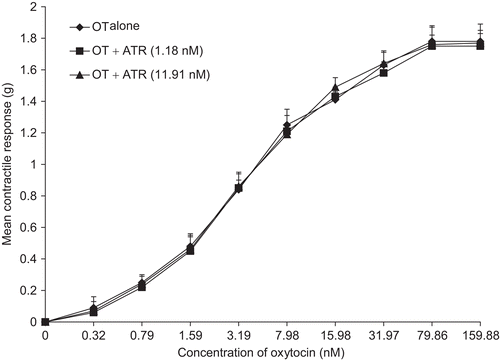
In the presence of diphenhydramine (DPH) and atropine (ATR), the contractile activity of the extract was inhibited. This was observed in the significant increases (p <0.01) in the EC50 of EEN (); however, there were no significant differences in the Emax (, respectively). There was no significant inhibition of oxytocin-induced contraction by either of the antagonists ( and , respectively).
Figure 4. Concentration-response curves of the effect of diphenhydramine (DPH) on oxytocin-induced uterine contraction. No inhibition was observed and there was no significant difference in the Emax of oxytocin (n = 6 rats).
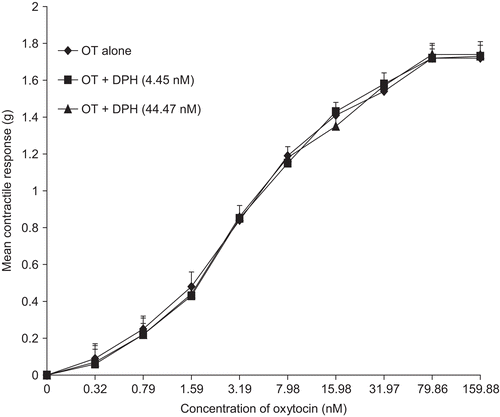
Figure 5. Concentration-response curves showing uterine response to EEN in the presence and absence of atropine (ATR). ATR inhibited the effect of the extract but there was no significant difference in the Emax (n = 6 rats).
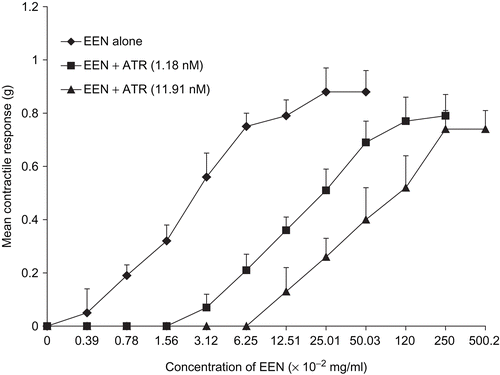
Figure 6. Concentration-response curves of oxytocin-induced uterine contraction in the presence of atropine (ATR). No inhibition was observed (n = 6 rats).
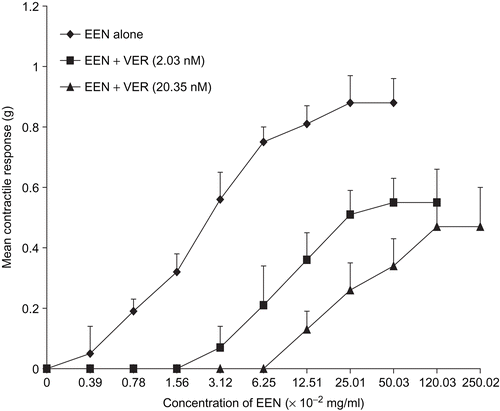
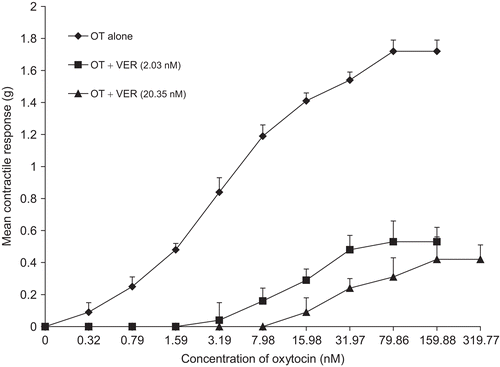
Effect of verpamil on EEN- and oxytocin induced uterine contractions
Verpamil (VER) significantly increased (p <0.01) the EC50 of EEN and oxytocin ( and ) and significantly depressed (p <0.01) the Emax ().
