Abstract
Objective: To investigate the in vitro antioxidant activity of methanol extracts of Ixora coccinea L. (Rubiaceae) flower, leaf and stem.
Materials and methods: The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, total antioxidant capacity (TAC) and xanthine oxidase inhibition assay were carried out to evaluate the antioxidant potential of the extract. The IC50 values were calculated for the DPPH and xanthine oxidase assays in order to evaluate the antioxidant efficiency of each of the I. coccinea extracts. The phenol contents were also determined.
Results: I. coccinea flowers revealed the best antioxidant property, presenting much lower IC50 value (6.6 mg/mL for DPPH assay). The flower extract showed a significantly higher antioxidant capacity compared to the other extracts. Furthermore, the highest phenolic content (polyphenols) was found in the flower extract (210.55 ± 6.31 µg GAE/mg extract). Moreover, I. coccinea extracts scavenged the superoxide radical generated by the xanthine/xanthine oxidase system. The xanthine oxidase inhibition activity was in the order of allopurinol > leaf > flower > stem with the percentage of inhibition ranged from 39.7% to 77.3% for the plant parts investigated. The highest phenolic contents (polyphenols) were found in the flower extracts (210.55 ± 6.31 µg GAE/mg extract).
Conclusions: I. coccinea could be considered as a potential source of natural antioxidant.
Introduction
Oxidation is essential to many living organisms for the production of energy to fuel biological processes. However, oxygen-centered free radicals and other reactive oxygen species (ROS), which are continuously produced in vivo, result in cell death and tissue damage. The role of oxygen radicals have been implicated in several diseases, including cancer, diabetes, cardiovascular disease and aging (CitationHalliwell & Gutteridge, 1999). Lipid peroxidation of fats and fatty acids in food not only results in their spoilage but is also a source of peroxyl and hydroxyl radicals that are associated with carcinogenesis, mutagenesis and aging (CitationYagi, 1987; CitationFinkel & Holbrook, 2000). Therefore antioxidants that scavenge these reactive oxygen species and free radicals are of major importance in preventing the onset and progression of many diseases caused by oxidative stress. Synthetic antioxidants such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) are very effective and are used for industrial processing, but they may possess side effects and toxic properties that affect human health (CitationAnagnostopoulou et al., 2006; CitationBranen, 1975; CitationIto et al., 1983). The search for antioxidants from natural sources has received much attention and efforts have been put into the identification of compounds that can act as suitable antioxidants (CitationWong et al., 2006).
Ixora coccinea L. (Rubiaceae) English name: Burning Love, Jungle Flame or Jungle Geranium; Local name: Jejarum is a small evergreen shrub (CitationLatha & Panikkar, 1998) found throughout Asia. Tropical plants are generally tolerant to high levels of environmental stress induced by ultraviolet radiation thereby explaining the high levels of phenolic compounds previously studied (CitationToyokuni et al., 2003). This plant has been used traditionally for a variety of ailments: the leaves are used to treat diarrhea; the roots are used to treat hiccough, fever, sores, chronic ulcers, skin diseases; and the flowers have been used in catarrhal bronchitis and dysentery (CitationSivarajan & Balachandran, 1994). Studies on this plant have revealed the presence of anthocyanins from flowers, methyl ester of palmitic, stearic, oleic, and linoleic acids in the root oil, octadecadienoic acid from the root bark (CitationChopra et al., 1956) and saponins and tannins (CitationGrainge & Ahmed, 1988). Phytochemical screening showed the presence of alkaloids, flavonoids, sapogenins, sterols, terpenes, and phenols (CitationAnnapurna et al., 2003). The various phenolic compounds present in I. coccinea contribute to its many pharmacological effects. The alcohol extract of I. coccinea flower has been proven to enhance the healing process of the wound that was pre-created in rats (CitationNayak et al., 2003). CitationLatha and Panikkar (1998) revealed in a study that the flowers of I. coccinea had cytotoxic and antitumor activity in mice. A previous study by CitationAnnapurna et al. (2003) revealed that the leaves of I. coccinea exhibited antimicrobial activity which was comparable to streptomycin, a standard antibiotic. It was also found that the aqueous extract of I. coccinea showed antinociceptive and anti-inflammatory effects in mice (CitationRatnasooriya et al., 2005a, Citation2005b). Studies on the antioxidant activity of I. coccinea are scarce except for a recent work which showed high antioxidant properties of the flower extract. This was attributed to the high amount of hydrophilic phenolic compounds found in the flower (CitationSaha et al., 2008). In view of this, we report the TPC (total phenolic content) and AOA (antioxidant activity) of the flower, leaf, and stem of I. coccinea. The evaluation of the antioxidant capacity was performed in vitro by DPPH radical scavenging assay, total antioxidant capacity, and xanthine oxidase assay.
