Abstract
Context: Calotropis procera (Ait.) R.Br. (Asclepiadaceae) is a shrub or small tree that grows wild in Egypt. Calotropis acts as a purgative, anthelmintic, anticoagulant, palliative (in problems with respiration, blood pressure), antipyretic, and analgesic, and induces neuromuscular blocking activity. Little research has been done to study the electrophysiological effects of this plant’s extracts on cardiac, smooth, and skeletal muscle activities.
Objective: The present study was conducted to determine the phytochemical composition and the effect of the total alcohol extract of the shoot of the plant, which contains almost all of C. procera’s cardiac glycosides, flavonoids, and saponins. Also, this study attempted to throw more light on the electrophysiological effects of the plant extracts on cardiac, smooth, and skeletal muscle activities and to clarify the mechanism(s) of their observed action(s).
Materials and methods: The aerial parts of the plant were air dried and their ethanol extracts partitioned with successive solvents. Cardiac, smooth, and skeletal muscles were used in this study to investigate the physiological and pharmacological effects of the plant extracts from different solvents. The data were analyzed by paired t-test.
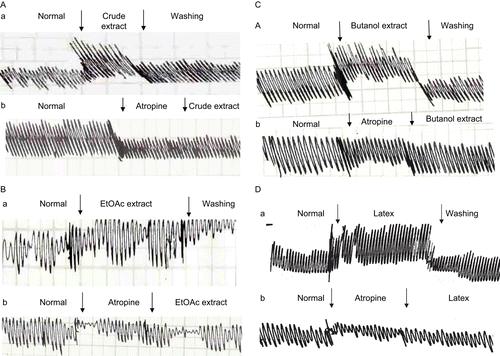
Results: The phytochemical investigation of Calotropis procera revealed the presence of cardenolides, flavonoids, and saponins. The effects of ethanol, n-butanol, and ethyl acetate (EtOAc) extracts were each evaluated on isolated toad heart and their mechanisms of action determined. Perfusion with 2 μg/mL ethanol, 0.2 μg/mL butanol, and 0.2 μg/mL EtOAc extracts caused a significant decrease in heart rate (bradycardia), significant increase in the force of ventricular contraction, and increase in T-wave amplitude. In addition, the effects of different extracts of the studied plant on smooth muscle and skeletal muscle were investigated in this study. The different extracts and latex of C. procera induced a negative chronotropic effect and decreased the heart rate (HR) of isolated toad heart. The different extracts increased the power of contraction of the duodenum (trace a). Pretreatment with atropine sulfate as a muscarinic receptor blocker abolished the stimulatory effect of the different plant extracts and latex of C. procera (trace b).
Discussion: The present data suggest that ethanol, butanol, and EtOAc extracts of Calotropis procera have negative chronotropism and positive inotropism. Verapamil could abolish the inotropic effect of ethanol as well as that of butanol and EtOAc extracts. Meanwhile, atropine did not abolish the observed negative chronotropic effect. The ethanol extract increased the power of contraction of rabbit duodenum, but atropine abolished this effect. It also decreased the skeletal muscle contraction; this effect could be through blocking of the nicotinic receptors. Butanol and EtOAc extract data for smooth and skeletal muscles are very close to those for the corresponding ethanol extract of the studied plant. The present data for C. procera indicate its direct action on the myocardium, its increase of smooth muscle motility, and its relaxation of skeletal muscle contraction. The chemical constituents could directly affect the cell membrane probably through receptors coupling to G proteins. They regulate the ion channel physiology as in the myocardium.
Conclusion: The present data on the extracts of C. procera indicate a direct action on the myocardium, stimulatory effect on smooth muscle motility, and relaxant action on skeletal muscle contraction. Chemical constituents could directly affect the cell membrane probably through receptors coupling to G proteins. They regulate the ion channel physiology as in the myocardium.
