Abstract
Context: The ischemic brain lesions induced in rats by temporary occlusion of the bilateral common carotid arteries and restoration of blood flow to an ischemic brain region is associated with generation of reactive oxygen species with consequent reperfusion injury.
Objective: The present study investigated the neuroprotective potential of Hibiscus rosa sinensis L. (Malvaceae) in a bilateral common carotid artery (BCCA) occlusion model of global cerebral ischemic reperfusion.
Materials and methods: The animals underwent 30 min BCCA occlusion and 45 min reperfusion. The methanol extract of H. sinensis (100, 200, 300 mg/kg/day for 6 days, p.o.) was administered 30 min before induction of BCCA occlusion.
Results: The bilateral common carotid artery occlusion resulted in increase in lipid peroxidation, and reduction in superoxide dismutase (SOD), catalase (CAT) and glutathione reductase (GSH) activity. The extract attenuated the ischemic reperfusion-induced increase in lipid peroxidation and fall in SOD, CAT, and GSH levels. The cerebral hypoperfusion caused a propensity towards anxiety and was accompanied by deficits of learning and memory. The extract ameliorated anxiety and there was improvement of learning and memory.
Discussion: The administration of H. sinensis prevented the oxidative stress and the biochemical changes associated with cerebral ischemic reperfusion injury. The mechanism of such protection of H. sinensis may be due to cerebral adaptation, through augmentation of cellular antioxidants such as GSH, SOD and CAT. The results suggest the protective role of H. sinensis in ischemic reperfusion injury.
Conclusion: This study indicates the beneficial role of H. sinensis in cerebrovascular insufficiency states and dementia.
Introduction
Worldwide, stroke remains the third most common cause of death. Stroke is a major cause of severe long-term disability and is characterized by sudden loss of motor, sensory or cognitive function (CitationSaleem et al., 2006). Dramatic reduction of oxygen in stroke may lead to ischemia of the whole brain (global ischemia) or of defined cerebral territories (focal ischemia) depending on cerebral artery occlusion. The pathophysiological mechanisms leading to neuronal injury in ischemic stroke are complex and multifactorial. Ischemic-induced brain damage is accompanied by biochemical alterations and neurological sequelae. Reactive oxygen species (ROS) are produced in the brain during ischemia and reperfusion injury. ROS such as superoxide radical, hydroxyl radical and hydrogen peroxide contributed to ischemic brain damage (CitationGupta & Sharma, 2006). Cerebral ischemic injury induced by bilateral clamping of carotid arteries induces transient metabolic changes in various brain regions. Reperfusion injury results when cell damage induced by ischemia is heightened by post-ischemic reperfusion. The oxygen free radicals initiate lipid peroxidation and inflict damage on macromolecular components of the cells. The acute ischemic reperfusion injury leads to reduction in cerebral blood flow and brain energy metabolism caused behavioral and cognitive defects (CitationRaghavendra et al., 2007).
The molecular mechanisms and potential treatment of acute and chronic neurological disorders have been research areas of paramount importance (CitationSaleem et al., 2006). Elucidation of the role of oxidative injury is important because therapy with agents that scavenge reactive oxygen species and augment endogenous antioxidant capacity may prove useful in therapeutic modulation of these devastating neurological conditions (CitationNakashima et al., 1999).
Hibiscus rosa sinensis L. (Malvaceae), also known as China rose, is a popular herb in the traditional system of Indian medicine. Ethnomedical information states that this herb is used for the treatment of cough, fever, dysentery, venereal disease, and also applied topically to cancerous swelling (CitationCSIR, 1956; CitationMhaskar et al., 2000). Experimental reports indicate that H. sinensis possesses a protective effect against the tumor promotion stage of cancer development (CitationSharma et al., 2004). The flowers and leaves of the plant were found to exhibit significant hypoglycemic and lipid lowering activity (CitationSachdewa & Khemani, 2003). The experimental and clinical studies have shown that the dried flower powder of H. sinensis has significant protective effect in ischemic heart disease (CitationGauthaman et al., 2006). The roots of H. sinensis were found to possess post-coital antifertility activity (CitationVasudeva & Sharma, 2008). It has hepatoprotective action through antioxidant effect (CitationObi & Uneh, 2003). Moreover, it is also known to have a radical scavenging effect (CitationMasaki et al., 1995). The pharmacological activities of H. sinensis were attributed due to the chemical constituents like quercetin, carotene, niacin, riboflavin, malvalic acid, gentisic acid, margaric acid, lauric acid, anthocyanin, and anthocyanidine (CitationNadkarni, 1976; CitationCSIR, 1956).