Materials and methods
Chemicals
Folin-Ciocalteu reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH) reagent, gallic acid, allopurinol, ascorbic acid, xanthine, and xanthine oxidase were purchased from Sigma-Aldrich (St. Louis, MO). HPLC grade methanol, hydrochloric acid (HCl), and phosphate buffer solution (pH 7.5) were purchased from Merck (Darmstadt, Germany). Butylated hydroxytoluene (BHT) was purchased from Fluka (Buchs, Switzerland). Sulfuric acid, sodium phosphate, ammonium molybdate, and sodium carbonate (Na2CO3) were purchased from Fisher Scientific (Toronto, Ontario).
Plant material
Samples of the different parts of I. coccinea were collected from Semeling in the state of Kedah, Malaysia, in March 2008. An initial quality evaluation of the plant material was carried out to validate its authenticity and also to ensure its quality, using techniques adopted from the WHO guidelines on herbal quality control (CitationWHO, 1998). The authenticity study was done by S. Sreeramanan, a botanist from the School of Biological Sciences, Universiti Sains Malaysia where a specimen of the plant material was deposited.
The flowers, leaves, and stems were dried in the oven (45°C) for 10 days and powdered with a heavy duty blender.
Solvent extraction
The powdered plant material (200 g) was soaked in methanol (300 mL) for 48 h. The extract was filtered and the solvent was completely removed by a rotary evaporator. The extract was dried in the oven at 60°C for a day to obtain a thick paste. The crude extracts obtained were used for the estimation of total phenolic contents and also for the assessment of antioxidant potential through various chemical assays.
Determination of total phenolic content
The total phenolic content was determined using Folin-Ciocalteu reagent according to the method of CitationSingleton et al. (1999). A total amount of 40 µL crude methanol extract (1 mg/mL) was mixed with 200 µL of Folin-Ciocalteu reagent and 1160 µL of distilled water. After 3 min, 600 µL of 20% sodium carbonate (Na2CO3) was added. The mixture was shaken for 2 h at room temperature, and absorbance was measured at 765 nm. All tests were performed in triplicate. Gallic acid was used as a standard for the calibration curve. The concentration of total phenolic compounds was expressed as µg of gallic acid equivalents per 1 mg of extract using the following equation obtained from a standard gallic acid graph.
where C is expressed as µg of gallic acid equivalent per mg of dry weight of the extract; A is the equivalent concentration of gallic acid established from calibration curve (µg); and B is the dry weight of extract (mg).
DPPH radical scavenging assay
The DPPH (1,1-diphenyl,2-picrylhydrazyl) radical scavenging assay was carried out according to the method of CitationSangetha et al. (2008). Quantitative measurement of the radical scavenging properties was carried out in a universal bottle. An aliquot of 5 mL 0.04% DPPH radical solution was added to the extract solution ranging from 1 to 10 mg/mL. The absorbance of the DPPH solution in the absence of the plant extract was measured as the control. Discoloration was measured at 517 nm after incubation for 30 min. The commercial antioxidant BHT was used for comparison as a positive control. Measurements were made in triplicate. Percentage scavenging of the DPPH free radical was measured using the following equation:
where A0 was the absorbance of the control and A1 was the absorbance of the sample (CitationOktay et al., 2003).
