Abstract
Context: The search for innovative therapeutic approaches is gaining more interest in clinical oncology.
Objective: In the present investigation we reported the chemical profile and the photo-induced cytotoxic activity of two endemic Calabrian Citrus species (Rutaceae): Citrus bergamia Risso & Poit. and Citrus medica L. cv. Diamante.
Materials and methods: Essential oils were obtained by hydrodistillation and analyzed by GC and GC/MS. In order to evaluate the cytotoxic activity two melanoma models, such as amelanotic melanoma C32 and malignant melanoma A375, were used.
Results: The essential oil of C. bergamia was characterized by limonene, linalyl acetate, γ-terpinene, linalool and β-pinene as major components. The most abundant compounds of C. medica cv. Diamante oil were limonene, γ-terpinene, citral, geranial, β-pinene and α-pinene. Two coumarins, bergapten and citropten, were also identified in C. bergamia and C. medica cv. Diamante, respectively and tested for biological activity. Both C. bergamia and C. medica cv. Diamante oils exhibited a selective interesting activity against the A375 cell line with IC50 values of 79.3 and 89.1 µg/mL, respectively, after 100 min exposure to UV irradiation. The strong antiproliferative activity demonstrated with bergapten (IC50 value of 71.3 µg/mL after 20 min of irradiation) was not found with citropten.
Discussion and conclusion: Our study suggested that UV irradiation is effective in activating essential oils and in particular bergapten. This phototoxicity may be considered as a treatment option in some cases of lentigo maligna or lentigo maligna melanoma.
Introduction
Malignant melanoma is an aggressive and therapy-resistant malignancy of melanocytes. The incidence of melanoma has been steadily increasing worldwide, resulting in an increasing public health problem. The current spectrum of malignant melanoma includes two clinical extremes. At one end of the spectrum thin primary cutaneous melanoma is characterized by a relatively uniform treatment and a high cure rate. At the opposite end, metastatic melanoma is characterized by no proven effective therapy and poor outcomes. In recent decades a heightened awareness of melanoma has led to an increased rate of diagnosis of early stage disease. Notwithstanding the fact that treatment of advanced melanoma still remains in the realm of experimental, tremendous research efforts in melanocyte biology, melanoma genetics, and tumor immunology are providing hope for a cure. Although more than 95% of tumors are found in the skin, melanoma is not exclusively a skin cancer. Sites of primary extracutaneous melanoma include ocular, mucosal, gastrointestinal, genitourinary, leptomeninges, and lymph nodes (CitationMarkovic et al., 2007).
Citrus bergamia Risso & Poit. (Bergamot) (Rutaceae) constitutes an agricultural production of Calabria, a region of southern Italy. Of worldwide bergamot cultivation, 95% occurs in the Ionic area of Calabria. The cultivation of bergamot can become a leading economic resource of the zones of production in southern Europe. Bergamot is mainly cultivated for its essential oil, an important widely used raw substance that has great commercial value. It is necessary in the international perfumery industry as it has not only the function of fixing the aromatic bouquets of perfumes, but also that of blending all the other essences contained in them, exalting notes of freshness and fragrance. The essential oil of bergamot is an important ingredient in many alimentary, cosmetic and sanitary preparations (CitationVerzera et al., 2003). As compared with other Citrus oils, bergamot oil is marked by a lower amount of limonene (25.6%–53%) and higher amounts of linalool (1.7%–20%) and linalool acetate (15.6%–40.4%) (CitationVerzera et al., 1996). Generally, these volatile compounds make up approximately 93%–96% of the bergamot oil. The characteristic flavor and pharmacological properties of Citrus oils are mainly provided by the oxygenated compounds, which consist of alcohols, aldehydes, and esters, such as linalool, citral and linalool acetate, respectively. Previously, CitationGupta & Anderson (1987) reported that psoralens were used in the therapy of psoriasis and vitiligo. In addition, recent electrophysiological experiments carried out using the patch-clamp technique demonstrate that natural and synthetic psoralens, e.g. bergapten and 8-methoxypsoralen, respectively, block voltage-gated K+ channels in Ranvier nodes, Schwann cells maintained in vitro (CitationDuring et al., 2000). Inhibition of K+ currents by alkoxypsoralens has also been reported in cultured immune cells together with inhibition of immune cell proliferation response, interferon-gamma gene expression and cytokine production supporting a role in the modulation of the immune response which follows, for instance, encephalitogenic stimuli (CitationStrauss et al., 2000). In addition, experimental evidence demonstrates that bergamottin, another important component of the non-volatile fraction of bergamot essential oil, might be endowed with Ca2+ antagonist in vitro properties (CitationOcchiuto & Circosta, 1996; Citation1997). It is worth noting, however, that very limited information is available in the literature concerning the biological effects of the bergamot essential oil as such. Interestingly, in industrialized countries this oil, as well as other essential oils, is increasingly used in aromatherapy to minimize the effects of stress-induced anxiety and/or to facilitate sleep induction (CitationKomori et al., 1995; CitationLehrner et al., 2000) suggesting a possible modulatory activity on neurotransmission in the mammalian central nervous system.
