Abstract
The constituents of the ethanol extract from the root of Polygala tenuifolia Willd. (Polygalaceae) were investigated for antioxidant activity in senescence-accelerated mice. Consequently, two relevant samples were obtained, a fraction separated by macroporous resin (YZ-OE), and a major pure crystal of 3,6′-disinapoyl sucrose (DISS). Based on HPLC-ESI-MS analysis, the most constituents in the YZ-OE fraction from the extract of P. tenuifolia were oligosaccharide esters. The antioxidant activities of these two samples were evaluated using the accelerated senescence-prone, short-lived mice (SAMP) in vivo. The activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-PX) were increased significantly in SAMP mice fed oligosaccharide esters (YZ-OE 50 mg/kg) and its constituents (DISS 50 mg/kg). However, the content of malondialdehyde (MDA) was increased in the blood and liver of SAMP mice. But when given YZ-OE, it could be decreased, by 44.3% and 47.5%, respectively, compared with the SAMP model. Results from the analyses indicated that the oligosaccharide esters (YZ-OE) from roots of P. tenuifolia had a high in vivo antioxidant activity.
Introduction
The free-radical theory has proposed that aging is the cumulative result of oxidative damage to cells and tissues, and the damage is considered primarily as a result of aerobic metabolism (CitationWickens, 2001). It is clearly known that the use of antioxidant nutrients may reduce or prevent the risk of having many diseases (e.g., chronic inflammation and cardiovascular diseases) caused by oxidative stresses. Currently, the function of antioxidant nutrients has been associated with a reduced risk of cancer, and their special function may be due to its ability to avoid the damage to DNA caused by free radicals (CitationFraga et al., 1990).
During the evaluation of natural antioxidants, investigators often use different types of assays, such as the β-carotene and linoleic acid systems, 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity, and inhibition of hemolysis of rat erythrocytes induced by peroxyl radicals (CitationCheung et al., 2003). However, the evaluation of the effects from natural antioxidants with a sensitive method in vivo, using the obtained results for human reference, is still a problem.
The high degree of oxidative stresses observed in the strains of accelerated senescence-prone, short-lived mice (SAMP) mice suggested that these SAMP strains can be used as a valuable tool for evaluating the effect of natural antioxidants in vivo (CitationMasanori, 2002; CitationLi et al., 2005), while the strains of the accelerated senescence-resistant, longer-lived (SAMR ) mice were used as a control.
Polygalae radix (recorded as “yuanzhi” in the Pharmacopoeia of the People’s Republic of China) is the prepared roots of Polygala tenuifolia Willd. (Polygalaceae) (CitationZheng et al., 2000). It has been used as a traditional medicine as an expectorant, tonic, tranquillizer and antipsychotic agent, etc. Recent reports have suggested that P. tenuifolia has anti-inflammatory activity in the central nervous system (CitationKim et al., 1998) and enhances memory and cognitive function in the scopolamine-induced amnesia model of rat (CitationPark et al., 2002). Studies have also shown that P. tenuifolia exerts a strong hemolytic effect in vitro and reduces the elevated lipid peroxide levels in senescence-accelerated mice (CitationNishiyama et al., 1994). As part of our long-term search for antioxidant activity from medicinal sources, we found that the extract of Polygalae radix showed an antioxidant activity in vitro. Therefore, the objectives of this study were to screen the active fractions and components with antioxidant activity, which isolated from the ethanol extract of P. tenuifolia roots, using the senescence-accelerated mice in vivo as a model.
Materials and methods
Plant material
The roots of P. tenuifolia were purchased from the Traditional Chinese Medicinal (TCM) Pharmacy, the Chinese People’s Liberation Army (PLA) General Hospital (Beijing, China). The voucher specimens of the plants, identified by Ping Liu and registered under the numbers NU-80617 were deposited at the Herbarium of the Traditional Chinese Medicinal Pharmacy, the Chinese People’s Liberation Army General Hospital.