Discussion and conclusions
Contraction of smooth muscles in general is dependent on an increase in the concentration of free cytoplasmic calcium which activates the contractile elements. The source of activator calcium is determined by the entry and efflux of calcium and its release and re-uptake into the sarcoplasmic recticulum (CitationMatthew et al., 2004). The relative contribution of calcium from these sources to contraction depends largely on the type of smooth muscle, the contractile agent in question, the concentration of the agent and the component of the contractile response being examined (CitationTriggle, 1985).
Oxytocin is known to contract the uterinesmooth muscle by acting on G-protein coupled membrane receptors, which have been found to be of two sizes in humans; 3.6 kb in breast and 4.4 kb in ovary, endometrium, and myometrium (CitationMichelini et al., 1995; CitationInoue et al., 1994). The possible existence of oxytocin receptor subtypes has been investigated (CitationVerbalis, 1999). Such subtypes have been suggested to be present in the rat uterus (CitationChen et al., 1994) to explain differential pharmacological profiles or immunoreactivity patterns. In the human myometrium, these receptors are coupled to Gq and G11α class GTP binding proteins that stimulate together with Gßγ the activity of phospholipase Cß isoforms, with the generation of inositol 1,4,5,-triphosphate and 1,2-diacylglycerol. Inositol trisphosphate triggers Ca2+ release from intracellular stores, whereas diacylglycerol stimulates protein kinase C, which phosphorylates unidentified target proteins. Consequently, the intracellular calcium level is increased through the receptor-operated calcium channel (ROC). This Ca2+ release activates voltage-operated calcium channels that lead to influx of Ca2+ from the extracellular fluid. The relative contributions of these two sources of calcium depend on both the contractile agents and their concentrations (CitationEdwards et al., 1996). Finally, in response to an increase of intracellular [Ca2+], a variety of cellular events are initiated. In smooth muscle cells, the Ca2+-calmodulin system triggers the activation of myosin light-chain kinase activity which initiates smooth muscle contraction, e.g., in myometrial or mammary myoepithelial cells (CitationSanborn et al., 1998).
Atropine and related parasympatholytic drugs are competitive antagonists to choline esters or the parasympathomimetic alkaloids at those sites where parasympathomimetic or muscarinic effects are exerted (CitationMeyers & Abreu, 1952). Thus, the antagonism of EEN by atropine suggests that the extract may interact with muscarinic receptors in the uterus, also atropine may have had greater affinity for the receptor sites than the active component of the crude extract at the concentrations used, such that EEN no longer acted to depolarize the uterine cell and initiate uterine contraction with the receptors being occupied by atropine (CitationMeyers & Abreu, 1952).
Similarly, the inhibition of the contractile effect of EEN by diphenhydramine suggests possible interaction of the extract with histamine H1-receptors in the myometrium. However, CitationSohen et al. (2001) reported that diphenhydramine also has muscarinic blocking effects, thus increasing the possibility that EEN interacts with muscarinic receptors.
Phentolamine, a competitive α-adrenergic receptor blocker combines with its receptors on the uterus to antagonize α-adrenoceptor-mediated uterine contraction (CitationHoffmann, 2001). From the results, the contractile effect of the plant extract was not inhibited by phentolamine. Phentolamine also blocks receptors of serotonin (5-HT) (CitationMcPherson, 1993) suggesting that although there are α-adrenergicreceptors (CitationMhaouty-Kodja et al., 2001) and 5-HT receptors in the uterine smooth muscles (CitationHoffmann, 2001), the contractile activity of the EEN may not be the result of activation of either or both of these two receptors.
The possible involvement of voltage-operated calcium channels (VOCs) in EEN-induced contraction is apparent by the observed inhibition of EEN by verapamil, an L-type calcium channel antagonist of the phenylalkylamine group (CitationKerins et al., 2001).