C. medica L. cv. Diamante (Rutaceae) cultivation is limited to only one area, Calabria in Southern Italy. Its fruits are used in food industry to prepare candy and liqueurs, while the oil is used as flavoring in sweets and beverages (CitationCutuli et al., 1985). Recent investigations reported the chemical composition of Diamante citron peel essential oil (CitationPoiana et al., 1998; CitationVerzera et al., 2005) but few studies evaluated its biological activity (CitationConforti et al., 2007).
Bergamot and citron oils are used not only in the food industry but also in the pharmaceutical and perfumery industries (CitationHuet, 1991; CitationLanças & Cavicchioli, 1990). Citrus plants are rich sources of various physiologically active compounds, including coumarins that have recently drawn much attention due to their broad pharmacological activities (CitationBorges et al., 2005). Many coumarins and their derivatives exert in vitro and in vivo scavenging of reactive oxygen species (ROS), anti-coagulant, anti-inflammatory and anti-viral effects (CitationManolov et al., 1993; CitationFylaktakidou et al., 2004; CitationKostova et al., 2006). In sensitive neoplasms, coumarins and derivatives cause significant changes in cell growth and differentiation (CitationRiveiro et al., 2004, Citation2007). Furthermore, coumarin derivatives modulate multidrug resistance transporters which are intimately related with the resistance developed to diverse chemotherapeutic therapies (CitationKawase et al., 2005).
The use of drugs in association with ultra-violet light for the treatment of skin diseases can be traced back to ancient Egypt, India and Greece, where plant extracts, containing psoralens, were applied on the skin in association with light to treat psoriasis and vitiligo (CitationDaniel & Hill, 1991). This concept, known as photochemotherapy, represents a common basis for different therapeutic procedures, such as PUVA (psoralen + UVA) and photodynamic therapy. The mechanism of cellular damage mediated by photochemotherapy includes a variety of biochemical and molecular reactions, leading to inhibition of the hyperproliferation of skin keratinocytes or tissue destruction (CitationRopp et al., 2004).
On the basis of these considerations, in this paper we evaluated the photo-induced cytotoxic activity of C. bergamia and C. medica cv. Diamante peel essential oils and its coumarin components against two melanoma models C32 and A375.
Materials and methods
Chemicals
Dimethylsulfoxide (DMSO), dichloromethane and anhydrous sodium sulfate (Na2SO4) were obtained from VWR International (Milan, Italy). Limonene, sabinene, α-pinene, β-pinene, β-myrcene, α-terpinene, γ-terpinene, terpinolene, δ-cadinene, β-caryophyllene, α-humulene, citropten, bergapten, RPMI 1640, Dulbecco’s modified essential medium (DMEM), l-glutamine, penicillin/streptomycin, fetal bovine serum, sulforodamine B (SRB), trichloroacetic acid (TCA), vinblastine sulfate salt, and tris[hydroxymethyl]aminomethane were purchased from Sigma-Aldrich (Milan, Italy).