Introduction
Plants belonging to the Asclepiadaceae family have a wide range of therapeutic activities. The genus Calotropis is used in traditional medicine for the treatment of leprosy, ulcers, tumors, diseases of the spleen and liver, and piles. Also it acts as purgative, anthelmintic, anticoagulant, antipyretic, analgesic, anti-inflammatory, and antimicrobial, and as a palliative in problems with respiration and blood pressure. It also has a neuromuscular blocking activity (CitationJalalpure et al., 2009; CitationMukherjee et al., 2009; CitationPatra et al., 2009; CitationSeddek et al., 2009). Members of the family are rich in cardiac glycosides and also are regarded as promising sources for antitumor agents (CitationBarrett & Kieffer, 2001). The target compounds are found in almost all parts of C. procera (Asclepiadaceae), but the total amount or relative distribution in any given plant may vary with ecological factors (CitationOluwaniyi & Ibiyemi, 2007). They are important agents in the treatment of heart failure (CitationEAPCCT, 2008). Calotropis procera is a shrub or small tree that grows in Egypt, and it is the only species recorded in that region. It is widely distributed in Nile-Faiyum, oases of the Libyan Desert, Arabian Desert east of the Nile, Red Sea coastal region, and Gebel Elba. It is situated in the southeast corner of Egypt at the Sudan frontier and Sinai proper (CitationAbdel-Khalik & Bakker, 2007). CitationKumar and Shivkar (2004) studied the effect of the latex of Calotropis procera on intestinal smooth muscle in vivo and in vitro. The plant has the highest percentage of cardenolides, viz., uzarigenin, syriogenin, calotropagenin, proceroside, calotropin, calactin, frugoside, coroglaucigenin and corotoxigenin, calotoxin, uscharidin, uscharin, voruscharin, and 3-episarmentogenin (CitationLarhsini et al., 2001; CitationIbrar et al., 2003; CitationOluwaniyi & Ibiyemi, 2007; CitationRamos et al., 2007; CitationMemon et al., 2008). Two flavonoid constituents were isolated from this plant, which are known as rutin and quercetin 3-O-galactoside (CitationWheeler et al., 2003). It is known that C. procera is considered a source of digitalis-like therapeutic agents and is highly toxic to the land snail Thepa pisana (Muller) (CitationChoedon et al., 2006; CitationBanday et al., 2008).
The present study detected the phytochemical composition and the effect of the total alcohol extract of aerial parts of the plant which contained cardiac glycosides, flavonoids, and saponins. Also, this study attempted to throw more light on the electrophysiological effects of the plant extracts on cardiac, smooth, and skeletal muscle activities and to clarify the mechanism(s) of the observed action(s).
Materials and methods
Preliminary phytochemical screening
C. procera (syn. Asclepias procera Ait.) was collected in June 2002 during the flowering stage from the Suez Canal University Garden, Ismailia, Egypt. The identity of the plant was established by Prof. Dr. Hamdy K. Atta-Alla, Professor of Floriculture and Medicinal Plants, Department of Horticulture, Faculty of Agriculture, Suez Canal University. A voucher specimen (number AMYM-1005) was deposited in the Herbarium of the Botany Department, Faculty of Science, Suez Canal University, Ismailia, Egypt.
The aerial parts of the plant were air dried and ground together as a fine powder. Phytochemical screening was determined in accordance with CitationEleyinmi (2007).
The dried plant powder (2 kg) was percolated with 70% ethanol until exhaustion. The whole ethanol solution was concentrated in vacuo and this yielded about 728 g, which was then dissolved in water and filtered. The precipitate was discarded while the filtrate was partitioned with petroleum ether (pet. ether) (40–60°C). The mother liquor fraction was partitioned with successive portions of chloroform, ethyl acetate (EtOAc), and n-butanol. The extracts were dried separately over anhydrous sodium sulfate then evaporated in vacuo. The amounts of chloroform, EtOAc, and n-butanol extracts were approximately 43.68, 47.32, and 218.4 g, respectively.
The plant latex was collected at the same time as collection of the plant from the same area. Samples were collected into flasks surrounded by crushed ice. The crude latex contains an inert coagulum of white elastic material, so it was partially purified by centrifugation (CitationKumar & Roy, 2008).
Thin layer chromatography (TLC) and paper chromatography (PC) were carried out for detection of the components of the different plant extracts and latex by using different solvent systems and adsorbents. Pre-coated silica gel 60F254 glass plates (Sigma-Aldrich) and Whatman No. 1 MM chromatography paper were used as adsorbents. The solvent systems used were: (I) n-butanol:acetic acid:water (BAW) (4:1:5) upper layer and (II) 15% acetic acid for flavonoids; (III) n-butanol:ethanol:water (BEW) (4:1:5) upper layer, (IV) benzene:ethanol (9:1), (V) chloroform:methanol (9:1), (VI) chloroform:methanol (17:3), (VII) chloroform:methanol:formamide (90:6:1), and (VIII) EtOAc:methanol (97:3) for cardiac glycosides. These solvents were arranged according to increasing polarity.
Flavonoids were detected on chromatograms by their characteristic yellowish color in daylight and fluorescence in ultraviolet (UV) light (365 nm), before and after exposure to ammonia vapor. Also, they could be detected by spraying with aluminum chloride solution reagent by forming colored complexes. Cardiac glycosides were detected on chromatograms by reacting with Kedde’s reagent (CitationWeaver, 2005), which, when positive, formed colored complexes (reddish-violet or purplish-violet color). On the other hand, trace elements were estimated in the crude extract and its fractions, latex, and digoxin (as control for cardiac glycosides, commercially procured from Canadian Health Care Co., Canada) by using flame atomic absorption spectrophotometry (atomic absorption/emission spectrophotometer 200-A; Buck Scientific, UK). The glutathione (GSH) content was measured by the method of CitationSurapaneni and Venkataramana (2007). Catalase was measured according to CitationBouskela and Donyo (1997).
