Abstract
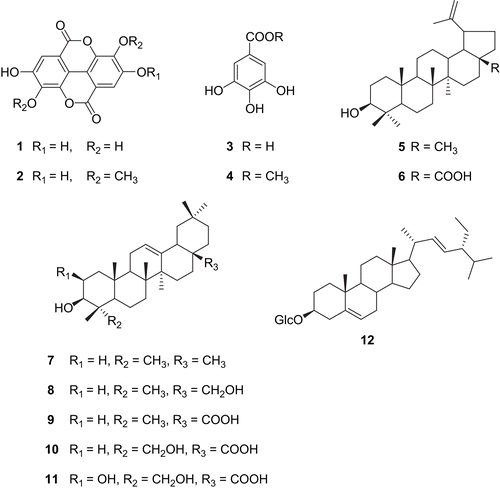
Bioassay-guided fractionation of the methanol extract of the stem bark of Klainedoxa gabonensis Pierre ex Engl. (Irvingiaceae) afforded 12 compounds, namely, ellagic acid (1), ellagic acid 3,3′-dimethylether (2), gallic acid (3), methyl gallate (4), lupeol (5), β-amyrin (7), erythrodiol (8), oleanolic acid (9), betulinic acid (6), hederagenin (10), bayogenin acid (11), and stigmasterol-3-O-β-d-glucopyranoside (12). Compounds 1-3 and 7-12 were isolated for the first time from this genus. The structures were established on the basis of 1D/2D NMR experiments and mass spectrometric data. Crude extract, fractions (A, B, C and D) and pure compounds were tested for their antimicrobial activity using paper disk agar diffusion assay. The test delivered a range of low to high activities for phenolic compounds 1-4, low or missing activities for terpenoid compounds 5-11, and impressive very high antibacterial/antifungal values for two fractions C and D probably due to synergistic effects of compounds. The broth microdilution assay revealed MICs of 15.4-115.1 μg/mL for phenolic compounds, MICs higher than 1 mg/mL for terpenoids and MICs of 4.5-30.3 μg/mL for fractions C and D. The determination of the radical scavenging activity using 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay gave high antioxidant values for the methanol extract and fraction D (IC50 10.45 and 5.50 μg/mL) as well as for the phenolic compounds 1-4 (IC50 45.50-48.25 mM) compared to the standard 3-t-butyl-4-hydroxyanisole (BHA) (IC50 44.20 mM).
Introduction
In our program to valorise Cameroonian rain-forest medicinal plants with respect to the discovery of potential antitumoral, anti-inflammatory, antibacterial, antimalarial, and antioxidant compounds, we investigated Klainedoxa gabonensis Pierre ex Engl. Klainedoxa is one of the three genera of the Irvingiaceae family; two species of this genus (K. gabonensis and K. grandifolia Engl.) exist in Cameroon (CitationAubreville, 1962). K. gabonensis is a big tree with orange or golden brown heartwood, turning to dark brown with black veining when exposed; the sapwood is not clearly demarcated, the texture is fine to medium, the grain is straight to interlocked; the tree is without characteristic odor or taste. It predominantly occurs in evergreen forest on sandy soil (CitationAdjanohoun et al., 1988) and can also be found in Guinea, Congo, Uganda, Sudan and Gabon. In Cameroon it is used locally for the treatment of rheumatism, swelling, inflammation, bacterial infections, and diarrhea (CitationBouquet, 1969). In Congo, it is one of the main plants used by traditional healers for the treatment of a disease locally known as “mwandza” dermatitis for which modern physician’s treatments are yet unsuccessful (CitationOnanga et al., 1999). Previous phytochemical investigation of K. gabonensis reported the isolation of methyl gallate from the methanol extract of the trunk bark (CitationEkouya et al., 2005, Citation2006), betulinic acid, lupeol, β-sitosterol, β-amyran-3-one, and 3,3′,4′-tri-O-methylellagic acid (CitationDongo et al., 2009), geranyl acetone (13.8%), β-bourbonene (11.1%) and (Ε)-α-ionone (10.5%) from the leaves; linalool (17.4%), 1, 8-cineole (9.9%), and 1-octen-3-ol (8.0%) from the stem bark, with 1, 2, 3-trimethylbenzene (9.8%), 1-ethyl-2-methyl benzene (9.1%), pentyl benzene(9.1%), and methyl salicylate (9.1%) occurring in higher proportions in the root oil (CitationOgunwande et al., 2008). In this paper we report the isolation of some constituents from K. gabonensis, together with the antimicrobial and antioxidant activities of crude extract and isolated compounds.
