Abstract
The hepatoprotective activity of jigrine, a polypharmaceutical herbal formulation, at a dose of 1 mL/kg/day p.o. was evaluated against galactosamine (400 mg/kg b.wt.)-induced hepatopathy in rats. Biochemical parameters such as alanine trasaminase (ALT), alkaline phosphatase (ALP), and bilirubin were estimated to assess liver function. Jigrine was also evaluated for its effect on the possible behavioral alterations secondary to liver damage produced by galactosamine (d-Gal) administration in rats. The d-Gal-induced elevation in serum levels of ALT, ALP, and bilirubin was significantly reduced (p values <0.01, <0.01, and <0.05, respectively) in jigrine- and silymarin-pretreated rats. Jigrine pretreatment also exhibited beneficial effects on d-Gal-induced behavioral abnormalities in rats. Silymarin (25 mg/kg/day p.o.) was used as reference standard. The biochemical observations were supplemented with histopathological examination of rat liver sections. Histopathological evaluation showed marked improvement in the livers of jigrine- and silymarin-treated animals.
Introduction
Jigrine [Hamdard (Wakf) Laboratories] is a polypharmaceutical herbal hepatoprotective syrup formulation containing aqueous extracts of 14 medicinal plants used in the Unani traditional Indian system of medicine for liver ailments (). A few studies are reported on its constituents (CitationNajmi et al., 2002), safety evaluation (CitationValecha et al., 1990), mechanism of hepatoprotective action (CitationVivek et al., 1994; CitationAftab et al., 1999, Citation2002), and anti-inflammatory activity (CitationKarunakar et al., 1997). In general, investigators have concentrated on behavioral study in hepatic damage only when the hepatic toxicant was administered in multiple and high doses to produce hepatic encephalopathy (CitationShimomura et al., 1992; CitationBaraldi et al., 1995), although behavioral and psychiatric deficits are some of the earliest signs in cirrhotic patients (CitationWatanabe et al., 1995). The present investigation was designed to evaluate the hepatoprotective effect of jigrine against d-galactosamine (d-Gal)-induced hepatotoxicity in rats. The aim of this investigation was also to determine whether a single dose of d-Gal could produce any behavioral impairment in rats. The effect of jigrine on the possible behavioral alterations secondary to liver damage produced by d-Gal in rats was also evaluated.
Table 1. Medicinal plant ingredients of jigrine (a phytopharmaceutical formulation).
Materials and methods
Drugs and chemicals
Jigrine was provided by Hamdard (Wakf) Laboratories, Ghaziabad, India. Silymarin was purchased from Micro Laboratories, Holar, TN, India. Galactosamine was purchased from SRL, India. All the biochemicals and chemicals used were of analytical grade.
Animals
Albino rats of Wistar strain weighing 150–200 g were used for the study. Animals were supplied by the Central Animal House Facility of Hamdard University, and were kept under standard laboratory conditions in a 12 h light/dark cycle at 25 ± 2°C. Animals were provided with a pellet diet (Lipton, India) and water ad libitum.
Experimental protocol
Rats were randomly divided into four groups of six animals each. Group I served as normal control and received normal saline for 21 days. Group II served as toxic control and received normal saline (1 ml/kg, p.o.) for 21 days. Groups III and IV were treated prophylactically with jigrine (1 ml/kg, p.o.) and silymarin (25 mg/kg, p.o.) for 21 days, respectively. Groups II, III, and IV also received d-Gal (400 mg/kg, i.p.) on the 21st day (CitationMitra et al., 2000). All the groups were observed in a number of behavioral tests 24 h after d-Gal administration. Behavioral observations were carried out from 7.00 to 11.00 h. Immediately after the behavioral study, blood was collected from the tail vein under light ether anesthesia for estimation of various marker enzymes. Liver samples were also collected for histopathological evaluation. The blood samples were allowed to clot at 37°C for 30–40 min. Serum was separated by centrifugation and used for the estimation of various biochemical parameters. All the procedures carried out on animals were approved by the institutional animal ethics committee (JHAEC).