Physiological experiments
Three types of muscle were used in this study to investigate the physiological and pharmacological effects of the different plant extracts.
Cardiac muscle
Experiments on cardiac muscle were carried out on isolated toad heart preparations. Three groups each of 10 animals were used. They were directly perfused with 1 mL of ethanol extract (2 μg/mL), butanol extract (0.2 μg/ mL), and EtOAc extract (0.2 μg/mL). Furthermore, verapamil and atropine sulfate were used to investigate the mechanism of action. Electrocardiography (ECG) data were recorded from the surface of the heart before and after drug application. Signals were recorded every 5 min for half an hour on a multi-pen rectilinear recorder (DBE, UK) with paper speed options of 2 and 10 mm/s.
Smooth muscle
Extracts of C. procera were studied on several preparations of rabbit duodenum to clarify the mechanism of action of the plant. Spontaneous activity of smooth muscle was recorded by ink kymograph (model 10500, Bioscience, UK) with a paper speed of 1 mm/s.
Skeletal muscle
Toad gastrocnemius muscle/sciatic nerve preparation was used to study the effect of C. procera. The muscle was directly perfused with 2 μg/mL of ethanol extract solution, and 0.2 μg/mL butanol and EtOAc extracts. Activity of the skeletal muscle was recorded on a universal kymograph (Harvard Apparatus Ltd., UK) at a paper speed of 1.4 mm/s. The sciatic nerve was electrically stimulated with 1 V using 0.1 ms width and 1.25 mm/s speed with 5 Hz square-pulse waves.
Antagonists
A dose of 4 μg/mL of atropine sulfate (Memphis Co. for Pharmaceuticals and Chemicals, Egypt) in Ringer’s solution was used on heart preparations and a dose of 5 × 10−6 μg/mL in saline was added to smooth muscle preparations. Verapamil hydrochloride (ADWIC Pharmaceutical Division, El Nasr Pharm. Chem. Co., Egypt), a calcium channel antagonist, was applied directly to the perfused toad hearts at a dose of 5 μg/mL. Gallamine triethiodide (Alexandria Co. Pharmaceuticals, Egypt), a nicotinic receptor antagonist for skeletal muscle, was used at a dose of 3 μg/mL in Ringer’s solution.
Statistical analysis
Changes of the heart rate (HR) and other ECG parameters (P–R interval, R-wave amplitude, and T-wave amplitude) are expressed as mean ± standard error. The data were analyzed by paired t-test (CitationSujii et al., 2001).
Results
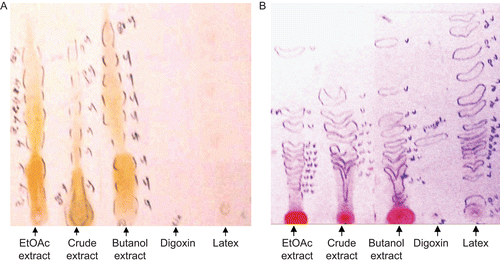
The results obtained from the preliminary phytochemical screening of the total extract of C. procera revealed the presence of cardiac glycosides, flavonoids, saponins, carbohydrates, and/or glycosides as major components. Also, alkaloids, coumarins, tannins, sterols, and/or triterpenes were detected, as shown in .
Table 1. Results of phytochemical screening of total extract of C. procera.
Detection of flavonoids
Solvent system I showed the best resolution, revealing the presence of at least seven spots of flavonoids (Rf 0.03, 0.08, 0.13, 0.21, 0.36, 0.55, 0.61) in the ethyl alcohol extract, eight flavonoids (Rf 0.03, 0.08, 0.13, 0.21, 0.36, 0.44, 0.55, 0.62) in the EtOAc extract, and nine flavonoids (Rf 0.03, 0.13, 0.21, 0.33, 0.42, 0.49, 0.59, 0.67, 0.79) in the butanol extract, while latex and digoxin were free from flavonoids (). The pet. ether extract was also free from flavonoids.