Materials and methods
General experimental procedures
Ultraviolet spectra were recorded on a Hitachi UV 3200 spectrophotometer in MeOH. Infrared spectra were recorded on a JASCO 302-A spectrophotometer. ESI-HR mass spectra were recorded on a Bruker FTICR 4.7 T mass spectrometer. EI-MS spectra were recorded on a Finnigan MAT 95 spectrometer (70 eV) with perfluorokerosene as reference substance for EI-HR-MS. GC-MS analyses were carried out in a GC chromatograph coupled with an ion trap mass detector, using a DB5-MS column (30 m × 0.32 mm × 1 mm). The 1H- and 13C-NMR spectra were recorded at 500 MHz and 125 MHz respectively on Bruker AMX 500 NMR spectrometers. Methyl, methylene and methine carbons were distinguished by DEPT (Distortionless Enhancement by Polarisation Transfer) experiments. Homonuclear 1H connectivities were determined by using the COSY (COrrelation SpectroscopY) experiment. One bond 1H-13C connectivities were determined with HMQC (Heteronuclear Multiple Quantum Connectivity by 2D-multiple) gradient pulse factor selection. Two- and three-bond 1H-13C connectivities were determined by HMBC (Heteronuclear Multiple Bond Connectivity by 2D-multiple Quantum) experiments. Chemical shifts are reported in δ (ppm) using TMS as internal standard, and coupling constants (J) were measured in Hz. Column chromatography was carried out on silica gel (70-230 mesh, Merck) and flash silica gel (230-400 mesh, Merck). TLC (Thin Line Chromatographic) was performed on Merck precoated silica gel 60 F254 aluminum foil, and spots were detected using ceric sulfate spray reagent. Phenolic compounds were detected using FeCl3 reagent. The purity of the compounds was investigated by means of GC-MS, 1H-NMR and ESI-MS. The degree of the purity of all tested compounds was > 96%. All other substances, if not otherwise specified, were purchased from Sigma-Aldrich (Germany). All reagents used were of analytical grade.
Plant material
Stem bark of K. gabonensis was collected in March 2004 at the Douala-Edea forest reserve, Littoral Province, Cameroon. The identification was made by Mr. Victor Nana at the Cameroon National Herbarium, where the voucher specimen was deposited under ref. no. 6517 SRF/CAM.
Extraction and isolation
The air-dried and powdered stem bark (2 kg) of K. gabonensis was macerated in methanol (8 L) at room temperature for 72 h. The filtrate was concentrated under vacuum to give a dark brown crude extract (KGB) (350 g). For bioassay-guided fractionation based on antimicrobial and antioxidant activities (), 150 g of KGB was subjected to column chromatography over silica gel 60 (230-400 mesh) (210 g) using a gradient system of n-hexane, CH2Cl2, ethyl acetate and MeOH. A total of 250 sub-fractions (approximately 250 mL each) were collected and combined according to TLC (Thin Line Chromatographic) analysis leading to four main fractions A-D. Fraction A (25 g) resulted from combined sub-fractions 1-60 eluted with a mixture of n-hexane-CH2Cl2 (6:4). Fraction B (17 g) was constituted of sub-fraction 61-103 eluted with a mixture of n-hexane-CH2Cl2 (1:1-1:3). Fraction C (25 g) was obtained from sub-fractions 104-190 eluted with CH2Cl2-EtOAc (1:1). Fraction D (35.5 g) was the combination of sub-fractions 191-250 eluted with pure EtOAc-MeOH (9:1). Antimicrobial and antioxidant activity tests of these main fractions registered the bioactivity to be concentrated in fractions C and D. Fraction A was chromatographed over silica gel 60 column with an n-hexane-CH2Cl2 gradient. A total of 35 fractions of approximately 100 mL each were collected and combined on the basis of similar TLC. Fractions 1-25 were further chromatographed on silica gel 60 with a mixture of