Xanthine oxidase assay
The xanthine oxidase (XO) activities with xanthine as the substrate were measured spectrophotometrically using the procedure of CitationMarcocci et al. (1994). The xanthine solution (150 mM) was prepared by initially dissolving xanthine in phosphate buffer solution (PBS), and adjusting the pH to 7.5. The XO solution was prepared by diluting XO to a final concentration of 0.01 U/ mL in cold 50 mM potassium phosphate buffer (pH 7.5). The extract was serially diluted to achieve a concentration range of 12.5 to 100 μg/mL. The assay mixture was prepared consisting of 1 mL of the extract, 2.9 mL of phosphate buffer solution (pH 7.5) and 2 mL of xanthine solution. This assay mixture was pre-incubated at 25°C for 15 min. The reaction was initiated by adding 0.1 mL XO solution and the samples were incubated at 25°C for 30 min. The reaction was then stopped by the addition of 1N hydrochloric acid (HCl) and the absorbance was read using a UV-spectrophotometer at 290 nm. Allopurinol was used as positive control. Allopurinol is a known inhibitor of xanthine oxidase. As for the blank, the enzyme solution was added after the addition of 1N HCl. The antioxidant activity is expressed as the percentage of xanthine oxidase inhibition using the following formula:
where A contains the enzyme without the extract, B is the control of A without the extract and the enzyme, C contains the extract with the enzyme and D contains the extract without the enzyme.
Total antioxidant capacity
The total antioxidant capacity of the methanol extract was determined using the phosphomolybdenum method of CitationPrieto et al. (1999). The extract (0.1 mL) with varying concentrations was combined with 1 mL of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The reaction mixture was incubated at 95°C for 90 min. The mixture was cooled to room temperature and the absorbance of the mixture was measured at 695 nm against a blank. The antioxidant activity of BHT (0.5 mg/mL) was also assayed for comparison. Ascorbic acid was used as standard. The antioxidant activity was expressed as µg/ml ascorbic acid equivalents (AAE).
Statistical analysis
All analyses were run in triplicate and expressed as mean ± SD. Statistical analyses were performed using SPSS version 16.0 software. Analyses of variance were performed using the one-way analysis of variance (ANOVA). Significant differences between means were determined by Duncan’s multiple range test. P values less than 0.05 were considered statistically significant.
Results and discussion
shows the total phenolic contents where the highest amount was found in the flower (210.55 ± 6.31 µg/ mg) followed by the leaf (180.56 ± 1.27 µg/mg) and stem (100.31 ± 4.63 µg/mg) extracts. The presences of phenols content in the flower is consistent with the studies reported by CitationSaha et al. (2008). A preliminary phytochemical screening revealed that the flower extract of I. coccinea possessed flavonoids and tannins. Other studies demonstrated the presences of the pentacyclic triterpenoid, ursolic acid, oleanolic acid, and sitosterol in the flower extract (CitationRagasa et al., 2004; CitationLatha & Panikkar, 1999).
Table 1. Total phenolic contents of flower, leaf and stem extracts of Ixora coccinea.
The antioxidant activity of flower, leaf and stem methanol extracts were determined by using the DPPH scavenging assay. The DPPH assay is often used to evaluate the ability of antioxidants to scavenge free radicals which are known to be a major factor in biological damage caused by oxidative stress. This assay is known to give reliable information concerning the antioxidant ability of the tested compounds (CitationHuang et al., 2005). The principle of the assay is based on the color change of the DPPH solution from purple to yellow as the radical is quenched by the antioxidant (CitationKaragözler et al., 2008). The flower methanol extract exhibited a significant dose-dependent inhibition of DPPH activity, with a 50% inhibition (IC50) at a concentration of 6.6 mg/mL (). The corresponding IC50 for leaf and stem extracts were 109.95 and 272.42 mg/ mL, respectively. The IC50 value of flower extract was low but comparable to BHT (butylated hydroxytoluene) which had an IC50 value of 5.11 mg/ mL.