Detection of cardiac glycosides
Solvent system VII showed the best resolution, revealing the presence of eight spots of cardiac glycosides (Rf 0.09, 0.23, 0.30, 0.34, 0.40, 0.44, 0.47, 0.60) in the ethyl alcohol extract, 11 spots of cardiac glycosides (Rf 0.11, 0.16, 0.20, 0.23, 0.28, 0.30, 0.32, 0.34, 0.45, 0.51, 0.82) in the EtOAc extract, nine spots of cardiac glycosides (Rf 0.07, 0.15, 0.26, 0.30, 0.35, 0.43, 0.48, 0.62, 0.86) in the butanol extract, and 15 cardiac glycosides (Rf 0.06, 0.17, 0.22, 0.28, 0.31, 0.32, 0.35, 0.38, 0.42, 0.50, 0.56, 0.69, 0.79, 0.91, 0.98) in the latex and one spot of cardiac glycoside (Rf 0.36) in digoxin (). The pet. ether extract was also free from cardiac glycosides.
Estimation of trace elements
summarizes the concentration of sodium, potassium, calcium, zinc, copper, magnesium, and manganese trace elements in C. procera extracts, latex, and digoxin. These measurements were previously recorded for the crude plant extract and digoxin (CitationNabil et al., 2006).
Table 2. Concentration of trace elements (ppm), non-enzymatic antioxidant GSH (μg/g), and catalase (U/g) in different plant extracts, latex, and digoxin.
Effect of C. procera extracts and latex on myocardial activity
The effect of direct perfusion of the plant extracts and its latex solution on the electrical activity of isolated toad heart was studied. Heart rate, conduction time, and depolarization and repolarization voltage of the isolated toad hearts are listed in –.
Table 3. Effect of direct perfusion of crude extract (2 μg/mL), EtOAc extract (0.2 μg/mL), butanol extract (0.2 μg/mL), and latex (0.2 μg/mL) of C. procera on the heart rate (beats/min) of isolated toad heart.
Table 4. Effect of direct perfusion of crude extract (2 μg/mL), EtOAc extract (0.2 μg/mL), butanol extract (0.2 μg/mL), and latex (0.2 μg/mL) of C. procera on the heart P–R interval (ms) of isolated toad heart.
Table 5. Effect of direct perfusion of crude extract (2 μg/mL), EtOAc extract (0.2 μg/mL), butanol extract (0.2 μg/mL), and latex (0.2 μg/mL) of C. procera on the ventricular contraction [R-wave amplitude (mV)] of isolated toad heart.
Table 6. Effect of direct perfusion of crude extract (2 μg/mL), EtOAc extract (0.2 μg/mL), butanol extract (0.2 μg/mL), and latex (0.2 μg/mL) of C. procera on the repolarization voltage [T-wave amplitude (mV)] of isolated toad heart.
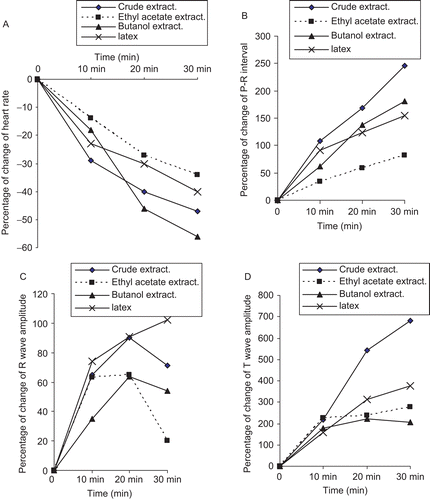
demonstrates that the different extracts and latex of C. procera induced a negative chronotropic effect, which is indicated by the recorded significant bradycardia. Direct perfusion of alcohol crude extract (2 μg/mL), ethyl acetate extract (0.2 μg/mL), butanol extract (0.2 μg/ mL), and latex (0.2 μg/mL) of C. procera significantly (p < 0.01) decreased the heart rate (HR) of isolated toad heart after different time intervals (10, 20, and 30 min).
The negative chronotropism following application of plant extracts and latex was accompanied by a significant increase, or elongation, of the conduction time (P–R interval), indicating a negative dromotropic effect. Direct perfusion of crude extract (2 μg/mL), EtOAc extract (0.2 μg/mL), butanol extract (0.2 μg/mL), and latex (0.2 μg/mL) of C. procera significantly (p < 0.01) increased the conduction time of the isolated toad heart after 10, 20, and 30 min ().
The effect of the three tested plant extracts (crude, ethyl acetate, and butanol) and the plant latex on cardiac contractility (depolarization voltage) showed a significant increase in the R-wave amplitude after plant application, reflecting a positive inotropic influence as demonstrated ().
shows that the repolarization voltage (T-wave) was significantly increased after the administration of different plant extracts and latex as well. Moreover, the percentage change in the HR and the different electrocardiogram parameters [P–R (ms), R-wave amplitude (mV), and T-wave amplitude (mV)] are graphically represented in .