In spite of the reported antioxidant property of H. sinensis in a variety of models, there is no major investigative report available pertaining to its neuroprotective effect. This study investigated the neuroprotective potential of H. sinensis on bilateral carotid artery occlusion-induced cerebral ischemic reperfusion injury.
Materials and methods
Plant material
The roots were collected in the month of November (2007) from local area of Nashik (India) by V.S. Nade and authenticated by P.S.N. Rao (Director, Botanical Survey of India, Pune). A voucher specimen of the plant has been deposited at Botanical Survey of India, Pune (Voucher Specimen No. NVHR3). The plant material was shade dried and coarsely powdered. The powdered plant material (1 kg) was de-fatted with petroleum ether (60°–80°C) by Soxhlet extractor. The de-fatted marc was further extracted with methanol for 72 h. The extract was filtered and concentrated under reduced pressure. The yield of methanol extract of H. rosa sinensis roots (HRS) was found to be 6.2% w/w. The dried extract was suspended in 0.5% carboxymethyl cellulose in distilled water and administered orally (p.o.).
Animals
Male Wistar strain rats (200-230 g) were used for the study. The animals were housed in polypropylene cages and maintained under standard laboratory environmental conditions; temperature 25° ± 2°C, 12 h light:12 h dark cycle, and 50 ± 5% relative humidity with free access to food and water ad libitum. Animals were acclimatized to laboratory conditions before the test. Each group consisted of five (n = 5) animals. All the experiments were carried out during the light period (08:00-16:00 h). The studies were carried out in accordance with the guidelines given by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi (India). The Institutional Animal Ethical Committee of M.V.P.S. College of Pharmacy, Nashik approved the protocol of the study (IAEC/2008/02).
Drugs and chemicals
Thiobarbituric acid (TBA) (Research-Lab Fine Chem Industries, Mumbai), nitroblue tetrazolium (NBT) (Himedia Laboratories, Mumbai), 5,5-dithiobis (2-nitro benzoic acid) (DTNB) (Alfa Aesar, Johnson Mathey, Chennai). All other chemicals used were of analytical grade and purchased from standard manufacturers.
Surgical procedure
Surgical procedure for induction of cerebral ischemia was followed according to the method described by CitationYanpallewar and Acharya (2004). Briefly, under ketamine (100 mg/kg i.p.) anesthesia, a midline skin incision in the neck was made. Bilateral common carotid arteries were identified and separated carefully from the vagus nerve. Body temperature was maintained at about 37°C during the period with the help of a heat lamp. Then the neck incision area was sutured. The rats were kept under the heat lamp for 2 h until recovery to prevent post-ischemic hypothermia. After recovery the animals were returned to their home cage.
Experiment
For acute studies the animals were divided into five groups (n = 5 for each group). The first group served as control. In the second group, vehicle treated animals underwent 30 min BCCA occlusion and 45 min reperfusion. In the third, fourth and fifth groups HRS 100, 200, and 300 mg/kg (p.o.) were administered 30 min before BCCA occlusion, respectively. HRS (100, 200, and 300 mg/kg per day, p.o.) was then continued up to day 6 post-surgery. On day 6, 60 min after the last dose of HRS, all the animals were subjected to behavioral testing in an open field paradigm and elevated plus maze.
Sampling techniques–dissection and homogenization
At the end of the experiment the rats were sacrificed by cervical dislocation and brains were taken out. They were rinsed thoroughly with ice-chilled 0.9% NaCl and weighed. A 10% (w/v) tissue homogenate was prepared in 0.1 M phosphate buffer (pH 7.4). The post-nuclear fraction for catalase assay was obtained by centrifugation (Remi–C-30, Remi Industries, Mumbai) of the homogenate at 1,000 g for 20 min at 4°C; for other enzyme assays, centrifugation was at 12,000 g for 60 min at 4°C. A Shimadzu -160A spectrophotometer was used for subsequent assays (CitationNaidu et al., 2003).