Behavioral study
Each animal was subjected to a series of tests in the following order: first, the animal was observed inside a plus-maze, then in an open field arena, and finally in an “actophotometer.” Between each observation the animal was returned to its home cage for 30 min (CitationVale et al., 1999).
Spontaneous alternation behavior
Spontaneous alternation testing was conducted by placing a rat on the central platform of the plus-maze and allowing 12 min of unimpeded exploration. The number and sequence of arm entries were recorded for calculation of a “percent alternation score.” An alternation was defined as entry into four consecutive arms on overlapping quintuple sets. Five consecutive arm choices within the set of arm choices made up a quintuple set. A quintuple set consisting of arm choices ABDAC was considered an alternation. A quintuple set consisting of arm choices ABDAB was not considered an alternation. Using this procedure, possible alternation sequences are equal to the number of arm entries minus 4. The percent alternation score is equal to the ratio of (actual alternation/possible alternation) × 100.
The plus-maze was composed of four arms joined to a central platform. Each arm was 55 cm long and 10 cm wide, with 12 cm high walls. The central platform was 25 cm across. The floor and walls were made of gray wood. The maze floor was wiped with a cotton swab dipped in 70% alcohol between animals to prevent accumulation of odors (CitationRagozzino et al., 1996).
Open field test
Rats were placed singly into the open field arena and the following behavioral parameters were scored: ambulation (number of areas entered with all four paws); frequency of rearing; and frequency of grooming. Fecal pellets were removed after every occupation and the floor wiped with clean damp tissues after every occupation. The open field consisted of a circular arena 85 cm in diameter, divided into 25 segments of approximately equal area by black painted lines on the white floor. The arena was bounded by a wall 30 cm high (CitationTricklebank et al., 1978).
Locomotor activity
The spontaneous motor activity of a rat was recorded by placing the animal in a photoelectric actimeter (actophotometer). This apparatus consisted of a square chamber, and the activity of the animal was measured by light beams connected to a photoelectric cell. The total number of beam breaks was measured for 6 min (CitationRenault et al., 1999).
Biochemical estimations
Serum alanine trasaminase (ALT) (CitationReitman & Frankel, 1957), alkaline phosphatase (ALP) (CitationKing & King, 1954), and bilirubin (CitationVarley, 1980) were estimated according to the reported procedures.
Histological studies
Livers were quickly removed and preserved in neutral buffered formalin. Histological liver sections were prepared as previously described (CitationLuna, 1968).
Statistical analysis
Results are expressed as mean ± SEM. For biochemical results, the total variation present in a set of data was estimated by analysis of variance (ANOVA) followed by Dunnet’s post hoc test. For behavioral study, the Mann–Whitney U-test was used. p < 0.05 was considered significant.
Results
A significant increase in ALT, ALP, and bilirubin was observed in animals treated with d-Gal (group II) as compared to the normal control animals (group I). The d-Gal-induced elevation in levels of the above indices was decreased significantly by prophylactic treatment of the rats with both jigrine and silymarin (). Spontaneous alternation behavior evaluation revealed that in d-Gal-treated animals (group II), the alternation score was decreased as compared to the normal group (group I). Jigrine pretreatment increased the percent alternation score significantly. Silymarin also increased the percent alternation score (). Motor activity was decreased by d-Gal administration. Jigrine and silymarin pretreatment restored the motor activity significantly (). Results for open field activity were not significant ().
Table 2. Effect of jigrine and silymarin on various serum biochemicals in d-Gal-induced hepatopathy in rats.
Table 3. Effect of jigrine and silymarin on spontaneous alternation score in d-Gal-induced hepatopathy in rats.
Table 4. Effect of jigrine and silymarin on locomotor activity in d-Gal-induced hepatopathy in rats.
Table 5. Effect of jigrine and silymarin on open field activity in d-Gal-induced hepatopathy in rats.