Figure 2. (A) Percentage change of heart rate and ECG parameters; (B) P–R interval; (C) R-wave amplitude; (D) T-wave amplitude of isolated toad heart after perfusion with crude alcohol extract (2 μg/mL), EtOAc extract (0.2 μg/mL), butanol extract (0.2 μg/mL), and latex (0.2 μg/mL) of C. procera.
Examples of in vitro electrocardiographic representations that followed the application of C. procera extracts and latex are demonstrated in –.
Figure 3. ECG traces showing (A) the effect of direct perfusion of isolated toad heart with crude extract solution (2 μg/ml) of C. procera on the electrocardiogram of isolated toad heart at different time intervals; (B) the effect of adding atropine (4 μg/mL) after crude extract application, and (C) the effect of adding verapamil (5 μg/mL) after crude extract application (a) before treatment, (b) 20 min after treatment, (c) after adding blocker.
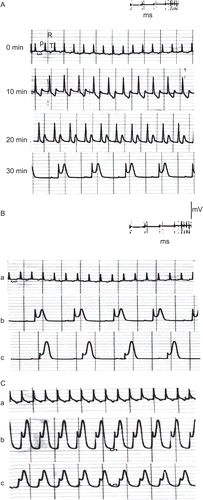
Figure 4. ECG traces showing (A) the effect of direct perfusion of isolated toad heart with EtOAc extract solution (0.2 μg/mL) of C. procera on the electrocardiogram of isolated toad heart at different time intervals; (B) the effect of adding atropine (4 μg/mL) after EtOAc extract application, and (C) the effect of adding verapamil (5 μg/mL) after EtOAc extract application (a) before treatment, (b) 20 min after treatment, (c) after adding blocker.
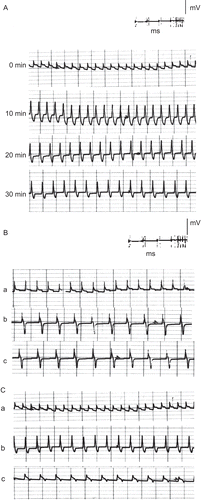
Figure 5. ECG traces showing (A) the effect of direct perfusion of isolated toad heart with butanol extract solution (0.2 μg/mL) of C. procera on the electrocardiogram of isolated toad heart at different time intervals; (B) the effect of adding atropine (4 μg/mL) after butanol extract application, and (C) the effect of adding verapamil (5 μg/mL) after butanol extract application (a) before treatment, (b) 20 min after treatment, (c) after adding blocker.
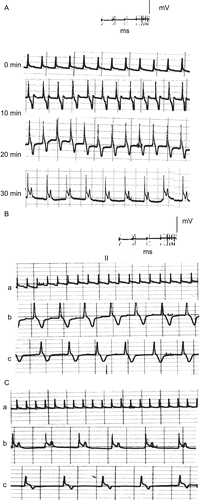
Figure 6. ECG traces showing (A) the effect of direct perfusion of isolated toad heart with latex solution (0.2 μg/mL) of C. procera on the electrocardiogram of isolated toad heart at different time intervals; (B) the effect of adding atropine (4 μg/mL) after latex application, and (C) the effect of adding verapamil (5 μg/mL) after latex application (a) before treatment, (b) 20 min after treatment, (c) after adding blocker.
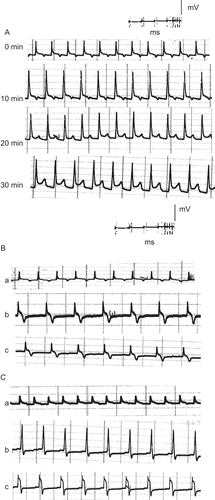
In an attempt to reveal the mechanism of action of the plant’s extracts, atropine sulfate was used as a muscarinic cholinergic antagonist following perfusion of the different extracts and latex of Calotropis procera. – show that atropine did not abolish the negative chronotropism (bradycardia) and the negative dromotropism that were caused by the different extracts and latex. On the other hand, the addition of verapamil as an L-type calcium blocker onto the hearts perfused with plant extracts abolished their positive inotropic effect, which was indicated by a reduction in the power of ventricular contraction (–).
Effect of C. procera extracts and latex on the spontaneous activity of smooth muscle
represents the mechanical activity of smooth muscle (duodenum of rabbit) before and after the application of crude extract (2 μg/mL), EtOAc and butanol extracts, and latex of C. procera (0.2 μg/mL each). The figure shows that the different extracts increased the power of contraction of the duodenum (trace a). Pretreatment with atropine sulfate as a muscarinic receptor blocker abolished the stimulatory effect of the different plant extracts and latex of C. procera (trace b).