Extraction, isolation and preparation of oligosaccharide esters
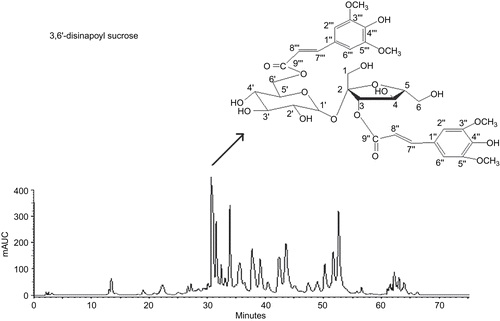
After air-drying for three months, the roots of P. tenuifolia (965.27 g) were extracted with 60% EtOH (8:1) at room temperature for two weeks. The dry extract obtained was then subjected to open column chromatography (CC) packed with macroporous resin (1300 Version). The column was eluted stepwise with each of four different concentrations of aqueous ethanol (30, 50, 70, and 95% v/v). The 50% aqueous-ethanol fraction was then concentrated under reduced pressure using a rotary evaporator and lyophilized into powders, designated as “resin fractionated YZ oligosaccharide esters” (YZ-OE, 86 g) for the following study, standardized using HPLC (). Then, YZ-OE was further chromatographed on the silica gel column and eluted by CHCl3-MeOH-H2O to obtain the relevant components, as described previously by CitationTu et al. (2008). Among them, the 3,6′-disinapoyl sucrose (DISS, 1.09 g) was the major component in YZ-OE, determined on the basis of spectroscopic analysis with chemical structure as shown in .
Characterization of extracted fractions
The HPLC (Waters 2695-2487) separation was performed on an Agilent C18 column (2.5 cm × 4.6 mm i.d.), with mobile phase-A: H2O (0.05% phosphoric acid), mobile phase-B: CH3CN; a gradient from 11% to 45% solvent B within 75 min; flow rate: 1 mL/min; detection wavelength: 318 nm; injection volume: 20 µL. Electrospray ionization mass spectrometry (ESI-MS) was performed on a QTOF MicroTM mass spectrometer (Micromass, Manchester) equipped with an electrospray interface operating in negative- and positive-ion mode at an optimized sample cone voltage of 40V (CitationPiccoli et al., 1994). The identification of constituents was carried out by HPLC/DAD and HPLC/ESI/MS analysis, and/or by comparison and combination of their retention times, UV–vis and mass spectra of the peaks with those of authentic reference samples, or isolated compounds or data reported on literature.
Animal handling and experimental design
Senescence-accelerated mice, both SAMP and SAMR, were employed in this study. The mice were provided by Professor Zhang, Institutes of Medical Plant Development. They were acclimated to the surroundings for 1 week before experimentation and were individually housed in a temperature (22° ± 2°C), humidity (55% ± 10%), and light (12 h light:12 h dark cycle; lights on at 7 a.m.) controlled environment and were fed food and water ad libitum. Animals used in this study were specifically cared for and treated humanely according to the Principles of Laboratory Animal Care (CitationNIH, 1985) and the Guide for the Care and Use of Laboratory Animals (GUIDE). Considering the average life span of SAMP to be about one year, therefore, 9-month-old SAMP mice were used in this experiment to represent the old mice as treated group, and SAMR (accelerated senescence-resistant, longer-lived) strains were used as normal control group. The mice were exposed to a 12 h light-dark cycle with access to food and water. SAMR group and one group of SAMP as normal control group received only water. SAMP in the treated group received YZ-OE and 3,6′-disinapoyl sucrose at the dose of 50 and 25 mg/kg body weight (choice of the doses by the previous test screening and part of previous result) for 90 days, once a day at 9:00 am for 90 days. On the last day in experiment, the rats were sacrificed. The liver was removed and washed thoroughly with ice-cold saline. The tissues were immediately frozen in liquid nitrogen and kept at -80°C until analysis. Serum was separated from whole blood by centrifugation (10 min, 4°C) and stored at -20°C for two days untill used.
Determination of malondialdehyde (MDA)
The frozen liver (1 g) was homogenized in 10 mL of buffer, pH 7.4, containing 0.01 mol/L Tris-HCl, 0.01 mol/L sucrose, 0.0001 mol/L EDTA-2Na and 0.8% NaCl. Following centrifugation (16,000 g, 10 min), the supernatant was analyzed for MDA values according to the method described by CitationOhkawa et al. (1979) following the instructions of the kit. MDA were calculated using thiobarbituric acid reactive substances (TBARS).
Measurement of serum enzyme levels in mice
Superoxide dismutase (SOD) activity and glutathione peroxidase (GSH-PX) activity were determined following the instructions of the kit, respectively. The kits used were purchased from Nanjing Jiancheng Institute (Nanjing, China). The activity of GSH-PX was determined using t-butyl hydroperoxide as a substrate following the method of Citation(Tamura et al., 1982), and the activity was expressed as nmol NADPH oxidized. Superoxide dismutase (SOD) activity was determined by the epinephrine method (CitationMisra & Fridovich, 1972), based on the measurement of the inhibitory rate of epinephrine autoxidation by SOD contained in the examined samples in 50 mM sodium carbonate buffer (pH 10.2), within the linear range of autoxidative curve. The concentration of total protein was determined by the burette method (CitationLowry et al., 1951) using bovine serum albumin as standard.
Statistical analysis
All data presented were as mean values with standard errors. Differences in means between treatments were compared using the ANOVA test. Differences at *P <0.05 and **P<0.01 were considered significant.
