Abstract
Context: The clinical applications of well-known benzodiazepines as anxiolytic agents are limited because of their side effects. Therefore, the development of new pharmacological agents, from medicinal plants, is well justified.
Objective: Among medicinal plants, Gelsemium sempervirens (L.) Aiton. (Loganiaceae) has been recommended for relief of anxiety in traditional folk medicines. Nevertheless, no pharmacological studies have so far evaluated it in this regard. The purpose of the present study was to investigate the anxiolytic effects of various extracts of the roots and rhizomes of G. sempervirens.
Materials and methods: Petroleum ether, chloroform, methanol, and water extracts of G. sempervirens were prepared by successive extractions using a Soxhlet apparatus, and subsequently evaluated for antianxiety activity using the elevated plus maze model. Diazepam was used as standard drug.
Results: Among various extracts, the methanol extract of G. sempervirens exhibited significant increases in open arm entries and mean time spent in open arms at the dosage of 150 mg/kg. A fraction (F9.4) derived from the methanol extract was also observed to exhibit significant anxiolytic activity at the dose level of 10 mg/kg in the elevated plus maze test.
Conclusion: The present study clearly demonstrated that the methanol extract exerts an anxiolytic effect on mice, and it could serve as a new approach for the treatment of anxiety.
Introduction
Despite significant advances in the understanding and management of neuropsychiatric disorders during the past few decades, anxiety and depression still remain leading causes of mortality. According to a world health report (CitationWHO, 2001), 450 million people suffer from a mental or behavioral disorder, yet only a small portion of them receive even the most basic treatment (CitationBhattamisra et al., 2008). This amounts to 12.3% of the global burden of the diseases, which will rise to 15% by 2020 (CitationReynolds, 2003). Currently, the most widely prescribed medicines for anxiety disorders are benzodiazepines; however, the clinical use of these agents is limited by their side effects (CitationEmamghoreishi et al., 2005; CitationMitte et al., 2005). In spite of their relative safety, benzodiazepines can lead to disturbing effects such as amnesia, dependence liability, and sedation, which cause considerable concern (CitationRaupp et al., 2008). Therefore, the exploration of new medications from traditional medicinal plants possessing anxiolytic effects without the complications of benzodiazepines would be of great importance for the relief of anxiety-like disorders. On the basis of these considerations, the purpose of the present study was to evaluate the anxiolytic effects of various extracts prepared from the roots and rhizomes of Gelsemium sempervirens (L.) Aiton. (Loganiaceae).
G. sempervirens, commonly known as yellow jasmine, is native to the southern United States, from Virginia to Florida.
Since ancient times, G. sempervirens has been known for its uses in the treatment of cold, influenza (CitationVoorhees & Nachman, 2002), psoriasis, neurodermatitis (CitationCalarasu, 1989), and myopia (CitationNebera et al., 2003). G. sempervirens is frequently used for mental irritability, anxiety, and insomnia (CitationFelter & Lloyd 1983).
However, until now there have been no reports on the pharmacological evaluation of the anxiolytic effects of G. sempervirens. Thus, in the present study, the anxiolytic activity of various extracts prepared from G. sempervirens was investigated using the elevated plus maze (EPM) test. Bioactivity directed fractionation and chromatographic methods were also employed to obtain the final bioactive fraction containing the major phytoconstituents liable for the potential anxiolytic-like effects of this plant.
Materials and methods
Plant material
The dried roots and rhizomes of G. sempervirens were procured from a commercial source, Himalayas Herbs, Saharanpur (UP, India). The identity of the roots and rhizomes of G. sempervirens was confirmed by one of the authors (V.D.) through the National Institute of Science Communication and Information Resources (NISCAIR), Delhi. A voucher specimen (NISCAIR/RHMD/Consult/-2008-09/1146/178) was deposited in NISCAIR, Delhi for further reference.
Chemicals and instruments
Solvents, viz., petroleum ether (40–60°C), chloroform, and methanol (AR grade; S.D. Fine-Chem Ltd., Mumbai, India), were employed for extraction of the plant material. Kieselguhr was used for column chromatography. Precoated thin layer chromatography (TLC) sheets (E. Merck) and 2 μL capillary tubes were utilized for developing thin layer chromatograms. The chromatograms were visualized under ultraviolet (UV) light and also by spraying 0.5% anisaldehyde (Thomas Baker, Mumbai, India). Diazepam I.P. was procured form Ranbaxy Ltd., Gurgaon, Haryana, India.