From the foregoing, though the leaf extract of N. laevis has uterine contracting activity as oxytocin, they do not seem to have the same mechanism of action.
Early phytochemical studies in the Republic of Congo on the leaves and bark of N. laevis revealed the absence of flavonoids, saponins, quinones, terpenes, and steroids (CitationOliver-Bever, 1986). Recent phytochemical studies in Nigeria revealed the presence of flavonoids, tannins, terpenes, steroidal and cardiac glycosides while alkaloids and saponins were found to be absent (CitationUsman & Osuji, 2007).
Tannins are common constituents of medicinal plant extracts and have been reported to have pharmacological actions of their own. Studies on these actions are likely to be helpful in interpreting data obtained with tannin-rich crude plant extracts (CitationCalixto et al., 1986) as found in N. laevis. Tannins have been reported to affect calcium availability for contraction of uterine smooth muscles and cardiac muscles (CitationCalixto et al., 1986; CitationPolya et al., 1995) while flavonoids have been reported to inhibit uterine contractions (CitationRevuelta et al., 1997). Thus the tannins and flavonoids in N. laevis may not have been responsible for the contractile effect observed on the uterus. However, cardiac glycosides reported to be present in N. laevis affect the uterus of animals of various species. CitationSugimoto (1913) reported stimulation of the non-pregnant guinea pig uterus with strophanthin, and CitationRansom (1920) reported that the tincture of strophanthin sensitized the cat myometrium to calcium. CitationRothlin and Hamet (1934), however, reported that digitalin counteracted the motor effect of adrenaline on the non-pregnant rabbit uterus. On the human uterus CitationNorris (1961) found that digoxin and ouabain increased both tone and the frequency of contractions in vitro, while digoxin significantly alleviated dysmenorrhea symptoms in a double-blind trial. CitationSullivan (1963) reported that ouabain caused a persistent contraction both of the spontaneously contracting and of the electrically stimulated uterus, the response depending upon the presence of calcium. Sullivan also reported that ouabain had a biphasic effect on the uterus which was not observed with the other agonists used in his experiment. These reports lend credence to the possibility that cardiac glycosides present in the ethanol leaf extract of N. laevis contributes to its uterine contractile effect.
The use of this plant to induce or augment labor by traditional practitioners may therefore be due to the contractile effect of the leaf extract of N. laevis mediated via muscarinic receptors and/or histamine H1-receptors, and may also interact with voltage-operated calcium channels. However, further studies are required to determine the action of EEN on other receptors present in the uterine smooth muscle.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Akaneme FI (2007): Identification and preliminary phytochemical analysis of herbs that can arrest threatened miscarriage in Orba and Nsukka towns of Enugu state. Afr J Biotech 7: 006–011.
- Calixto BJ, Mauro N, Rae GA (1986): Pharmacological actions of tannic acid. I. Effects on isolated smooth and cardiac muscles and on blood pressure. Planta Med 52: 32–35.
- Chen DL, Chan WY, Manning M (1994): Agonist and antagonist specificities of decidual prostaglandin-releasing oxytocin receptors and myometrial uterotonic oxytocin receptors in pregnant rats. J Reprod Fertil 102: 337–343.
- Den Hertog CEC, De Groot ANJA, Van Dongen PWJ (2001): History and use of oxytocics. Eur J Obstet Gynecol Reprod Biol 94: 8–12.
- Edwards CR, Benediktsson R, Lindsay RS, Seckl J (1996): 11 beta-Hydroxysteroid dehydrogenases: Key enzymes in determining tissue specific glucocorticoid effects. Steroids 4: 263–269.
- Eferekeya A, Nworgu Z (1985): Inhibitory effects of gallopamil and hydralazine on early and late pregnant uteri. Nig J Pharm Sci 1: 67–71.
- Hoffmann BB (2001): Catecholamines, sympathomimetic drugs, and adrenergic receptor antagonists, in:Hardman JG, Limbird LE, Gilman GA, eds. The Pharmacological Basis of Therapeutics, 10th Edition, McGraw-Hill, New York, USA. pp. 233–246.