Isolation of the essential oils
The fruits of C. medica cv. Diamante used in this study were collected in October 2006 in Calabria (southern Italy), loc. Santa Maria del Cedro (Cosenza). The authentication of the fruits was carried out by Dr. N. Passalacqua at at the Natural History Museum of Calabria and Botanic Garden, University of Calabria, Italy. Fresh peels (1.8 kg) of C. medica cv. Diamante were subjected to hydrodistillation for 3 h using a Clevenger-type apparatus (CitationClevenger, 1928). The white-yellow essential oil was dried (anhydrous Na2SO4) to remove traces of moisture (yield 0.1% v/w). The C. bergamia essential oil was obtained by the “Consorzio del bergamotto” of Reggio Calabria (Italy) by cold pressure procedure. Both oils were stored at 4-8°C in a bottle covered with aluminum foil to prevent the negative effect of light until tested and analyzed.
Gas chromatography-mass spectrometry
In order to determine the Citrus bergamia and C. medica essential oils composition, analyses were carried out using a gas chromatography (GC) system (Hewlett-Packard 6890 N) with a fused capillary column (30 m length; 0.25 mm i.d.; 0.25-µ film thickness; static phase methyl silicone SE-30) directly coupled to a selective mass detector (Hewlett Packard 5973 N). Electron impact ionization was carried out at energy of 70 eV. Helium was used as carrier gas. Injector and detector were maintained at 250°C and 280°C, respectively. The analytical conditions were: 50°C for 5 min, 50–250°C with a rate of 5°C/min, 250°C for 10 min. Constituents of essential oils were identified by gas chromatography by comparison of their GC retention indices with those of the literature or with those of standards available in our laboratories. The retention indices were determined in relation to a homologous series of n-alkanes (C8–C24) under the same operating conditions. Further identification was made by comparison of their mass spectra on both columns with those stored in Wiley 138 and NIST 98 libraries or with mass spectra from literature () (CitationAdams, 1995).
Table 1. Components of Citrus bergamia Risso & Poit. (Bergamot oil) and C. medica L. cv. Diamante (Diamante oil) essential oils identified by GC-MS analysis.
Cell culture
Amelanotic melanoma C32 (ATCC no.: CRL-1585) and malignant melanoma A375 (ECACC no. 88113005) were used. The C32 cell line was cultured in RPMI 1640 medium, while the A375 cell line was cultured in DMEM. Both media were supplemented with 10% fetal bovine serum, 1% L-glutamine, and 1% penicillin/streptomycin. The cell lines were maintained at 37°C in a 5% CO2 atmosphere with 95% humidity.
Evaluation of in vitro photo-induced cytotoxic activity
Cytotoxic screening models provide important preliminary data to select plants with potential anticancer compounds, and the sulforodamine B (SRB) assay used in this study is commonly employed (CitationLoizzo et al., 2005). The SRB assay, developed by the National Cancer Institute for the in vitro anticancer screening, was used to estimate cell number indirectly by providing a sensitive index of total cellular protein content that is linear to cell density. The cells were trypsinized, counted, placed in 96-well plates in a concentration ranging from 5 × 104 to 15 × 104, and incubated to allow for cell attachment. After 24 h the cells were treated with serial dilutions of the samples. Each sample was initially dissolved in DMSO and further diluted in medium to produce different concentrations. Aliquots of 100 µL/well of each dilution were added to the plates in six replicates to obtain the final concentrations ranging from 5 to 100 µg/mL for oils and compounds. The final mixture used for treating the cells contained not more than 0.5% of the solvent (DMSO), the same as in the solvent-control wells. Treated and non-treated cells were irradiated for 20, 40, 60 and 100 min using a light source equipped with a halogen lamp emitting in the wavelength range 254-365 nm (Vilber Lourmat, Deutschland). The device is routinely used in laboratories for fluorescent techniques. The irradiation was done at λ = 365 nm and distribution of the light source is not fully homogenous at a few cm distances from the light guide. Therefore, the light guide was held close to the cell dish or plate. Irradiation was performed by moving the light guide slowly across the bottom side of the cell dish or plate. This irradiation procedure contributes to a homogenous irradiation distribution and dose to each cell. Also, light delivery from the bottom of the cell dish avoids absorption of light through the cell medium and decreases the light path to the cells growing in monolayer. After 48 h of exposure 100 µL of ice-cold 40% trichloroacetic acid (TCA) was added to each well, left for 1 h at 4°C, and washed with distilled water. The TCA-fixed cells were stained for 30 min with 50 µL of 0.4% (w/v) SRB in 1% acetic acid. The plates were washed with 1% HOAc and air dried overnight. For reading the plate, the bound dye was solubilized with 100 µL of 10 mM tris base (tris[hydroxymethyl]aminomethane). The absorbance of each well was read on a Molecular Devices SpectraMax Plus Plate Reader (Molecular Devices, Celbio, Milan) at λ = 564 nm. Cell survival was measured as the percentage absorbance compared to the untreated control. Vinblastine sulfate salt was used as positive control.