Biochemical analysis
Lipid peroxidation assay
The quantitative measurement of lipid peroxidation (LPO) in brain was done by the CitationWills (1966) method. The amount of malondialdehyde (MDA) formed was measured by reaction with thiobarbituric acid at 532 nm. The results were expressed as nM of MDA per mg of protein, using the molar extension coefficient of chromophore (1.56 × 105 M−1 cm−1).
Superoxide dismutase activity
Superoxide dismutase (SOD) activity was assayed according to the method of CitationKono (1978), wherein the reduction of nitroblue tetrazolium chloride (NBT) was inhibited by the superoxide dismutase which was measured at 560 nm spectrophotometrically. Briefly, the reaction was initiated by the addition of hydroxylamine hydrochloride to the reaction mixture containing NBT and post-nuclear fraction of brain homogenate. The results were expressed as units per mg of protein, with one unit of enzyme defined as the amount of SOD required to inhibit the rate of reaction by 50%.
Catalase activity
Catalase activity (CAT) was assessed by the CitationLuck (1971) method, where the breakdown of H2O2 was measured at 240 nm. Briefly, the assay mixture consisted of 3 mL H2O2 phosphate buffer (0.0125 M H2O2) and 0.05 mL supernatant of brain homogenate (10%) and the change in the absorbance were measured at 240 nm. The enzyme activity was calculated using the millimolar extension coefficient of H2O2 (0.07). The results were expressed as micromole of H2O2 decomposed per min per mg of protein.
Estimation of reduced glutathione
Reduced glutathione (GSH) in the brain was estimated according to the CitationEllman (1959) method. An aliquot of 0.1 mL homogenate was precipitated with 0.75 mL of 4% sulfosalicylic acid. The assay mixture contained 0.5 mL of supernatant and 4.5 mL of DTNB in 0.1 M phosphate buffer, pH 8. The yellow color developed was read immediately at 412 nm. The results were expressed as nanomoles of GSH per mg of protein.
Protein estimation
The protein content was measured according to the CitationLowry et al. (1951) method, using bovine serum albumin as standard and expressed as µg protein per mg of tissue.
Behavioral testing
Open field test
The locomotor activity was evaluated in an open field paradigm. The apparatus consisted of a wooden box (60 × 60 × 30 cm). The floor of the box was divided into 16 squares (15 × 15 cm). The apparatus was illuminated with a 40-W lamp suspended 100 cm above. Each animal was placed at one corner of the apparatus and for the next 5 min it was observed for the ambulations (number of squares crossed), total period of immobility (in s), number of rearings, grooming, fecal pellets (CitationLister, 1990).
Elevated plus maze test
The elevated plus maze test (EPM) consisted of two open arms (35 × 5 cm) crossed with two closed arms (35 × 5 × 20 cm). The arms were connected together with a central square of 5 × 5 cm. The apparatus was elevated to the height of 50 cm in a dimly illuminated room. Animals were placed individually at the end of either of the open arms facing away from the central platform. The time taken by each animal to move from open arm to either of the closed arms was recorded. This duration of time was called transfer latency (TL). If the animal did not enter into any of the enclosed arms within 120 s, it was gently pushed into any of the enclosed arms and TL was considered as 120 s. Later the animal was allowed to explore the plus maze for 5 min and send back to its home cage. TL was then noted on day 1 and day 6. TL measured on day 1 served as a parameter for acquisition (learning) while TL on day 6 indicated retention (memory) (CitationJaiswal & Bhattacharya, 1992).
Statistical analysis
Results are expressed as mean ± SEM, and the statistical analysis of data was done using one-way analysis of variance (ANOVA) followed by Dunnett’s test. Probability level less than 0.05 was considered statistically significant.