Results and discussion
This study was first undertaken to assess the effect of resin-fractionated YZ oligosaccharide esters (YZ-OE, at 25 and 50 mg/kg) and its major component 3,6′-disinapoyl sucrose (at 25 and 50 mg/kg) on the activity of antioxidant enzymes in the serum and in the homogenated liver of SAMP. Effects on antioxidant activities of YZ in mouse serum and liver are shown in and , respectively.
Figure 2. Effect of resin-fractionated YZ oligosaccharide ester and its component, 3,6′-disinapoyl sucrose, on the content of SOD, GSH-PX, MDA in serum of SAMP and SAMR mice. Values are presented as mean ± SEM of the results from eight mice. Significance was determined at **P <0.01 (*P <0.05, two way ANOVA) versus model group and ##P <0.01 (#P <0.05, two way ANOVA) versus normal control.
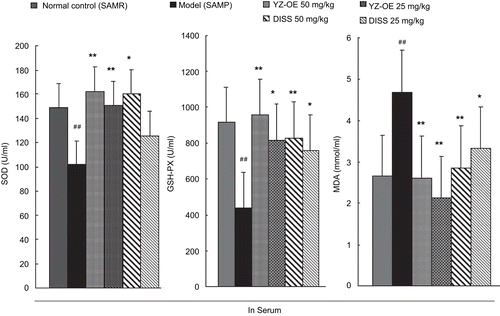
Figure 3. Effect of resin-fractionated YZ oligosaccharide ester and its component, 3,6′-disinapoyl sucrose, on the content of SOD, GSH-PX, MDA in liver of SAMP and SAMR mice. Values are presented as mean ± SEM of the results from eight mice. Significance was determined at **P <0.01 (*P <0.05, two way ANOVA) versus model group and ##P <0.01 (#P <0.05, two way ANOVA) versus normal control.
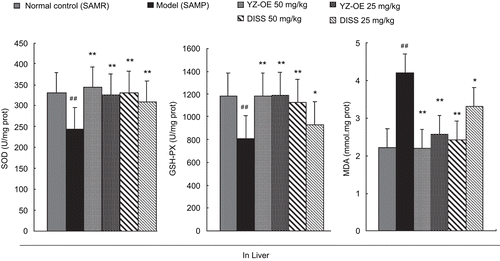
The crucial components of the antioxidant defense system in the body are cellular antioxidant enzymes (superoxide dismutase, SOD and glutathione peroxidase, GSH-PX). Among them, SOD and GSH-PX serve as potential markers of susceptibility, early reversing tissue damage, and decreasing antioxidant defense. Our results showed that activities of SOD and GSH-PX in the serum of the SAMP model group were significantly decreased, by 31.6% and 51.9%, respectively, while in liver were decreased by 25.8% and 31.4%, respectively, compared with those of the normal control. Also, in our study, the age-related decrease in the activity of SOD documented was in agreement with the previous investigations that showed an age-associated decline of SOD activity in the whole blood (CitationArivazhagan et al., 2000). But the activities of SOD and GSH-PX in the serum and liver were enhanced in the mice fed YZ-OE and DISS. The best results showed that YZ-OE (50 mg/kg) inhibited the decreasing of the SOD and GSH-PX activities in SAMP, and even made it to normal level (see and ).
On the other hand, the lipid peroxidation level exhibited an antioxidant capacity that was determined by the MDA. A significant increase in MDA was detected, 77% in serum and 78% in liver, in the SAMP model group, compared with the normal group (SAMR). SAMP treated with DISS (50 mg/kg) showed the best effect, it decreased MDA in serum and liver by 44.3% and 47.5%, respectively. Free radicals react with lipids and cause peroxidative changes, resulting in enhanced lipid peroxidation, thus leading to protein fragmentation and loss of cell viability, and causing cellular injury by inactivation of membrane enzymes and receptors, so inducing the change of antioxidant enzymes (CitationGirotti, 1985). In this study, supplementation with YZ-OE and DISS brought MDA and the antioxidant enzymes to the normal level, suggesting that YZ-OE could inhibit the lipid peroxidation, then increase the activity of the antioxidant enzymes against oxidative stresses induced by age.
The results obtained using the SAMP model successfully demonstrated the antioxidant activities of YZ-OE and DISS in vivo, YZ-OE showing the best effect. We used HPLC-ESI-MS to identify different components in the fraction and found nine compounds in the YZ-OE extract (). Most of these components belonged to different classes of oligosaccharide ester constituents. It is clear that DISS had antioxidant effects and thus provided protection against free radical-induced damage. However, as DISS is only a part in oligosaccharide ester-enriched fractions, the best antioxidant activity of YZ-OE was probably dependent on the synergistic effect with the other oligosaccharide ester constituents.