- Inoue T, Kimura T, Azuma C, Inazawa J, Takemura M, Kikuchi T, Kubota Y, Ogita K, Saji F (1994): Structural organization of the human oxytocin receptor gene. J Biol Chem 269: 32451–32456.
- Kerins DM, Robertson RM, Robertson D (2001): Drugs used for the treatment of myocardial ischemia, in: Hardman JG, Limbird LE, Gilman GA, eds. The Pharmacological Basis of Therapeutics, 10th Edition, McGraw-Hill, New York, USA. pp. 857.
- Matthew A, Shymgol A, Wray S (2004): Ca2+ entry, efflux and release in smooth muscle. Biol Res 37: 617–624.
- McPherson GA (1993): Current trends in the study of potassium channel openers. Gen Pharmacol 24: 275–281.
- Meyers FH, Abreu BE (1952): A comparison of the central and peripheral effects of atropine, scopolamine and some synthetic atropine-like compounds. J Pharmacol Exp Therap 104: 387–395.
- Mhaouty-Kodja S, Houdeau E, Cohen-Tannoudji J, Legrand C (2001): Catecholamines are not linked to myometrial phospholipase C and uterine contraction in late pregnant and parturient mouse. J Physiol 536: 123–131.
- Michelini S, Urbanek M, Dean M, Goldman D (1995): Polymorphism and genetic mapping of the human oxytocin receptor gene on chromosome 3. Am J Med Genet 60: 183–187.
- Norris PR (1961): The action of cardiac glycosides on the human uterus. J Obstet Gynaec Brit Cwlth 68: 916–929.
- Okoli RI, Aigbe O, Ohaju-Obodo JO, Mensah JK (2007): Medicinal herbs used for managing some common ailments among Esan people of Edo State, Nigeria. Pak J Nutr 6: 490–496
- Oliver-Bever B (1986): Medicinal Plants in Tropical West Africa. London: Cambridge University Press, pp. 117–118.
- Polya GM, Wang BH, Foo YL (1995): Inhibition of signal-regulated protein kinases by plant-derived hydrolysable tannins. Phytochemistry 38: 307–314.
- Ransom F (1920): The reaction of the cat’s uterus to strophanthus and calcium. J Pharmacol Exp Ther 15: 181–188.
- Revuelta MP, Cantabrana B, Hildago A (1997): Depolarization-dependent effect of flavonoids in rat uterine smooth muscle contraction elicited by CaCl2. Gen Pharmacol 29: 847- 857.
- Rothlin E, Hamet R (1934): Action de la digitaline sur l’uterus isole de lapine. C R Soc Biol (Paris) 116: 504–506.
- Sanborn BM, Dodge K, Monga M, Qian A, Wang W, Yue C (1998): Molecular mechanisms regulating the effects of oxytocin on myometrial intracellular calcium. Adv Exp Med Biol 449: 277–286.
- Sohen S, Ooe H, Hashima M, Nonaka T, Fukuda K, Hamanishi C (2001): Activation of histamine H1 receptor results in enhanced proteoglycan synthesis by human articular chondrocyte: Involvement of protein kinase C and intracellular Ca. Pathophysiol 8: 93–98.
- Sugimoto T (1913): Pharmakologische Untersuchungen am uberlebenden Meerschweinchenuterus. Naunyn-Schmiedeberg’s Arch Exp Path Pharmak 74: 27–40.
- Sullivan TJ (1963): Action of ouabain on the guinea pig isolated uterus. Brit J Pharmacol 21: 226–234.
- Triggle DJ (1985): Calcium and respiratory smooth muscle function. Brit J Clin Pharmacol 20: 2135–2140.
- Usman H, Osuji JC (2007): Phytochemical and in vitro antimicrobial assay of the leaf extract of Newbouldia laevis. Afr J Trad Comp Alt Med 4: 476–480
- Verbalis JG (1999): The brain oxytocin receptor(s)? Front Neuroendocrinol 20: 146–156.