Discussion
In our study, at least eight cardiac glycosides were detected in the alcohol extract of the plant. Fifteen cardiac glycosides were detected in the latex, and almost all of them had the same retardation value (Rf). The latex was free of flavonoids, while the total alcohol extract contained at least seven flavonoids. Previous investigations (CitationLarhsini et al., 2001; CitationIbrar et al., 2003; CitationOluwaniyi & Ibiyemi, 2007; CitationRamos et al., 2007; CitationMemon et al., 2008) of latex revealed the presence of 11 cardiac glycosides. Hitherto, there had been no concrete studies on cardiac glycosides of the total alcohol extract of the plant, but two flavonoids were isolated from the alcohol extract of the plant as glycosides: rutin and quercetin 3-O-galactoside (CitationWheeler et al., 2003).
In the present study, the total alcohol extract of the plant was fractionated to estimate the effectiveness of each fraction. This fractionation was carried out by partitioning with successive solvents: pet. ether, EtOAc, and butanol. We found that the pet. ether extract was free of flavonoids, cardiac glycosides, and saponins. While the total alcohol extract contained seven flavonoids and eight cardiac glycosides, the greater majority were present as aglycones, less polar compounds. The EtOAc extract contained eight flavonoids and 11 cardiac glycosides. On the other hand, the butanol extract contained nine flavonoid and nine cardiac glycoside components (CitationMemon et al., 2008). These compounds are highly polar compounds. The butanol extract contained flavonoids with retardation values similar to those of the flavonoids in the EtOAc extract.
The pharmacology of cardiac glycosides involves a complex set of direct and indirect effects (CitationKatzung & Parmely, 2001; CitationLopatina, 2008). Their most important effect is to enhance the force and velocity of myocardial contraction (positive inotropic effect). In the present study, C. procera increased the power of ventricular contraction. The effect could have been directly on the T-calcium channels, since verapamil (L-type calcium channel antagonist) abolished the increase in ventricular voltage. The direct positive inotropic effect of the plant extracts on the myocardium is similar to that of phosphodisterase in its mode of action. It is well known that the synthetic phosphodiesterase (PDE) inhibitors are used for treatment of cardiac failure (CitationBrown & Kozlowski, 1997). Through an increase in intracellular cyclic adenosine monophosphate (CAMP), which in turn opens calcium channels, enhancing the inward calcium current, the therapy produces a positive inotropic effect. It is apparent from the present study that a group of steroid compounds from the plant extracts can alter the electrical functions of the heart through inhibition of Na+/K+-ATPase. Consequently, there is increased Na+/ Ca++ exchange leading to an increase of calcium entry (CitationKatzung & Parmely, 2001; CitationBanday et al., 2008). In addition, the cardiac glycosides examined in the present study decreased the heart rate (negative chronotropic effect), thereby suggesting therapeutic potential during cardiac failure that is commonly accompanied by ventricular tachycardia. Digitalis depresses atrioventricular nodal conductivity (negative dromotropic effect), but it may also increase myocardial excitability and automaticity (positive bathmotropic effect), leading to the development of arrhythmia. The present results showed that different extracts of C. procera induced negative chronotropism and negative dromotropism on the isolated hearts. This local mechanism would appear to result from the plant’s rich constituents and minerals including potassium, calcium, sodium, and zinc. For example, the action of potassium ions could manifest the bradycardia or negative chronotropism. A moderate increase in extracellular K+ concentration causes the membrane potassium conductance to increase, thereby bringing the potential close to the potassium equilibrium potential, membrane hyperpolarization, and myocardial relaxation (CitationDraid et al., 2005). On the other hand, the increased repolarization voltage of isolated hearts in the present study suggests hyperkalemia in the myocardium (CitationWeaver, 2005). The muscarinic cholinergic antagonist atropine did not abolish the observed effect, which stimulates an argument of direct action on the myocardium (CitationVassort, 2001). The present data also suggest that the duration of atrioventricular conduction was prolonged by the extracts of the studied plant. On the other hand, it is highly probable that plant extract was responsible for the direct effects observed, which were mediated in part by disruption of the cellular electrolyte gradient associated with ATPase inhibition. It is also known that the positive inotropic effect and toxic action of digitalis on the heart is diminished or eliminated by raising the K+ concentration in the medium. The basis of this antagonism may be competition between digitalis and K+ for the same binding site on the outer surface of the cell. Because of their very narrow therapeutic margin, therapy with cardiac glycosides is difficult. In some patients, toxic symptoms are observed at doses that are required to compensate the failing heart at least partially (CitationSundararajan et al., 2005; CitationXiaohua et al., 2007; CitationEAPCCT, 2008). Flavonoids inhibit Na+/K+-ATPase, and can therefore affect the function of the plasma membrane transport of Na+/K+-ATPase, mitochondrial ATPase, and Ca++-ATPase. The sarcoplasmic reticulum Ca++-ATPase of muscle is effectively inhibited by several flavonoids (CitationEbadi, 2002). From the present study, it was found that the alcohol extract of C. procera contained a high concentration of K+ ions, which diminished the toxicity of digitalis glycosides resulting from hypokalemia. The increase in smooth muscle activity following the application of the different extracts can be postulated to be the indirect stimulatory effect of vagal tone, since atropine abolished the observed effect due to the plant extracts.