Table 2. IC50 values of the DPPH radical scavenging activity and xanthine oxidase inhibition for the different samples.
The high content of phenolic compounds in the flower extract might explain its higher antioxidant capacity compared to the leaf and stem extract. There is evidence of a good correlation between phenolic contents of the different plant part extracts (R2 = 0.9833) and their DPPH scavenging activity. This is consistent with previous reports of significant negative linear correlation between the phenolic contents and the IC50 antioxidant activity values (CitationGonzález et al., 2003; CitationLlobera & Cañellas, 2007; CitationMakris et al., 2007; CitationLecumberri et al., 2007). This negative linear correlation proves that the extracts with the highest phenolic contents show lower IC50 values. Apart from that, CitationHavsteen (1983) and CitationBrandi (1992) also reported that flavonoids, which are a type of polyphenol, possess enzyme inhibitory and antioxidant activities and are responsible for their therapeutic effects in traditional medicine.
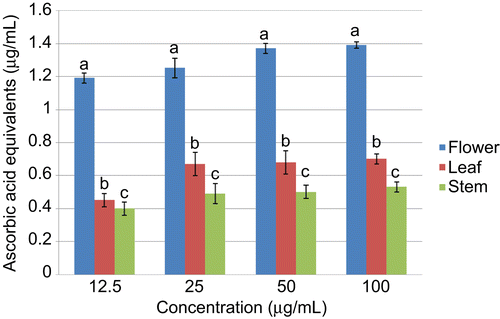
The total antioxidant capacity (TAC) of the flower, leaf, and stem extracts were also determined and presented in . TAC is expressed as µg/ml of ascorbic acid equivalents (AAE). The flower extract showed a significantly higher antioxidant capacity when compared to the other extracts tested in this study.
Figure 1. Total antioxidant capacity of the different extracts. The TAC is expressed as µg/mL of ascorbic acid equivalents. Values are expressed as mean ± SD (n = 3); p <0.05.
The xanthine oxidase assay results are shown in . It has been reported that xanthine oxidase inhibitors may be useful for the treatment of hepatic disease and gout, which is caused by the generation of uric acid and superoxide anion radical (CitationLin et al., 2001). The xanthine oxidase inhibition activity was in the order of allopurinol > leaf > flower > stem. The percentage of inhibition ranged from 39.7% to 77.3% in a dose-dependent manner (12.5–100 μg/mL) for studied plant parts. The IC50 values of the leaf, flower and stem extracts were 26.5, 31.3, and 36.7 μg/mL, respectively, and a moderate coefficient of correlation value was noted between the phenolic content of the extracts and the xanthine oxidase antioxidant activity (R2 = 0.5234).
In the DPPH assay IC50 value of the flower extract was reasonably higher (6.6 mg/mL) when compared to that (IC50: 0.1 mg/mL) reported by CitationSaha et al. (2008). This difference in the IC50 could be possibly due to the different methods of extraction of the flower. CitationSaha et al. (2008) used heat Soxhlet extraction while the present study utilized maceration procedures. Maceration has generally been reported to give lower yields of plant extracts due to its insolubility at room temperature compared to Soxhlet extraction (CitationIbrahim et al., 1997).
All the antioxidant assays showed that the flower extracts possess better antioxidant activity than that of leaf and stem. This is evident by lower flower extract IC50 and higher phenolic content. It was interesting to note that, although flower extracts had the highest DPPH and TAC antioxidant activity, they were not the most effective ones in xanthine oxidase assay, where the leaf extract displayed the highest inhibition effect. This difference can possibly be due to the fact that the antioxidant activities of putative antioxidants have been attributed to various mechanisms. Among these are prevention of chain initiation, binding of transition metal ion catalysts, decomposition of peroxides, prevention of continued hydrogen abstraction and radical scavenging (CitationDiplock, 1997). Both the DPPH and the total antioxidant assays utilize the mechanism of reduction while the xanthine oxidase utilizes the mechanism of competitive inhibition of xanthine oxidase. Therefore, it is possible that the leaf extract probably had more compounds which can act as competitive inhibitors of xanthine oxidase compared to the flower extract, thus showing a higher antioxidant activity.