n-hexane-CH2Cl2 (8:2) to yield fatty acids (25 mg) (CitationAndersen & Gorbert, 2002), a mixture of sterols (β-sitosterol and stigmasterol) (30.4 mg) (CitationMorris et al., 1984), and lupeol (5) (19.3 mg) (CitationWansi et al., 2006). Fraction B was chromatographed over a silica gel 60 column with an n-hexane-CH2Cl2 gradient. A total of 40 fractions of approximately 100 mL each were collected and combined on the basis of similar TLC. Fractions 1-10 were further chromatographed over silica gel 60 with a mixture of n-hexane-CH2Cl2 (1:1) to yield β-amyrin (7) (15.5 mg) and erythrodiol (8) (20.5 mg) (CitationWansi et al., 2006). Fraction C was chromatographed over a silica gel 60 column with a CH2Cl2, CH2Cl2-EtOAc gradient. A total of 25 fractions of approximately 100 mL each were collected and combined on the basis of similar TLC. Fractions 21-30 were further chromatographed over silica gel 60 with a mixture of CH2Cl2- EtOAc (2:1) to yield oleanolic acid (9) (13.5 mg), betulinic acid (6) (20.5 mg), hederagenin (10) (10.5 mg), and bayogenin acid (11) (8.0 mg) (CitationWansi et al., 2007; CitationWandji et al., 2000). Similarly, fraction D was chromatographed over a silica gel 60 column with a CH2Cl2-EtOAc, EtOAc-MeOH gradient. A total of 45 fractions of approximately 100 mL each were collected and combined on the basis of similar TLC. Fractions 1-30 were further chromatographed over silica gel 60 with a mixture of EtOAc-MeOH (4:1) to yield ellagic acid (1) (43.5 mg), ellagic acid 3,3′-dimethyl ether (2) (50 mg), gallic acid (3) (30.5 mg), methyl gallate (4) (450.5 mg), and 3-O-β-d-glucopyranosyl of stigmasterol (12) (25 mg) (CitationWansi et al., 2007). The chemical structures of the isolated compounds are presented in .
Table 1. Zone of inhibition (Ø in mm) of MeOH extract (KGB), fractions A-D and compounds 1–12 from K. gabonensis in agar diffusion test with 20 μg/disk (Ø in mm, paper disk Ø, 6 mm).
Table 2. Minimum inhibition concentrations (μg/mL) of MeOH extract (KGB), fractions C-D and compounds 1-4 from K. gabonensis.
Table 3. Free radical scavenging activity of crude extract (KGB), fractions A-D and compounds 1-4, 6, 9 and 10 against DPPH (1,1-diphenyl-2-picrylhydrazyl).
Biological activities
Antimicrobial assays
Agar diffusion tests were performed as described by CitationMaskey et al. (2002) with the bacteria Bacillus subtilis and Escherichia coli (on peptone agar), Staphylococcus aureus (Bacto nutrient agar), Streptomyces viridochromogenes (M2 agar), the fungi Mucor miehei and Candida albicans (Sabouraud agar), and three micro green algae Chlorella vulgaris, Chlorella sorokiniana, and Scenedesmus subspicatus (Bold’s basal agar). Streptomyces viridochromogenes Tü57, Mucor miehei and the micro green algae Chlorella vulgaris, Chlorella sorokiniana, and Scenedesmus subspicatus originate from the Tübingen Strain Collection and the Göttingen Algae Collection, Germany respectively. Other strains were clinical isolates from the Centre Pasteur du Cameroun, Yaoundé, Cameroon and were carefully purified.
Compounds were dissolved in chloroform/MeOH (87:13) azeotrope and paper disks (Ø 6 mm) were impregnated with each 20 μg for pure compounds and fractions, and 2 mg for the crude extract, dried for 1 h under sterile conditions and placed on the pre-made agar test plates. Bacteria and fungi plates were kept in an incubator at 37°C for 12-16 h, micro algae plates in indirect natural light at room temperature for three days. The diameters of inhibition zones were measured in mm. Nystatin (Maneesh Pharmaceuticals, Govandi, Mumbai, India) was used as positive control for fungi and algae and gentamycin (Jinling Pharmaceutical Group, Nanjing, China) for bacteria.