Statistical analysis
All experiments were carried out in triplicate. Data were expressed as means ± SD. Differences were evaluated by the one-way analysis of variance (ANOVA) test completed by a multicomparison Dunnett’s test. Differences were considered significant at p < 0.01. The inhibitory concentration 50% (IC50) was calculated by a non-linear regression curve with the use of Prism GraphPad Prism version 4.0 for Windows (GraphPad Software, San Diego, CA). The dose-response curve was obtained by plotting the percentage of cell viability versus the concentrations.
Results and discussion
The essential oils of C. bergamia and C. medica were subjected to a detailed GC-MS analysis to determine their chemical composition (). C. medica essential oil was characterized by forty-three components, representing 95.3% of the total oil. The most abundant compounds were the monoterpenes limonene (35.4%), γ-terpinene (24.5%), geranial (5.5%), neral (4.4%), β-pinene (2.6%), α-pinene (2.5%) β-myrcene (2.1%) and terpinen-4-ol (1.5%). Among the sesquiterpenes the most abundant component was β-bisabolene (1.2%). Twenty-eight constituents were identified in the C. bergamia essential oil, representing 97.5% of the total oil. This oil was characterized by limonene (38.1%), linalyl acetate (28.9%), γ-terpinene (7.3%), linalool (6.4%), β-pinene (5.4%) and bergapten (1.7%) as major components.
Several monoterpenes were previously analyzed for their antitumor activity. CitationCalcabrini et al. (2004) evaluated the potential activity of terpinen-4-ol against human melanoma M14 WT cells and their drug-resistant counterparts, M14 adriamicin-resistant cells. This compound was able to induce caspase-dependent apoptosis of melanoma cells and this effect was more evident in the resistant variant cell population. Our recent study demonstrated that linalool was found active against amelanotic melanoma cell line C32 with IC50 of 23.16 µg/mL (CitationLoizzo et al., 2007).
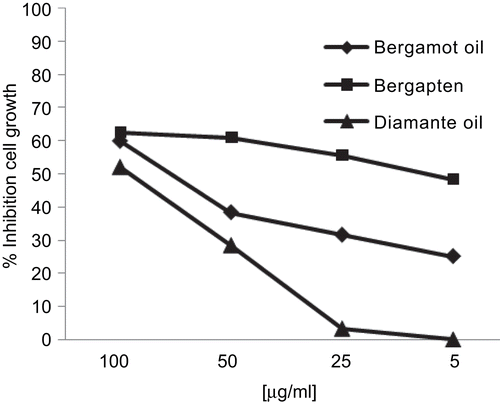
The search for innovative therapeutic approaches based on the use of new substances is gaining more interest in clinical oncology. In the conventional applications of photodynamic therapy, light-activated chemicals (photosensitizers) are used to destroy fast-growing cells and tissues. Upon exposure to light the sensitizer initiates a cascade of molecular reactions that can destroy those cells and the tissues they constitute. The destructive reactions are partly based on the sensitized formation of singlet oxygen and partly on the degradation of the sensitizer itself leading to free radical formation. Light activated drugs used in photodynamic therapy have a potential in the treatment of cancer located at a number of sites (e.g. skin, esophagus, lungs, head and neck, brain, mouth and bladder) (CitationSibata et al., 2000). Although photodynamic mechanisms (i.e. through endogenous photosensitizers) play a role in UVA phototherapy for the treatment of skin disorders such as eczema and psoriasis, photodynamic therapy employing exogenous photosensitizers are currently being used only for the treatment of certain forms of non-melanoma skin cancers and actinic keratoses. However, there are few reports on its use in treating melanomas. In this in vitro study the potential antitumor activity of Citrus oils was analyzed against two melanoma models. We showed that neither oils and coumarins exposure nor UV irradiation alone reduces cell viability in C32 cell line. On the contrary both bergamot and Diamante oils exhibited an interesting activity against A375 cell line after 100 min of UV irradiation at λ = 365 nm with IC50 values of 79.3 and 89.1 µg/mL, respectively (). It is commonly accepted that cancer formation can be prevented by the consumption of certain foods, and Citrus fruits and juices are one of the most prominent cancer-preventing foods (CitationBae et al., 2008). Citrus plants are rich