Histopathological observations
Histology of the liver sections of normal control animals (group I) showed normal hepatic cells with normal liver architecture (). The liver sections of d-Gal-treated animals (group II) showed hepatic cells with severe toxicity characterized by an inflammatory cell collection around the portal tract and central vein, scattered inflammation across the liver parenchyma, focal necrosis, and swelling of vascular endothelial cells (). Jigrine pretreatment in group III appeared to significantly reverse d-Gal toxicity, as revealed by few scattered inflammatory cells with no periportal clustering and necrosis (). Silymarin pretreatment (group IV) also exhibited protection from d-Gal-induced liver toxicity (, ).
Figure 1. Group I: liver section of normal control rat showing normal liver architecture (H&E, ×100).
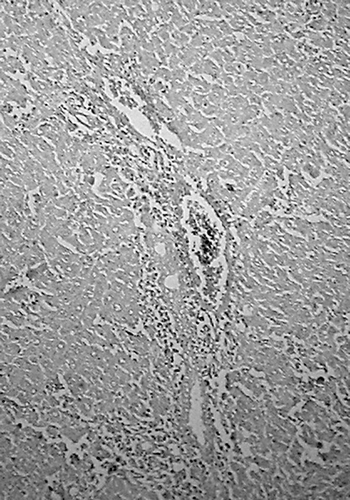
Figure 2. Group II: liver section of rat treated with d-Gal (400 mg/kg, i.p.) showing inflammatory cell collection around portal tract and central vein, scattered inflammation across liver parenchyma, and swelling of vascular endothelial cells (H&E, ×100).
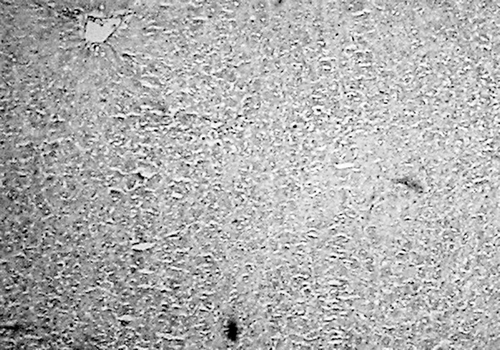
Figure 3. Group III: liver section of rat treated with jigrine (1 ml/kg, p.o.) + d-Gal (400 mg/kg, i.p.) showing only few scattered inflammatory cells (H&E, ×100).
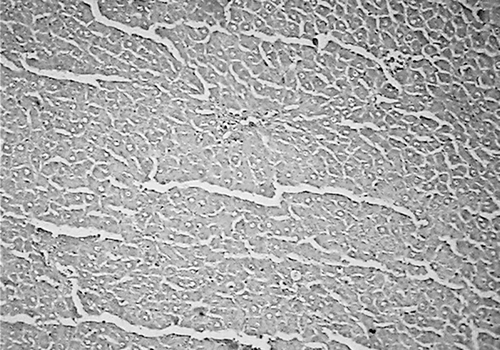
Figure 4. Group IV: liver section of rat treated with silymarin (25 mg/kg, p.o.) + d-Gal (400 mg/kg, i.p.) showing few scattered inflammatory cells and occasional periportal clustering (H&E, ×100).
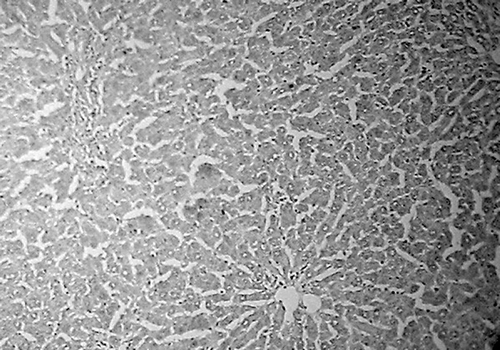
Table 6. Effect of jigrine and silymarin on the degree of liver damage induced by d-Gal administration.