Determination of minimum inhibition concentrations
The minimum inhibition concentrations (MICs) of test samples and reference drug were measured by the microdilution broth susceptibility assay (CLSI/CitationNCCLS, 2008). The inocula of bacterial and fungal strains were prepared from 12-h broth cultures, and suspensions were adjusted to 0.5 McFarland standard turbidity. The samples were dissolved in 10% DMSO and serial two-fold diluted in 96-well microtiter plates in duplicate, using BHI broth for bacterial and Sabouraud dextrose broth for fungal testlines. Standardized inocula of test strains were added, and after incubation at 37°C for 24 h on a rotary shaker at 200 rpm, MICs were read as the lowest concentration with inhibition of the visible growth of the test organism.
Determination of the radical scavenging activity
DMSO (5 μL) containing the test sample and 95 μL of DPPH (Sigma, 300 μM) in ethanol is given into a well of a 96-well microtiter plate (Molecular Devices, Germany) and incubated at 37°C for 30 min. The absorbance is measured at 515 nm. The percentage of radical scavenging activity is determined by comparison with the negative control. Extract and fractions (A-D) were tested at 20 mg/mL, compounds 1-4, 6, 9 and 10 at 1 mM, as well as the positive control BHA (3-t-butyl-4-hydroxyanisole) ().
Results and discussion
After exhaustive extraction of the air-dried and powdered stem bark of K. gabonensis with methanol, the crude extract underwent antimicrobial and antioxidant testing revealing MICs of 18.4-35.4 μg/mL in the microdilution broth susceptibility assay and an IC50 of 10.45 μg/mL in the radical scavenging (DPPH) assay, tested at 20 mg/mL. The extensive gradient column chromatography of the crude extract yielded four fractions (A-D). While fractions A and B were either not or barely active in both tests, fractions C and D gave MICs of 4.5-45.3 μg/mL in the microdilution assay accompanied by IC50 values in the DPPH assay of 75 μg/mL for fraction C and a very high value of 5.5 μg/mL for fraction D when tested at 20 mg/mL. All fractions were subjected to successive column chromatography (silica gel 60) and preparative TLC to yield compounds 1-12 namely ellagic acid (1), ellagic acid 3,3′-dimethyl ether (2), gallic acid (3), methyl gallate (4), lupeol (5), betulinic acid (6), β-amyrin (7), erythrodiol (8), oleanolic acid (9), hederagenin (10), Bayogenin acid (11), and 3-O-β-d-glucopyranosyl of stigmasterol (12). Compounds 1-3 and 7-12 were isolated for the first time from this genus.
Dried stem bark of K. gabonensis collected in Cameroon delivered 250.25 mg/kg of methyl gallate from the methanol extract, matching well the yields reported from Congo Brazzaville (CitationEkouya et al., 2005, Citation2006). Phenolic compounds and tannins are secondary metabolites discussed to be produced by plants as drought protectants, and since K. gabonensis was collected from Douala-Edea forest (Cameroon) characterized by very sandy and acid soils, the high levels of these compounds are explainable.
The antimicrobial and antioxidant activities of MeOH extract, fractions A-D, and compounds 1-12 are presented in , , and .
In the paper disk agar diffusion test, extract, fractions C and D as well as phenolic compounds 1-4 turned out to be active against all tested bacteria, fungi and algae. Interestingly, though fractions and compounds were tested at the same concentration of 20 μg/disk, fractions C and D were highly active throughout against bacteria and fungi (MICs. 4.5-26.4 μg/mL), while activities of compounds 1-4 were evenly distributed through the low to high active range (MICs 15.4-120.1 μg/mL). This result might reflect strong synergist effects between compounds in the fractions.
In contrast, for fractions A and B as well as terpenoid compounds 5-12 antimicrobial activities were nonexistent to low, and none of these compounds yielded antialgal activity.
The radical scavenging activity using DPPH assay gave significantly high antioxidant values for the crude extract with IC50 10.45 μg/mL, fraction D with IC50 5.50 μg/mL and the phenolic compounds 1-4 with IC50 45.50-48.25 mM compared to the phenolic synthetic antioxidant standard BHA with IC50 44.20 mM. The triterpenoid 6, 9 and 10 delivered low antioxidant values (IC50 105.50-151.25 mM).