sources of various physiologically active substances, including coumarins. Coumarins comprise a very large class of compounds found throughout the plant kingdom (CitationEgan et al., 1990). The biological activity of coumarins and more complex related derivatives appears to be based on the coumarin nucleus and several study reported the cytostatic activity of these natural products against neoplastic skin cell line (CitationJimenez-Orozco et al., 1999; CitationFinn et al., 2001). On the basis of these studies we included in our work the furanocoumarin bergapten and the coumarin citropten derived from Citrus species. A dose-response relationship could be found for photo-induced cytotoxic activity of bergapten that at least after 20 min of irradiation exhibited an IC50 of 71.3 µg/mL while after 100 min an IC50 value of 9.1 µg/mL (). On the contrary, bergamot oil exhibited activity only after 100 min of UV irradiation at λ = 365 nm (). This different behavior could be attributed at the percentage of the coumarin in this oil. The strong antiproliferative activity demonstrated with bergapten was not found with citropten that exhibited a percentage of inhibition of 33% at 100 µg/mL after 100 min of exposure. CitationAlesiani et al. (2008) reported that citropten exhibited an IC50 of 300 µM and 142 µM, respectively, against A375 cell line using MTT assay and Trypan blue exclusion test. Structure–activity relationship revealed that the antiproliferative activity of bergapten depends on the presence of a furano-ring in the structure since citropten is lacking in this ring. Treatment with the coumarin psoralen and its derivatives such as bergapten with UVA is referred to as PUVA, and is used for the treatment of psoriasis. PUVA treatments cause inflammation and redness in the skin to develop within 2-3 days after treatment. Such damage inhibits skin cell proliferation and reduces psoriasis plaque formation. It has been known for some time that PUVA can change DNA and cause genetic mutations. PUVA is known to increase the risk for squamous cell skin cancer and slightly increase the risk for basal cell skin cancer, both of which are nearly always curable. One study reported an increased risk of melanoma. For this reason during application of phototherapy is necessary to use lamps with protective shields that can select the area of treatment. In each case these side effects appear after long-term treatment with PUVA (CitationVussuki et al., 2006). Previously, CitationCarneiro Leite et al. (2004) reported that human melanoma cells treated with 8-methoxypsoralen, 4,5′,8-trimethylpsoralen and 7-methylpyridopsoralen, and exposed to UVA light (0.3 J/cm2) were markedly cytotoxic in a dose- and post-exposure time- dependent manner.
Table 2. Photo-induced cytotoxic activity of Citrus bergamia Risso & Poit. (Bergamot oil) and C. medica L. cv. Diamante (Diamante oil) essential oils, bergapten and citropten at different times of exposure (20-100′) at λ = 365 nm against human melanoma A375 (IC50 µg/mL).
Conclusions
Our study suggests that UV irradiation is effective in activating C. medica cv Diamante and C. bergamia essential oils, and in particular bergapten. This phototoxicity may be considered as a treatment option in some cases of lentigo maligna or lentigo maligna melanoma that are too large for surgical resection.
Acknowledgements
The authors wish to thank Consorzio del Bergamotto, Reggio, Calabria, Italy, for C. Bergamia oil and V. Filippelli for English revision of the manuscript.
Declaration of interest
The authors have declared no conflict of interest.
References
- Adams R (1995): Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. New York City, USA: Carol Stream, Allured Publishing.
- Alesiani D, Cicconi R, Mattei M, Montesano C, Bei R, Canini A (2008): Cell cycle arrest and differentiation induction by 5,7-dimethoxycoumarin in melanoma cell lines. Int J Oncol 32: 425–434.