Discussion
d-Gal administration in rats disrupts the permeability of the plasma membrane, causing leakage of enzymes from the cell, which leads to an elevation in serum enzymes (CitationMitra et al., 2000). d-Gal-induced hepatotoxicity is considered to be an experimental model of acute hepatitis (CitationKeppler & Decker, 1969), and resembles human viral hepatitis (CitationKeppler et al., 1968). Elevated levels of serum enzymes are indicative of cellular leakage and loss of functional integrity of the cell membrane in the liver (CitationDrotman & Lawhorn, 1978). Damage to liver cells causes leakage of cellular enzymes into the serum. A significant rise in the transaminase concentration can be taken as an index of liver damage. In our study, the rise in ALT and ALP levels induced by d-Gal administration was significantly reduced by jigrine pretreatment, suggesting that its hepatoprotective activity might be due to its effect against cellular leakage and loss of functional integrity of the cell membrane in the liver. The membrane-stabilizing property of jigrine has already been reported (CitationKarunakar et al., 1997). The d-Gal-induced elevation in bilirubin levels in serum was also significantly reduced by jigrine pretreatment. The biochemical results are also supported by histopathological examination of liver sections of various animals. Histopathological evaluation demonstrated significant protection of the liver cells of jigrine- and silymarin-pretreated animals against d-Gal-induced hepatic damage.
Central nervous system (CNS) functions are often affected secondary to liver damage (CitationGilberstadt et al., 1980). This effect is particularly observed on cognition and memory. d-Gal administration reduced the spontaneous alternation score (group II), indicating the spatial memory-impairing effect of this hepatotoxin. Pretreatment of these animals with jigrine increased the percent alternation score in these rats significantly. Silymarin treatment showed a statistically non-significant increase in the percent alternation score. d-Gal administration also reduced the motor activity (group II), and pretreatment of these animals with jigrine and silymarin increased the motor activity in these rats significantly. Open field results were not significant, although jigrine and silymarin both showed non-significant improvement. Jigrine might have produced these beneficial effects in d-Gal-treated animals due to an improvement of liver function, as observed by the reduction in elevated levels of serum enzymes. There are a few ingredients in jigrine, such as Vitex negundo Linn. (Verbenaceae) (CitationGupta et al., 1999) and Phyllanthus niruri Linn. & Hook (Euphorbiaceae) (CitationSantos et al., 1995), which have strong CNS actions. The above ingredients might have acted directly on the CNS to produce these beneficial effects. The overall improvement in behavioral activity of the animals may also be because of an improvement in liver function.
It can be concluded that a single dose (400 mg/kg) of d-Gal used to induce hepatic damage (CitationMitra et al., 2000), which resembles human viral hepatitis (CitationKeppler et al., 1968), also produces behavioral impairments in rats. Jigrine pretreatment not only protects from the hepatotoxic effects of d-Gal but also prevents the associated behavioral impairments in rats. Further studies are needed to establish the mechanisms of behavioral effects of these drugs.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aftab A, Pillai KK, Najmi AK, Ahmad SJ, Balani DK (1999): Evaluation of hepatoprotective potential of jigrine (a polyherbal Unani formulation) pretreatment on thioacetamide induced liver damage in rats. Indian J Pharmacol 31: 416–421.
- Aftab A, Pillai KK, Najmi AK, Ahmad SJ, Pal SN, Balani DK (2002): Evaluation of hepatoprotective potential of jigrine post treatment against thioacetamide induced hepatic damage. J Ethnopharmacol 79: 35–41.
- Baraldi M, Zeneroli ML, Zanoli P, Truzzi C, Venturini I, Davalli P (1995): Increased brain concentrations of polyamines in rats with encephalopathy due to a galactosamine-induced fulminant hepatic failure. Pharmacol Res 32: 57–61.
- Drotman RB, Lawhorn GT (1978): Serum enzymes are indicators of chemical induced liver damage. Drug Chem Toxicol 1: 163–171.