The present study demonstrated the relaxant effect of C. procera extracts on skeletal muscle contraction following its direct application. The blockade of nicotinic receptors at the neuromuscular junction before application of the extracts sustained normal contraction and ensured the action of the plant on the nicotinic receptors. The decrease in contraction was attributed to the non-depolarizing action of the plant extract that mimics the effect of conventional neuromuscular blockers. The data generated require further investigation for definite characterization of the relaxant property of C. procera on the neuromuscular junction, which could be used for local medication during such surgeries in which increased muscle tone is a disadvantage (CitationDavies et al., 2001).
The results obtained from chromatographic techniques showed that the crude alcohol extract contained both cardiac glycosides and flavonoid glycosides. They apparently acted together to potentiate the electrophysiological effect(s) of the plant’s crude extract through inhibition of Na+/K+-ATPase (CitationEbadi, 2002). On the other hand, the butanol fraction was composed of cardiac glycosides as a major component in addition to flavonoids. The EtOAc fraction contained flavonoids as major constituents in addition to cardiac glycosides. This may offer an explanation of the graded effects of these extracts on the cardiac muscle activity.
Conclusion
The present data for the traditional medicinal plant of C. procera provide documentation of its direct action on the myocardium, stimulatory effect on smooth muscle motility, and relaxant action on skeletal muscle contraction. The biologically active chemical constituents as well as the rich mineral content were beneficial to manifestation of the suggested mode(s) of action on the excitable tissues. Their probable effects could include direct action on the cell membrane probably through receptors coupling to G proteins. These chemical constituents regulate the ion channel physiology as in the myocardium and indirectly through activation of the cholinergic muscarinic receptors of the smooth muscle and/or inhibition of the skeletal nicotinic receptors.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abdel-Khalik KN, Bakker FT (2007): Nasturtiopsis integrifolia (Boulos) Abdel Khalik & Bakker (Brassicaceae), a new combination, and Cruciata articulate (L.) Ehrend. (Rubiaceae), a new record for the flora of Egypt. Turk J Bot 31: 571–574.
- Banday AH, Singh S, Alam MS, Reddy DM, Gupta BD, Kumar HMS (2008): Synthesis of novel steroidal D-ring substituted isoxazoline derivatives of 17-oxoandrostanes. Steroids 73: 370–374.
- Barrett B, Kieffer D (2001): Medicinal plants, science and health care. J Herb Spice Med Plant 8: 1–36.
- Brown H, Kozlowski R (1997): Physiology and Pharmacology of the Heart. Oxford, Blackwall Science Ltd., pp. 103–105.
- Bouskela E, Donyo KA (1997): Effects of oral administration of purified micronized flavonoid fraction on increased microvascular permeability induced by various agents and on ischemia/reperfusion in the hamster cheek pouch. Angiology 48: 391–399.
- Choedon T, Mathan G, Arya S, Kumar VL, Kumar V (2006): Anticancer and cytotoxic properties of the latex of Calotropis procera in a transgenic mouse model of hepatocellular carcinoma. World J Gastroenterol 12: 2517–2522.
- Davies A, Blakely A, Kidd C (2001): Human Physiology. Edinburgh, Churchill Livingstone, pp. 65–67.
- Draid M, Shiina T, El-Mahmoudy A, Boudaka A, Shimizu Y, Takewaki T (2005): Neurally released ATP mediates endothelium-dependent hyperpolarization in the circular smooth muscle cells of chicken anterior mesenteric artery. Braz J Pharm 146: 983–989.
- EAPCCT (2008): Abstracts of the XXVIII International Congress of the European Association of Poison Centers and Clinical Toxicologists. Clin Toxicol 46: 351–421.
- Ebadi M (2002): Pharmacodynamic Basis of Herbal Medicine. Boca Raton, FL, CRC Press, pp. 393–395.
- Eleyinmi AF (2007): Chemical composition and antibacterial activity of Gongronema latifolium. J Zhejiang Univ Sci B 8: 352–358.
- Ibrar M, Hashim S, Marwat KB (2003): Ethnobotanic study of the weeds of five crops in district abbottabad, N-W Pakistan. Pak J Weed Sci Res 9: 229–240.