- Bae JM, Lee EJ, Guyatt G (2008): Citrus fruit intake and stomach cancer risk: A quantitative systematic review. Gastric Cancer 11: 23–32.
- Borges F, Roleira F, Milhazes N, Santana L, Uriarte E (2005): L Simple coumarins and analogues in medicinal chemistry: Occurrence, synthesis and biological activity. Curr Med Chem 12: 887–916.
- Calcabrini A, Stringaro A, Toccacieli L, Meschini S, Marra M, Colone, M, Salvatore G, Mondello F, Arancia G, Molinari A (2004): Terpinen-4-ol, the main component of Melaleuca alternifolia (tea tree) oil inhibits the in vitro growth of human melanoma cells. J Inv Dermatol 122: 349–360.
- Carneiro Leite V, Ferreira Santos R, Chen Chen L, Andreu Guillo L (2004): Psoralen derivatives and longwave ultraviolet irradiation are active in vitro against human melanoma cell line. J Photochem Photobiol B 76: 49–53.
- Clevenger JF (1928): Apparatus for the determination of volatile oils. J Am Pharm Assoc 17: 341–346.
- Conforti F, Statti GA, Tundis R, Loizzo MR, Menichini F (2007): In vitro activities of Citrus medica L. cv. Diamante (Diamante citron) relevant to treatment of diabetes and Alzheimer’s disease. Phytother Res 21: 427–433.
- Cutuli G, Di Martino E, Lo Giudice V, Pennisi L, Raciti G, Russo F, Scuderi A, Spina P (1985): Trattato di agrumicoltura. Bologna, Edagricole, 21.
- Daniel MD, Hill JS (1991): A history of photodynamic therapy. Aust N Z J Surg 61: 340–348.
- During T, Gerst F, Hansel W, Wulff H, Koppenhofer E (2000): Effects of three alkoxypsoralens on voltage gated ion channels in Ranvier nodes. Gen Physiol Biophys 19: 345–364.
- Egan DA, O’Kennedy R, Moran E, Thornes RD (1990): The pharmacology, metabolism, analysis and applications of coumarin and coumarin related compounds. Drug Metabol Rev 22: 503–529.
- Finn GJ, Creaven B, Egan D (2001): Study of the in vitro potential of natural and synthetic coumarin derivatives, using human normal and neoplastic skin cell lines. Melanoma Res 11: 461–467.
- Fylaktakidou KC, Hadjipavlou-Litina DJ, Litinas KE, Nicolaides DN (2004): Natural and synthetic coumarin derivatives with anti-inflammatory/ antioxidant activities. Curr Pharm Design 10: 3813–3833.
- Gupta AK, Anderson TF (1987): Psoralen photochemotherapy. JAAD 17: 703–734.
- Huet R (1991): Les huiles essentielles d’agrumes. Fruits 46: 501–513.
- Kawase M, Sakagami H, Motohashi N, Hauer H, Chatterjee SS, Spengler G, Vigyikanne AV, Molnar A, Molnar J (2005): Coumarin derivatives with tumor-specific cytotoxicity and multidrug resistance reversal activity. In Vivo 19: 705–711.
- Komori T, Fujiwara R, Tanida M, Nomura J (1995): Potential antidepressant effects of lemon odor in rats. Eur Neuropsychopharmacol 5: 477–480.
- Kostova I, Raleva S, Genova P, Argirova R (2006): Structure-activity relationships of synthetic coumarins as HIV-1 inhibitors. Bioinorganic Chem Appl 68274: 1–9.
- Jimenez-Orozco FA, Molina-Guarneros JA, Mendozapatino N, Leon-Cedeno F, Flores-Perez B, Santos-Santos E, Mandoki JJ (1999): Cytostatic activity of coumarin metabolites and derivatives in the B-16-F10 murine melanoma cell line. Melanoma Res 9: 243–247.
- Lanças FM, Cavicchioli M (1990): Analysis of the essential oils of Brazilian citrus fruits by capillary gas chromatography. J High Res Chromatogr 13: 207–209.
- Lehrner J, Eckersberger C, Walla P, Pötsch G, Deecke L (2000): Ambient odor of orange in a dental office reduces anxiety and improves mood in female patients. Physiology & Behavior 71: 83–86.