- Gilberstadt SJ, Gilberstadt H, Zieve L, Buegel B, Collier RO (1980): Psychomotor performance defects in cirrhotic patients without overt encephalopathy. Arch Intern Med 140: 519–521.
- Gupta M, Mazumdar UK, Bhawal SR (1999): CNS activity of Vitex negundo Linn. in mice. Indian J Exp Biol 37: 143–146.
- Karunakar N, Pillai KK, Hussain SZ, Rao, M, Balani DK (1997): Further studies on the anti-hepatotoxic activity of jigrine. Indian J Pharmacol 29: 222–227.
- Keppler D, Decker K (1969): Studies on the mechanism of the galactosamine induced hepatitis. Eur J Biochem 19: 219–225.
- Keppler D, Lesch R, Reutter W, Decker K (1968): Experimental hepatitis induced by d-galactosamine. Exp Mol Pathol 9: 279–290.
- King PRN, King EJ (1954): Estimation of plasma phosphatase by determination of hydrolyzed phenol with amino antipyrine. J Clin Pathol 7: 322–326.
- Luna LG (1968): Manual of Histology, Staining Methods of Armed Forces Institute of Pathology, 3rd ed. New York, McGraw-Hill Book Co., p. 43.
- Mitra SK, Seshadhari SJ, Venkataranganna MV, Gopumadhavan S, Udupa UV, Sarma DNK (2000): Effect of HD-03 – a herbal formulation in galactosamine induced hepatopathy in rats. Indian J Physiol Pharmacol 44: 82–86.
- Najmi AK, Pillai KK, Ahmad SJ, Nazmi AS (2002): Jigrine: A reappraisal of its medicinal ingredients. Hamdard Medicus XLIV(4): 34–39.
- Ragozzino ME, Unick KE, Gold PE (1996): Hippocampal acetylcholine release during memory testing in rats: Augmentation by glucose. Proc Natl Acad Sci USA 93: 4693–4698.
- Reitman S, Frankel AS (1957): A colorimetric method for the estimation of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28: 53–56.
- Renault O, Huard GC, Meil H, Steibing S, Le Bourn S, Boulouard M (1999): Synthesis and CNS activity of 3-amino-3-arylpropionic acid derivatives. Pharm Pharmacol Commun 5: 217–223.
- Santos AR, Filho VC, Yunes RA, Calixto JB (1995): Analysis of the mechanism underlying the antinociceptive effect of plants from genus Phyllanthus. Gen Pharmacol 26: 1499–1506.
- Shimomura Y, Saito S, Nagamine T, Shimizu H, Takahashi M, Uehara Y, Sato N, Negishi M, Yamada S; Kobayashi I (1992): Feeding behavior and ambulatory activity in rats with d-galactosamine-induced hepatic failure. Physiol Behav 51: 1029–1033.
- Tricklebank MD, Smart JL, Bloxam DL, Curzon G (1978): Effects of chronic experimental liver dysfunction and l-tryptophan on behaviour in the rat. Pharmacol Biochem Behav 9: 181–189.
- Vale TG, Matos FJA, De Lima TCM, Viana GSB (1999): Behavioral effects of essential oils from Lippia alba (Mill) N.E. Brown chemotypes. J Ethnopharmacol 167: 127–133.
- Valecha N, Khan EA, Dandiya PC (1990): A note on the safety evaluation of jigrine, a Unani hepatoprotective herbal formulation. Indian Drugs 27: 411–415.
- Varley H, ed. (1980): Practical Clinical Biochemistry. London, William Heinemann Medical Books Ltd., pp. 1016–1017.
- Vivek K, Pillai KK, Hussain SZ, Balani DK (1994): Hepatoprotective activity of jigrine on liver damage caused by alcohol-CCl4 and paracetamol in rats. Indian J Pharmacol 26: 35–40.
- Watanabe A, Tuchida T, YataY Kuwara, Y (1995): Evaluation of neuropsychological functions in patients with liver cirrhosis with special reference to their driving ability. Metab Brain Dis 10: 239–245.