The high antioxidant and antimicrobial activities obtained from phenolic compounds were in accordance with those obtained from CitationAtta-Ur-Rahman et al. (2001) and CitationNdukwe and Zhao (2007). Concerning the antimicrobial activity of triterpenoids, CitationHoriuchi et al. (2007), CitationCunha et al. (2007), CitationKuete et al. (2008), as well as CitationMathabe et al. (2008) reported very low or no activities matching our data. However, the triterpenoids ursolic acid and oleanolic acid (9), which revealed very low activities in the paper disk diffusion test (8-12 mm inhibition zone at 20 μg/disk) against Streptomyces viridochromogenes Tü 57 and other bacteria, were tested active by CitationHoriuchi et al. (2007) against vancomycin-resistant enterococci (MICs 4-8 μg/mL), S. pneumoniae (MICs 8-16 μg/mL) and methicillin-resistant S. aureus (MICs 8-16 μg/mL). CitationCunha et al. (2007) reported activity of both compounds against dental caries causing streptococci and Enterococcus faecalis (MICs 30-80 μg/mL), suggesting that both hydroxy and carboxy groups are important in the structure-antibacterial activity relationship of triterpene acids.
In Cameroon, the alcohol extract of stem bark of K. gabonensis is used for the treatment of a condition called “bloody diarrhea”, as well as rheumatism and inflammation. In this study, the antimicrobial activities of the phenolic compounds 1-4 and fractions C and D against Bacillus subtilis, Staphylococcus aureus, and Escherichia coli point to effective agents in the treatment of diarrhea caused by bacterial infection. At the same time, due to their potent antioxidant and anti-inflammatory properties, compounds 1-4 and fraction D seem to be responsible for the relief of pain caused by rheumatic inflammation and healing of other multicausal inflammations.
In Congo, K. gabonensis is the main plant in the treatment of a type of dermatitis called “mwandza”. Since until now, effective modern drugs have not been available, the present study might provide important baseline information on the search for useful agents.
Acknowledgements
This paper is dedicated to the memory of our late supervisor Professor Z. Tanee Fomum.
Declaration of interest
We wish to acknowledge the International Foundation for Science (IFS) for providing financial support (F/3978-2), Alexander von Humboldt (AvH) Foundation, Germany, for the post doctoral fellowship to J.D. Wansi and K.P. Devkota, and the European Commission for the Marie Curie IIF fellowship of B.N. Lenta.
References
- Adjanohoun EJ, Ake Assi L, Floret T, Guinko S, Koumare M, Ahyi AMR, Raynal J (1988): Traditional Medicine and Pharmacopoeia, Contribution of the ethnobotanic and floristic studies in Popular Republic of Congo 39. Paris, Popular Republic of Congo –Report ACCT, p. 605.
- Andersen PC, Gorbert DW (2002): Influence of year and planting date on fatty acid chemistry of high oleic acid and normal peanut genotypes. J Agric Food Chem 50: 1298–1305.
- Atta-Ur-Rahman Ngoumou, FN, Iqbal MC, Malik S, Makhmoor T, Nur-E-Alam Zareen, S, Lontsi D, Ayafor JF, Sondengam BL (2001): New antioxidant and antimicrobial ellagic acid derivatives from Pteleopsis hylodendron. Planta Med 67: 335–339.
- Aubreville A (1962): Flora of Gabon, Irvingiaceae, Simaroubaceae, Burreraceae, National Museun of Natural History, p. 12.
- Bouquet A (1969): Traditional Doctors and Traditional Medicines of Congo Brazzaville. Paris, Orston. p. 220.
- NCCLS (National Committee for Clinical Laboratory Standards) (2008): Performance standards for antimicrobial susceptibility testing, 18th edition. CLSI/NCCLS M 100-S18. Wayne, PA, Clinical and Laboratory Standards Institute.
- Cunha LCS, Silva MLA, Furtado NAJC, Vinholis AHC, Gomes MCH, Filho AAS, Cunha WR (2007): Antibacterial activity of triterpene acid and semi-synthetic derivatives against oral pathogens. Z Naturforsch 62c: 668–672.