- Loizzo MR, Tundis R, Statti GA, Menichini F, Houghton PJ (2005). In vitro antiproliferative effects on human tumour cell lines of extracts and jacaranone from Senecio leucanthemifolius Poiret. JPP 57: 897–901.
- Loizzo MR, Tundis R, Menichini F, Saab AM, Statti GA, Menichini F (2007): Cytotoxic activity of essential oils from Labiatae and Lauraceae families against in vitro human tumor models. Anticancer Res 27: 3293–3299.
- Manolov I, Topashka-Ancheva M, Klouchek E (1993): The synthesis, toxicological and comparative cytogenetic characteristics of the anticoagulant warfarin. Ekspert Med Morfol 31: 49–60.
- Markovic SN, Erickson LA, Rao RD, Weenig RH, Pockaj BA, Bardia A, Vachon CM, Schild SE, McWilliams RR, Hand JL, Laman SD, Kottschade LA, Maples WJ, Pittelkow MR, Pulido JS, Cameron JD, Creagan ET.(2007): Melanoma Study Group of the Mayo Clinic Cancer Center. Malignant melanoma in the 21st century, part 1: Epidemiology, risk factors, screening, prevention, and diagnosis. Mayo Clin Proc 82: 364–380.
- Occhiuto F, Circosta C (1996): Antianginal and antiarrhythmic effects of bergamottin, a furocoumarine isolated from bergamot oil. Phytother Res 10: 491–496.
- Occhiuto F, Circosta C (1997): Investigations to characterize the antiarrhythmic action of bergamottin, a furocoumarine isolated from bergamot oil. Phytother Res 11: 450–453.
- Poiana M, Sicari V, Mincione B (1998): A comparison between the chemical composition of the oil, solvent extract and supercritical carbon dioxide extract of Citrus medica L. cv. Diamante. JEOR 10: 145–152.
- Riveiro EM, Shayo C, Monczor F, Fernandez N, Baldi A, De Kimpe N, Rossi J, Debenedetti S, Davio C (2004): Induction of cell differentiation in human leukemia U-937 cells by 5-oxygenated-6,7-methylenedioxycoumarins from Pterocaulon polystachyum. Cancer Lett 210: 179–188.
- Riveiro ME, Vazquez R, Moglioni A, Gomez N, Baldi A, Davio C, Shayo C (2007): Biochemical mechanisms underlying the pro-apoptotic activity of 7,8-dihydroxy-4-methylcoumarin in human leukemic cells. Biochem Pharmacol 75: 725–736.
- Ropp S, Guy J, Berl V, Bischoff P, Lepoittevin JP (2004): Synthesis and photocytotoxic activity of new alpha-methylene-gamma-butyrolactone-psoralen heterodimers. Bioorg Med Chem 12: 3619–3625.
- Sibata CH, Colussi VC, Oleinick NL, Kinsella TJ (2000): Photodynamic therapy: A new concept in medical treatment. Braz J Med Biol Res 33: 869–880.
- Strauss U, Wissel K, Jung S, Wulff H, Hänsel W, Zhu J, Rolfs A, Mix E (2000): K+ channel-blocking alkoxypsoralens inhibit the immune response of encephalitogenic T line cells and lymphocytes from Lewis rats challenged for experimental autoimmune encephalomyelitis. Immunopharmacol 48: 51–63.
- Verzera A, Lamonica G, Mondello L, Trozzi A, Dugo G (1996): The composition of bergamot oil. Perfum FlaVorist 21: 19-28, 30-32, 34.
- Verzera A, Trozzi A, Gazea F, Cicciarello G, Cotroneo A (2003): Effects of rootstock on the composition of bergamot (Citrus bergamia Risso et Poiteau) essential oil. J Agr Food Chem 51: 206–210.
- Verzera A, Trozzi A, Zappalá M, Condurso C, Cotroneo A (2005): Essential oil composition of Citrus meyerii Y. Tan. and Citrus medica L. cv. Diamante and their lemon hybrids. J Agr Food Chem 53: 4890–4894.
- Vussuki E, Ziv M, Rosenman D, David M (2006): Long-term effects of PUVA therapy on Israeli patients with vitiligo. Harefuah 145: 483-485, 552, 551.