Table 1. Chemical components identified by HPLC-ESI-MS in the resin-fractionated YZ oilgosaccharide esters extracted from the roots of Polygala tenuifolia.
Previous studies in our laboratory showed that YZ-OE and DISS administration significantly decreased immobility time in the tail suspension and forced swim tests in mice and has notable antidepressant effect in pharmacological depression models (CitationLiu et al., 2008). It is intriguing to speculate, therefore, their antioxidant capacity may participate in and be utilized to treat some neurodegenerative diseases, such as depression and Alzheimer’s disease.
Declaration of interest
This research was supported by the National Natural Science Foundation of China (grant no. 30801524 and no. 90209036).
References
- Arivazhagan P, Thilakavathy T, Panneerselvam C (2000): Antioxidant lipoate and tissue antioxidants in aged rats. J Nutr Biochem 11: 122–127.
- Cheung LM, Cheung Peter CK, Vincent EC Ooi (2003): Antioxidant activity of total phenolics of edible mushroom extracts. Food Chem 81: 249–255.
- Fraga CG, Shigenaga MK, Park JW, Degan P, Ames BN (1990): Oxidative damage to DNA during aging: 8-Hydroxy-20-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci USA 87: 4533–4537.
- Girotti MW (1985): Mechanisms of lipid peroxidation. Free Radical Bio Med 1: 87–95.
- GUIDE (1996): Guide for the Care and Use of Laboratory Animals. Institute of Laboratory Animal Research, Commission on Life Sciences, National Research Council. Washington DC: National Academy Press 1996.
- Kim HM, Lee EH, Na HJ, Lee SB, Shin TY, Lyu YS, Kim NS, Nomura S (1998): Effect of Polygala tenuifolia root extract on the tumor necrosis factor-alpha secretion from mouse astrocytes. J Ethnopharmacol 61: 201-208.
- Li L, Ng TB, Gao TW, Li W, Fu M, Niu SM, Zhao L, Chen RR, Liu F (2005): Antioxidant activity of gallic acid from rose flowers in senescence accelerated mice. Life Sci 77: 230–240.
- Liu P, Wang DX, Guo DH, Tu HH, Ma L, Xie TT, Kong LY (2008): Antidepressant effect of 3,6′-disinapoyl sucrose from Polygala tenuifolia Willd. in pharmacological depression model. Chinese Pharm J 43: 1391–1394.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951): Protein measurement with folin-phenol reagent. J Biochem 193: 265–275.
- Masanori H (2002): A higher oxidative status accelerates senescence and aggravates age-dependent disorders in SAMP strains of mice. Mech Ageing Dev 123: 1553–1561.
- Misra HP, Fridovich I (1972): The role of the superoxide anion in the autooxidation of epinephrine and simple assay for superoxide dismutase. J Biol Chem 247: 3170–3175.
- NIH (1985): Principles of Laboratory Animal Care. National Institutes of Health publication No. 86-23, revised 1985.
- Nishiyama N, Zhou Y, Saito H (1994): Ameliorative effects of chronic treatment using DX-938 a traditional Chinese prescription, on learning performance and lipid peroxide content in senescence-accelerated mouse. Biol Pharm Bull 17: 1481–1484.
- Ohkawa H, Ohishi N, Yagi K (1979): Assay for peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351–358.
- Park CH, Choi SH, Koo JW, Seo JH, Kim HS, Jeong SJ, Suh WH (2002): Novel cognitive improving and neuroprotective activities of Polygala tenuifolia Willdenow extract, BT-11. J Neurosci Res 70: 484–492.
- Piccoli G, Fiorani M, Biagiarelli B, Palma F, Potenza L, Amicucci A, Stocchi V (1994): Simultaneous high-performance capillary electrophoretic determination of reduced and oxidized glutathione in red blood cells in the femtomole range. Chromatogr A 676: 239–246.
- Tamura M, Oschino N, Chance B (1982): Some characteristics of hydrogen and alkylhydroperoxides metabolizing systems in cardiac tissue. J Biochem 92: 1019–1031.
- Tu HH, Liu P, Wang DX, Liao HB, Ma L, Hu Y, Liu YM (2008): Study on antidepressant components of sucrose ester from Polygala tenuifolia Willd. Zhong Guo Zhong Yao Zazhi 33: 1278–1280.
- Wickens AP (2001): Aging and the free radical theory. Respir Physiol 128: 379–391.
- Zheng XY, Li X (2000): Pharmacopoeia of the People’s Republic of China. State Pharmacopiea Commission of China (ed.). Chemical Industry Press, Beijing 297–298. (In Chinese).