Results
Biochemical effects
Lipid peroxidation assay
The level of MDA was investigated after day 6 of BCCA. The level of MDA was significantly increased (p < 0.01) in the vehicle group, as compared with the control group, while administration of HRS (100, 200, 300 mg/kg) significantly (p < 0.01) brought down the level of MDA compared with the vehicle group ().
Table 1. Effects of H. rosa sinensis root extract on bilateral common carotid artery occlusion-induced alterations in rat brain CAT, SOD, LPO and GSH.
Effect on brain SOD and CAT levels
The levels of the defensive antioxidant enzymes SOD and CAT were decreased after BCCA ligation in rats. In the vehicle-treated group, the SOD and CAT (p < 0.01) activity was decreased as compared with the control group. Pretreatment with HRS (100-300 mg/kg) resulted in elevation of SOD and CAT levels (p < 0.01) as compared with the vehicle group ().
Effect on brain GSH level
The content of GSH was depleted significantly (p < 0.01) in vehicle group, as compared with the control group, indicating the neurotoxicity induced by carotid artery occlusion in rats. On the other hand, the GSH level was found to be elevated significantly (p < 0.01) after HRS (100, 200, 300 mg/kg) treatment as compared with the vehicle group ().
Behavioral effects
Open field test
The animals with BCCA ligation showed marked alterations in the locomotor activities in the open field paradigm. The BCCA ligation was associated with a reduced number of ambulations, rearings and groomings along with an increase in the period of immobility (p < 0.01). Pretreatment with HRS prevented these alterations ().
Table 2. Effects of H. rosa sinensis root extract (HRS) on bilateral common carotid artery occlusion (BCCAO)-induced alterations in open field parameters.
Elevated plus maze test
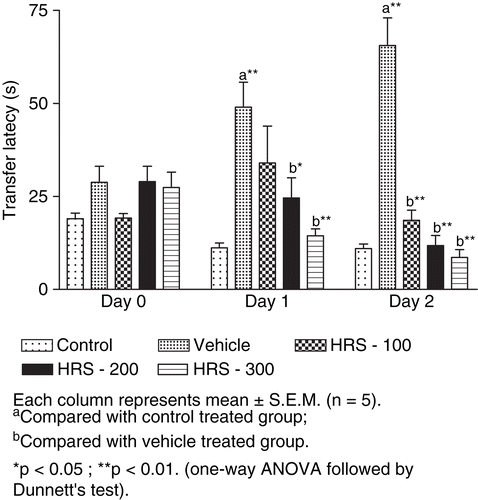
The transfer latency was increased at day 1 and day 6 in animals with BCCA ligation indicating impairment in learning and memory. Pretreatment with HRS (100, 200, 300 mg/kg) leads to significant decrease (p < 0.01) in transfer latency as compared to vehicle-treated animals, indicating improvement in retention of memory ().
Figure 1. Effect of H. rosa sinensis root extract on learning and memory in bilateral common carotid artery occlusion-induced alterations of transfer latency at day 0, 1 and 6 in the elevated plus maze. Each column represents mean ± SEM (n = 5). aCompared with control-treated group. bCompared with vehicle-treated group. *p < 0.05; **p < 0.01 (one-way ANOVA followed by Dunnett’s test).
Discussion
Cerebral ischemic injury induced by clamping of carotid arteries induces transient metabolic changes in various brain regions. Reperfusion injury results when cell damage induced by ischemia is heightened by post-ischemic reperfusion. Restoration of blood flow to an ischemic brain region is associated with generation of reactive oxygen species with consequent reperfusion injury (CitationMcCord, 1985). Bilateral common carotid occlusion for 30 min followed by reperfusion for 45 min was associated with increased generation of reactive oxygen species (CitationYanpallewar & Acharya, 2004).
The principal finding of the present study is that the cerebral ischemic reperfusion injury was associated with oxidative stress, as evidenced by increase in brain MDA level and depletion of cerebral endogenous antioxidant status (SOD, CAT and GSH). Similar observations were made earlier by other studies (CitationSorrenti et al., 1994; CitationNakashima et al., 1999). Increased levels of MDA reflect the membrane damage induced by toxic lipid peroxidation products. The depletion in SOD, CAT and GSH enzymes was observed and taken as a marker of oxidative stress. The administration of HRS prevented the oxidative stress and the biochemical changes associated with cerebral ischemic reperfusion injury. The mechanism of such protection of oral administration of HRS may be due to cerebral adaptation, through augmentation of cellular antioxidants such as GSH, SOD and CAT. In cerebral ischemic reperfusion injury, oxidative stress plays a central role in its etiopathogenesis. Protection against oxidative stress through this mechanism may be one of the effective therapeutic approaches.