- Dongo E, Hussain H, Miemanang RS, Tazoo D, Schulz B, Krohn K (2009): Chemical constituents of Klainedoxa gabonensis and Paullinia pinnata. Rec Nat Prod 3: 165–169.
- Ekouya A, Itoua GB, Ouabonzi A, Ouamba JM (2005): Tannin isolated from an antibacterial extract of Klainedoxa gabonensis. J Soc Ouest-afr Chim 10: 1–9.
- Ekouya A, Itoua GB, Ouabonzi A, Ouamba JM (2006): Isolement du gallate methyle et evaluation de l’activité antibactérienne et antitumorale de quelques extraits de Klainedoxa gabonensis Pierre ex Engl. Phytopharmacologie 3: 117–120.
- Horiuchi K, Shiota S, Hatano T, Yoshida T, Kuroda T, Tsuchiya T (2007): Antimicrobial activity of oleanolic acid from Salvia officinalis and related compounds on vancomycin-resistant enterococci (VRE). Biol Pharm Bull 30: 1147–1149.
- Kuete V, Wansi JD, Mbaveng AT, Kana Sop MM, Tcho TA, Penlap BV, EtoaF-X Wandji, J, Marion MJJ, Lall N (2008): Antimicrobial activity of the methanolic extract and compounds from Teclea afzelii (Rutaceae). S Afr J Bot 74: 572–576.
- Maskey RP, Asolkar RN, Kapaun E, Wagner-Döbler I, Laatsch H (2002): Phytotoxic arylethylamides from limnic bacteria using a screening with microalgae. J Antibiot 55: 643–649.
- Mathabe MC, Hussein AA, Nikolova RV, Basso AE, Meyer JJM, Lall N (2008): Antibacterial activities and cytotoxicity of terpenoids isolated from Spirostachys africana. J Ethnopharmacol 116: 194–197.
- Morris RJ, McCartney MJ, Bone Q (1984): The sterol chemistry of salps: The occurrence and distribution of saturated sterols. J Marine Biol Ass UK 64: 343–349.
- Ndukwe IG, Zhao Y (2007): Pharmacological activity of 2,3,8-tri-O-methyl ellagic acid isolated from the stem bark of Irvingia gabonensis. Afr J Biotechnol 6: 1910–1912.
- Ogunwande IA, Essien EE, Ogunbinu AO, Adebayo M, Karioti A, Saroglou V, Skalta H (2008): Essential oil constituents of Klainedoxa gabonensis Pierre Ex Engl. (Irvingiaceae), Brachystegia nigerica Hoyle et A. Jones (Caesalpinioideae) and Acalypha segetalis (Muell.) Arg., (Euphorbiaceae). J Essential Oil Res 20: 211–215.
- Onanga M, Ekouya A, Ouabonzi A, Itoua GB (1999): Ethnobotanical, pharmacological and chemical studies of plants used in the treatment of “Mwandza” dermatitis. Fitoterapia 70: 579–585.
- Talcott ST, Lee J-H (2002): Ellagic acid and flavonoid antioxidant content of Muscadine wine and juice. J Agric Food Chem 50: 3186–3192.
- Wandji J, Wansi JD, Fuendjiep V, Dagne E, Mulholland DA, Tillequin F, Fomum ZT, Sondengam BL, Nkeh BC, Njamen D (2000): Sesquiterpene lactone and friedelane derivative from Drypetes molunduana. Phytochemistry 54: 811–815.
- Wansi JD, Wandji J, Kamdem WAF, Ndom JC, Nguefa HE, Chiozem DD, Choudhary MI, Tsabang N, Tillequin F, Fomum ZT (2006): Triterpenoids from Drypetes chevalieri Beille (Euphorbiaceae). Nat Prod Res 20: 586–593.
- Wansi JD, Lallemand M-C, Chiozem DD, Toze FAA, Mbaze ML, Naharkhan S, Choudhary IM, Tillequin F, Wandji J, Fomum TZ (2007): α-Glucosidase inhibitory constituents from stem bark of Terminalia superba (Combretaceae). Phytochemistry 68: 2096–2100.