- Jalalpure SS, Salahuddin M, Shaikh MI, Manvi FV, Shivkar YM, Kumar VL (2009): Anticonvulsant effects of Calotropis procera root in rats. Pharm Biol 47: 162–167.
- Katzung BG, Parmely WW (2001): Cardiac glycosides and other drugs used in congestive heart failure. In: Katzung BG, ed., Basic and Clinical Pharmacology, 8th ed. New york, McGraw-Hill, pp. 200–208.
- Kumar VL, Roy S (2008): Protective effect of latex of Calotropis procera in Freund’s complete adjuvant induced monoarthritis. Phytother Res 23: 1–5.
- Kumar VL, Shivkar YM (2004): Effect of dry latex of Calotropis procera on gastrointestinal smooth muscle. J Ethnopharmacol 93: 377–379.
- Larhsini M, Oumoulid L, Lazrek HB, Wataleb S, Bousaid M, Bekkouche K, Jana M (2001): Antibacterial activity of some Moroccan medicinal plants. Phytother Res 15: 250–252.
- Lopatina EV, Karetsky AV, Penniyaynen VA, Vinogradova TV (2008): Role of cardiac glycosides in regulation of the growth of retinal tissue explants. Bull Exp Biol Med 146: 744–746.
- Memon AH, Rind FMA, Laghari MGH, Mughal UR, Memon N, Gilal RA, Khuhawar MY, Almani F (2008): Common folk medicinal and ethnomedicinal uses of thirty medicial plants of districts Dadu and Jamshoro, Sindh, Pakistan. Sindh Univ Res J (Sci Ser) 40: 89–108.
- Mukherjee PK, Sahoo AK, Narayanan N, Kumar NS, Ponnusankar S (2009): Lead finding from medicinal plants with hepatoprotective potentials. Expert Opin Drug Discov 4: 545–576.
- Nabil ZI, Hussein AA, Moustafa AMY, Ahmed SH, Omran MAA (2006): Assessment of the therapeutic effect of Calotropis procera extract compared to digoxin on myocardial pathology: An experimental study. Egypt J Nat Toxins 3: 51–76.
- Oluwaniyi OO, Ibiyemi SA (2007): Extractability of Thevetia peruviana glycosides with alcohol mixture. Afr J Biotechnol 6: 2166–2170.
- Patra A, Shivesh J, Murthy PN, Aher VD, Chattopadhyay P, Panigrahi G, Devdeep RD (2009): Anti-inflammatory and antipyretic activities of Hygrophila spinosa T. Anders leaves (Acanthaceae). Trop J Pharm Res 8: 133–137.
- Ramos MV, Freitas CDT, Stanisçuaski F, Macedo LLP, Sales MP, Sousa DP, Carlini CR (2007): Performance of distinct crop pests reared on diets enriched with latex proteins from Calotropis procera: Role of laticifer proteins in plant defense. Plant Sci 173: 349–357.
- Seddek AS, Mahmoud ME, Shiina T, Hirayama H, Iwami M, Miyazawa S, Nikami H, Takewaki T, Shimizu Y (2009): Extract from Calotropis procera latex activates murine macrophages. J Nat Med 63: 297–303.
- Sujii ER, Garcia MA, Fontes EMG, Da-Silva SMB, Meyer JFCA (2001): Soil temperature and diapause maintenance in eggs of the spittlebug, Deois Flavopicta (Hemiptera: Cercopidae). Braz J Biol 61: 605–613.
- Sundararajan R, Haja NA, Venkatesan K, Kakali M, Arun B, Kumar MP (2005): Exploring the effect of Asclepias curassavica on markers of oxidative stress in rats. Evid Based Integr Med 2: 87–93.
- Surapaneni KM, Venkataramana G (2007): Status of lipid peroxidation, glutathione, ascorbic acid, vitamin E and antioxidant enzymes in patients with osteoarthritis. Indian J Med Sci 61: 9–14.
- Vassort G (2001): Adenosine 5′-triphosphate: a P2-purinergic agonist in the myocardium. Physiol Rev 81: 767–793.
- Weaver CN (2005): The changing image of Hispanic Americans. Hisp J Behav Sci 27: 337–354.
- Wheeler GS, Massey LM, Southwell IA (2003): Dietary influences on terpenoids sequestered by the biological control agent Oxyops vitiosa: effect of plant volatiles from different Melaleuca quinquenervia chemotypes and laboratory host species. J Chem Ecol 29: 189–208.
- Xiaohua He, Sullivan EV, Stankovic RK, Harper CG, Pfefferbaum A (2007): Interaction of thiamine deficiency and voluntary alcohol consumption disrupts rat corpus callosum ultrastructure. Neuropsychopharmacology 32: 2207–2216.