In the present study H. Sinensis roots extract attenuated ischemic reperfusion injury. HRS reversed the ischemic reperfusion-induced changes in defensive enzyme levels such as SOD, CAT and GSH; and also attenuated MDA level. These findings support the earlier observation of CitationGauthaman et al. (2006) in which flowers of H. rosa sinensis were shown to exert a cardioprotective effect in an oxidative stress model of myocardial ischemic reperfusion injury and this cardioprotective effect was explained on the basis of antioxidant action of the plant. H. sinensis flowers contain anthocyanins, anthocyanidine and quercetin which may be responsible for its antioxidant effects. Augmentation of endogenous antioxidants by therapeutic substances has recently evoked scientific interest because any such property of a therapeutic agent can be expected to cause significant improvement in the endogenous defense against oxidative stress (CitationGauthaman et al., 2006). The observed beneficial effects of HRS on cerebral ischemic reperfusion injury-induced changes in biochemical parameters may thus be attributed to its various chemical constituents.
The reduction in total activity of the animals in the open field paradigm with significant reduction in the number of ambulations, groomings and rearings as compared to control animals suggest a propensity towards anxiety and restlessness. HRS has significantly prevented ischemic reperfusion-induced anxiety and restlessness.
The permanent BCCA occlusion was used as a model of neurodegenerative conditions and dementia. Reduction in blood flow and brain energy metabolism caused progressive dysfunction resulting in cognitive deficits (CitationNi et al., 1994). The hypoperfused animals had deficits of spatial learning and memory as indicated by EPM testing which is in accordance with earlier reports of ischemia-induced disturbances of spatial learning and memory (CitationRaghavendra et al., 2007). The animals consistently showed increased transfer latencies suggesting a defective registration of the learning task. The HRS-treated group showed a decrease in transfer latency indicating an improvement of learning and memory deficits.
Conclusion
The present study showed that the roots of H. sinensis could enhance cerebral endogenous antioxidants without producing any toxic effects. The administration of HRS may be able to attenuate the increased MDA level and improvement in the defensive antioxidant enzymes such as SOD, CAT, and GSH. These results strengthen the oxidative stress hypothesis of carotid artery occlusion-induced neurotoxicity. Therefore, the protection against cerebral ischemic reperfusion injury in the treated rats may be attributable to enhanced endogenous antioxidant activity. Thus, H. sinensis may be helpful in cerebral hypoperfusion states such as cerebrovascular insufficiency and dementia.
Declaration of interest
The authors are grateful to the All India Council for Technical Education, New Delhi, India for financial assistance.
References
- CSIR (1956): The Wealth of India. A Dictionary of Indian Raw Materials and Industrial Products. New Delhi, Council for Scientific and Industrial Research, pp. 91–92.
- Ellman GL (1959): Tissue sulfhydryl groups. Arch Biochem Biophys 82: 70–77.
- Gauthaman K, Maulik M, Kumari R, Manchanda SC, Dinda AK, Maulik SK (2001): Effect of chronic treatment with bark of Terminalia arjuna, a study on the isolated ischemic reperfused rat heart. J Ethnopharmacol 75: 197–201.
- Gauthaman KK, Salem MTS, Thanislas PT, Prabhu VV, Krishnamoorthy KK, Devaraj NS, Somasundaram JS (2006): Cardio protective effect of the Hibiscus rosa sinensis flowers in an oxidative stress model of myocardial ischemic reperfusion injury in rats. BMC Comp Alt Med 6: 32–40.
- Gupta S, Sharma SS (2006): Neuroprotective effects of Trolox in global cerebral ischemia in gerbils. Biol Pharm Bull 29: 957–961.