In a different study by CitationFarhoosh et al. (2007), hot water tea extracts had the highest antioxidant activity but they were the least effective ones in reducing power. The authors related this antioxidant difference to the variation in the antioxidant mechanisms.
Conclusion
The results of the present study indicated that the flower extracts provided the highest TAC and DPPH antioxidant activity. This is evident with the highest total phenolic content found in the flower extract compared to the leaf and stem extracts. However, the antioxidant activity of the flower extracts was not concomitant with the xanthine oxidase assay as the leaf extract demonstrated the highest xanthine oxidase inhibition activity. This suggests that the antioxidant activity of the leaf extracts likely involves other mechanisms than those of reductones alone. The flower and the leaf extracts could be used as potent natural antioxidative sources.
Declaration of interest
The author declare no conflict of interest.
References
- Anagnostopoulou MA, Kefalas P, Papageorgiou VP, Assimepoulou AN, Boskou D (2006): Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Food Chem 94: 19–25.
- Annapurna J, Amarnath PVS, Amar Kumar D, and Ramakrishna SV, Raghavan KV (2003): Antimicrobial activity of Ixora coccinea leaves. Fitoterapia 74: 291–293.
- Brandi ML (1992): Flavonoids: Biochemical effects and therapeutic applications. Bone Miner 19: S3–S14.
- Branen AL (1975): Toxicology and biochemistry of butylated hydroxyanisole and butylated hydroxytoluene. J Am Oil Chem Soc 52: 59–63.
- Chopra RN, Nayar SL, Chopra IC (1956): Glossary of Indian Medicinal Plants. New Delhi, Council of Scientific and Industrial Research.
- Diplock AT (1997): Will the good fairies please prove to us that vitamin E lessens human degenerative disease? Free Rad Res 27: 511–532.
- Farhoosh R, Golmovahhed GA, Khodaparast MHH (2007): Antioxidant activity of various extracts of old tea leaves and black tea wastes (Camellia sinensis L.). Food Chem 100: 231–236.
- Finkel T, Holbrook NJ (2000): Oxidants, oxidative stress and the biology of aging. Nature 408: 239–247.
- González EM, de Ancos B, Cano MP (2003): Relation between bioactive compounds and free radical-scavenging capacity in berry fruits during frozen storage. J Sci Food Agr 83: 722–726.
- Grainge M, Ahmed S (1988): Handbook of Plants with Pest Control Properties. New York, Wiley.
- Halliwell B, Gutteridge JMC (1999): Free Radicals in Biology and Medicine. Oxford, Oxford University Press.
- Havsteen B (1983): Flavonoids, a class of natural products of high pharmacological potency. Biochem Pharmacol 32: 1141–1148.
- Huang D, Ou B, Prior RL (2005): The chemistry behind antioxidant capacity assays. J Agric Food Chem 53: 1841–1856.
- Ibrahim MB, Owonubi MO, Onaolapo JA (1997): Antimicrobial effects of extracts of leaf, stem, and root-bark of Anogeissus leiocarpus on Staphylococcus aureus NCTC 8190, Escherichia coli NCTC 10418 and Proteus vulgaris NCTC 4636. J Pharmaceutical Res Dev 2: 20–26.
- Ito N, Fukushima S, Hasegawa A, Shibata M, Ogiso T (1983): Carcinogenicity of butylated anisole in F344 rats. J Natl Cancer Inst 70: 343–347.
- Karagözler AA, Erdag B, Emek YC, Uygun DA (2008): Antioxidant activity and proline content of leaf extracts from Dorystoechas hastata. Food Chem 111: 400–407.