Preparation of plant extract
Air dried and powdered (#10) roots and rhizomes of G. sempervirens (5 kg) were successively extracted separately with petroleum ether (60–80°C), chloroform, and methanol in a Soxhlet apparatus. Exhaustive extraction with each of the solvents was ensured. The marc was dried and further extracted with distilled water (boiling for 1 h) and filtered (CitationDhawan et al., 2001; CitationKumar & Sharma, 2006). The extracts were evaporated to dryness in vacuo and weighed, and their percentage yield was calculated. The extracts were kept in a refrigerator for further use.
Animals
Adult Swiss albino mice of either sex (18–25 g), procured from Haryana Agricultural University, Hisar, India, were kept in the Animal House of the Guru Gobind Singh College of Pharmacy, Yamunanagar, Haryana, India. They were housed in groups in polypropylene cages (11 cm × 17 cm × 28 cm) with wood shavings as bedding, under controlled conditions of light (12 h light–dark cycles) and temperature (25 ± 1°C). The mice had free access to food and water. The protocols for the experimental animals were duly approved by the Institutional Animal Ethical Committee (Reg. No. 873/ac/05/CPCSEA).
Treatments
The extracts/fractions (test materials) of G. sempervirens were separately suspended in a vehicle comprising 0.2% Tween 80 in normal saline solution (0.9% NaCl). Diazepam 2.5 mg/kg was suspended in the vehicle and used as a standard anxiolytic agent. The suspending vehicle was used as control. Doses of various test substances such as extracts/fractions/diazepam/vehicle were adjusted in such concentrations as to administer these to mice in a volume ranging from 0.20 to 0.28 mL via the oral route.
Acute toxicity studies
Acute toxicity studies were performed according to the Organization for Economic Co-operation and Development (CitationOECD, 1996). Swiss albino mice were selected by a random sampling technique. The animals were fasted for 4 h with free access to water only. The methanol extract of G. sempervirens was administered orally at the dose level of 5 mg/kg body weight, and mortality, if any, was observed for 3 days. If mortality was observed in two out of three animals, then the dose administered was considered the toxic dose. However, if mortality was observed in only one animal out of three, then the same dose was repeated to confirm the toxic effect. If no mortality was observed, then only the higher (50, 300, 1000 mg/kg) doses of the methanol extract of G. sempervirens were employed for further toxicity studies.
Elevated plus maze test
The plus maze apparatus, consisting of two open arms (16 × 5 cm) and two closed arms (16 × 5 × 12 cm) each having an open roof, with the apparatus elevated (25 cm) from the floor, was used to observe anxiolytic behavior in animals. Each mouse was placed at the center of the elevated plus maze with its head facing an open arm (CitationPeng et al., 2000; CitationRabbani et al., 2005). During this 5 min experiment, the behavior of each mouse was recorded as: (a) the number of entries into open arms, and (b) the average time spent by the mouse in the open arms (average time = total time spent in open arms/number of entries in arms). Test materials were administered orally using a tuberculin syringe fitted with an oral cannula. The dose administration schedule was adjusted so that each mouse had its turn on the EPM apparatus 45 min after administration of the dose (CitationKulkarni & Reddy, 1996). During the entire experiment, the animals were allowed to socialize. Every precaution was taken to ensure that no external stimuli, other than the height of the plus maze, could invoke anxiety in the animals. Similar observations were recorded for a standard group (diazepam 2.5 mg/kg) as well as for a control group.
Statistical analysis
The anxiolytic activities of the test substances, diazepam (standard), and control were assessed by analysis of variance (ANOVA), and test groups were compared with standard/control by studentized Tukey test. Differences were considered significant at p < 0.05.
Fractionation of methanol extract
The bioactive methanol extract (250 g) was subjected to column chromatography using kieselguhr as the stationary phase. Elution was done with chloroform and chloroform–methanol in increasing order of polarity. A total of 200 fractions, 250 mL each, were collected. On the basis of similar thin layer chromatograms, fractions were pooled. All the pooled fractions (F1–11) were screened for anxiolytic activity at various doses (5, 10, 15 and 25 mg/kg, p.o.) given to the mice, using the EPM apparatus.