- Jaiswal AK, Bhattacharya SK (1992): Effects of shilajit on memory, anxiety and brain monoamines in rats. Indian J Pharmacol 24: 12–17
- Kono Y (1978): Generation of superoxide radical during autooxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophy 186: 189–195.
- Lister RG (1990): Ethologically based animal models of anxiety disorders. Pharmacol Ther 46: 321–340.
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951): Protein measurement with Folin-phenol reagent. J Biol Chem 193: 265–275.
- Luck H (1971): Bergmeyer HU (Editor), Catalase, in: Methods of Enzymatic Analysis. New York, Academic Press, pp. 885–893.
- Masaki HS, Sakaki S, Atsumi T, Sakurai H (1995): Active oxygen scaven-ging activity of plant extracts. Biol Pharmacol Bull 18: 162–166.
- McCord JM (1985): Oxygen derived free radicals in post-ischemic tissue injury. N Engl J Med 312: 159 -163.
- Mhaskar KS, Blatter E, Calus JF (2000):Kirtikar KR and Basu BD, eds, Indian Medicinal Plants. New Delhi, Shri Satguru Publication, pp. 462–464.
- Nadkarni AK (1976): Indian Materia Medica, Vol.1. Mumbai, Popular Prakashan, p. 1199.
- Naidu PS, Singh A, Kulkarni SK (2003): Effect of Withania somnifera root extract on haloperidol-induced orofacial dyskinesia: Mecha-nisms of action. J Med Food 6: 107–114.
- Nakashima M, Niwa M, Iwai T, Uematsu T (1999): Involvement of free radicals in cerebral vascular reperfusion injury evaluated in a transient focal cerebral ischemia model of rats. Free Rad Biol Med 26: 722–729.
- Ni JM, Ohta H, Matsumoto K, Watanabe H (1994): Progressive cognitive impairment following chronic cerebral hypoperfusion induced by permanent occlusion of bilateral carotid arteries in rats. Brain Res 653: 231–236.
- Obi FO, Uneh E, (2003): pH dependent prevention of carbon tetrachloride-induced lipoperoxidation in rats by ethanolic extract of Hibiscus rosa sinensis petals. Biokemistri 13: 42–50.
- Raghavendra M, Anshuman T, Singh RK, Mitra S, Goel RK, Acharya SB (2007): Effect of ethanolic extract of Pongamia pinnata (L.) Pierre on oxidative stress, behavioral and histopathological alterations induced by cerebral ischemic reperfusion and long term hypoperfusion in rats. Indian J Exp Biol 45: 868–876.
- Sachdewa A, Khemani LD (2003): Effect of Hibiscus rosa sinensis Linn. ethanol flower extract on blood glucose and lipid profile in streptozotocin induced diabetes in rats. J Ethnopharmacol 89: 61–66.
- Saleem S, Ahmad M, Ahmad AS, Yousuf S, Ansari MA, Khan MB, Ishrat T, Islam F (2006): Behavioral and histological neuroprotection of aqueous Garlic extract after reversible focal cerebral ischemia. J Med Food 9: 537–544.
- Sharma S, Khan N, Sultana S (2004): Study on prevention of two-stage skin carcinogenesis by Hibiscus rosa sinensis extract and the role of its chemical constituent, gentisic acid, in the inhibition of tumor promotion response and oxidative stress in mice. Eur J Cancer Prevent 13: 53–63.
- Sorrenti V, Di Giaxomo C, Renis M, Russo A, La Della C, Perez-Polo JR (1994): Lipid peroxidation and survival in rats following cerebral post-ischemic reperfusion: Effect of drugs with different molecular mechanisms. Drugs Exp Clin Res 20: 185–189.
- Vasudeva N, Sharma SK (2008): Post-coital antifertility activity of Hibiscus rosa sinensis Linn roots. Evidence Based Comp Alt Med 5: 91–94.
- Wills ED (1966): Mechanism of lipid peroxide formation in animal tissues. Biochem J 99: 667–676.
- Yanpallewar SU, Acharya SB (2004): Nimodipine attenuates biochemical, behavioral and histopathological alterations induced by acute transient and long-term bilateral common carotid occlusion in rats. Pharmacol Res 49:143–150.