- Latha PG, Panikkar KR (1998): Cytotoxic and antitumour principles from Ixora coccinea flowers. Cancer Lett 130: 197–202.
- Latha PG, Panikkar KR (1999): Modulatory effects of Ixora coccinea flower on cyclophosphamide-induced toxicity in mice. Phytother Res 13: 517–520.
- Lecumberri E, Mateos R, Izquierdo-Pulido M, Rupérez P, Goya L, Bravo L (2007): Dietary fibre composition, antioxidant capacity and physico-chemical properties of a fibre-rich product from cocoa (Theobroma cacao L.). Food Chem 104: 948-954.
- Lin CC, Hsu YF, Lin TC (2001): Antioxidant and free radical scavenging effects of the tannins of Terminalia catappa L. Anticancer Res 21: 237–243.
- Llobera A, Cañellas J (2007): Dietary fibre content and antioxidant activity of Manto Negro red grape (Vitis vinifera): Pomace and stem. Food Chem 101: 659–666.
- Makris DP, Boskou G, Andrikopoulos NK (2007): Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J Food Comp Anal 20: 125–132.
- Marcocci L, Packer L, Droy-Lefaix MT, Sekaki A, Garde’s-Albert M (1994): Antioxidant action of Gingko biloba extract EGB 761. Methods Enzymol 234: 462–476.
- Nayak S, Udupa L, Udupa S (2003): Altered antioxidant enzyme profile in wound healing. Indian J Clin Biochem 18: 75–79.
- Oktay M, Gulcin I, Kufrevioglu OI (2003): Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extract. Lebensm-Wiss Technol 36: 263–271.
- Prieto P, Pineda M, Aguilar M (1999): Spectrophotometric quantitation of antioxidant capacity through the formation of phosphomolybdenum complex: Specific application to determination of vitamin E. Anal Biochem 269: 337–341.
- Ragasa CY, Tiu F, Rideout JA (2004): New cycloartenol esters from Ixora coccinea. Nat Prod Res 18: 319–323.
- Ratnasooriya WD, Deraniyagala SA, Bathige SD, Goonasekara CL, Jayakody JRAC (2005a): Antinociceptive action of aqueous extract of the leaves of Ixora coccinea. Acta Biol Hung 56: 21–34.
- Ratnasooriya WD, Deraniyagala SA, Galhena G, Liyanage SSP, Bathige SDNK, Jayakody JRAC (2005b): Anti-inflammatory activity of the aqueous leaf extract of Ixora coccinea. Pharm Biol 43: 147–152.
- Saha MR, Alam MA, Akter R, Jahangir R (2008): In vitro free radical scavenging activity of Ixora coccinea L. Bangladesh J Pharmacol 3: 90–96.
- Sivarajan VV, Balachandran I (1994): Ayurvedic Drugs and their Plant Sources. New Delhi, Oxford and IBH Publishing.
- Sangetha S, Zuraini Z, Sasidharan S, Suriani S (2008): Free radical scavenging activity of Cassia spectabilis and Cassia fistula. Int J Nat and Eng Sci 2: 111–112.
- Singleton VL, Orthofer R, Lamuela-Raventos RM (1999): Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu Reagent. Methods Enzymol 299: 152–178.
- Toyokuni S, Tanaka T, Kawaguchi W, Lai Fang NR, Ozeki M, Akatsuka S, Aruoma OI, Bahorun T (2003): Effects of the phenolic contents of Mauritian endemic plant extracts on promoter activities of antioxidant enzymes. Free Rad Res 37: 215–1224.
- WHO (1998): Quality Control Methods for Medicinal Plant Materials. Geneva, World Health Organization, pp. 128.
- Wong SP, Leong LP, Koh JHW (2006): Antioxidant activities of aqueous extracts of selected plants. Food Chem 99: 775–783.
- Yagi K (1987): Lipid peroxides and human disease. Chem Phys Lipid 45: 337–341.