Fractionation of F9
Fraction F9 was again column chromatographed over keiselguhr white. Elution was done with chloroform and chloroform–methanol. Fractions were pooled on the basis of similar TLC profiles. All seven fractions (F9.1–9.7) were evaluated for anxiolytic activity at various dose levels: 2, 5, 10, and 15 mg/kg.
Phytochemical screening of F9.4
The bioactive fraction F9 and sub-fraction F9.4 were subjected to a phytochemical screening test (CitationFarnsworth, 1966).
Effect on motor co-ordination
The effect on motor co-ordination was assessed using a rotarod apparatus as described earlier (CitationDunham & Miya, 1957). In brief, the mice were trained to remain for 5 min on the rod rotating at a speed of 25 rpm. On the next day, the animals were randomly divided into groups of five. The animals received F9.4 (10 mg/kg), diazepam (2 mg/kg), and vehicle. The animals were placed individually on the rotating rod, and the ability of the animal to remain on the rotating rod was assessed 45 min after drug administration. The time of fall from the rod was noted for each animal and the test was terminated after 3 min.
Results
Various extracts/fractions prepared from G. sempervirens were evaluated for anxiolytic activity at various doses. The percentage yields (w/w) of petroleum ether, chloroform, methanol, and water extracts were found to be 0.62, 1.41, 14.72, and 3.02%, respectively.
The methanol extract tested positive for alkaloids, steroids, and glycosides. All the extracts gave negative tests for saponins, flavonoids, and carbohydrates, whereas the chloroform extract showed a positive test for alkaloids. All the doses (5, 50, 300, and 1000 mg/kg, p.o.) of G. sempervirens employed for acute oral toxicity studies were found to be non-toxic. The methanol extract of G. sempervirens did not produce any mortality even at the highest dose (1000 mg/kg) employed. In addition, the general behavior remained unaltered, and similar observations were recorded for the controls.
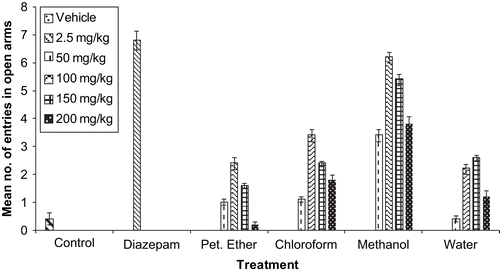
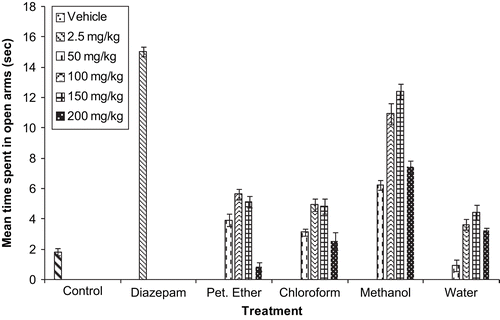
The antianxiety profile showing the mean number of entries and mean time spent in the open arms of the vehicle, standard drug diazepam, and various extracts of G. sempervirens are depicted in and , respectively. Results for column chromatography of the methanol extract of G. sempervirens are listed in , and the antianxiety activity profile of F1–11 is shown in . Results for column chromatography of F9 and the antianxiety activity of F9.1–9.7 are listed in and , resepctively. As F9.4 showed the maximum antianxiety profile amongst the various fractions, therefore F9.4 was processed further for pharmacological investigations. Mice treated with vehicle or F9.4 (10 mg/kg) remained on the rotating rod for the complete 180 s period, whereas diazepam (2 mg/kg i.p.) treated mice remained on the rotating rod for 27.8 ± 4.9 s only. Phytochemical screening of F9 tested positive for alkaloids, steroids, and iridiods, whereas sub-fraction F9.4 gave a positive test only for alkaloids and iridiods.
Table 1. Fractionation of bioactive methanol extract of G. sempervirens roots and rhizomes using column chromatography.
Table 2. Antianxiety activity of various fractions obtained from methanol extract of G. sempervirens in EPM model.
Table 3. Fractionation of F9 using column chromatography.
Table 4. Antianxiety activity of various fractions derived from F9 of G. sempervirens using EPM model.
Discussion
Despite the traditional use of G. sempervirens for treating nervous disorders, there is an absence of scientific reports on the evaluation of its anxiolytic effects. The elevated plus maze is currently one of the most widely used models of animal anxiety (CitationHoggs, 1996; CitationRodgers, 1997), and has been validated for use with both rats and mice (CitationLister, 1987). An anxiolytic agent increases the frequency of entries into open arms and increases the time spent in open arms of the EPM, and is thought to act via the GABAA (γ-aminobutyric acid type A) receptor complex, justifying the use of diazepam as a positive control in the study (CitationPellow et al., 1985). In the present study, the oral administration of a methanol extract prepared from the roots and rhizomes of G. sempervirens increased the number of entries and time spent in open arms. As expected, diazepam produced a significant increase in open arm entries and mean time spent in open arms. These increments were accompanied by a statistically significant change in motor activity. Since the other plant extracts, i.e., petroleum ether, chloroform, and water extracts, did not produce meaningful effects in the EPM test, therefore only the methanol extract of G. sempervirens was processed further for pharmacological investigation. Maximum anxiolytic activity of the methanol extract was confirmed at the dose level of 150 mg/kg using the EPM model in mice. The methanol extract of G. sempervirens was subjected to column chromatography, for isolation of the active fraction. Fraction F9 showed maximum anxiolytic potential amongst the various fractions, significant at a dose level of 10 mg/kg, using the EPM model.
Column chromatography of F9 yielded seven sub-fractions (), out of which only F9.4 exhibited significant anxiolytic activity at the dose of 10 mg/kg (). Further, F9.4 at the dose level of 10 mg/kg was without any effect on motor co-ordination. F9.4 gave a positive test as expected for alkaloids and iridiods. A literature survey revealed that alkaloids (CitationWenkert, et al., 1972; CitationKogure et al., 2005) and iridoids (CitationJensen et al., 1987) are the active constituents in G. sempervirens. The antianxiety activity of fraction F9.4 may be attributed to individual or combined effects of the abovementioned constituent groups of the plant.
Conclusion
It is concluded that a fraction F9.4 derived from the methanol extract of G. sempervirens showed significant antianxiety activity at the dose of 10 mg/kg in mice using the EPM model of anxiety. Studies are still in process for isolation of the compounds from the bioactive fraction F9.4.
Acknowledgement
The authors are grateful to S. Bhupinder Singh Jauhar, Chairman, Guru Nanak Khalsa Group of Educational Institutions, Yamunanagar, Haryana, India for providing the necessary facilities to carry out this research work.
Declaration of interest
The authors report no declaration of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bhattamisra SK, Khanna VK, Aggarwal AK, Singh PN, Singh SK (2008): Antidepressant activity of standardized extract of Marsilea minuta Linn. J Ethnopharmacol. 117: 51–57.
- Calarasu C (1989): Pharmaceutical containing diphenhydramine and Echinaceae and Eupatorium and Gelsemium and Lachesris for the treatment of psoriasis and neurodermititis. German Patent No. 3641220, 4, 1988. Chem Abstr. 110: 141559.
- Dhawan K, Kumar S, Sharma A (2001): Antianxiety studies on extract of Passiflora incarnata Linneaus. J Ethnopharmacol. 78: 165–170.
- Dunham MW, Miya TS (1957): A note on a simple apparatus for detecting neurological deficits in rats and mice. J Am Pharm Assoc Sci. 46: 208–209.
- Emamghoreishi M, Khasaki M, Aazam MF (2005): Coriandrum sativum: Evaluation of its anxiolytic effect in the elevated plus-maze. J Ethnopharmacol. 96: 365–370.
- Farnsworth NR (1966): Biological and phytochemical screening of plants. J Pharm Sci. 55: 225–286.
- Felter HW, Lloyd JU (1983): Gelsemium (U.S.P.) – Gelsemium. In: King’s American Dispensatory [online]. Avaliable at: http://www.ibilio.org/herbmed/kings/gelsemium.htm. Accessed 23 August 2006.
- Hoggs S (1996): A review of the validity and variability of the elevated plus maze as an animal model of anxiety. Pharmacol Biochem Behav. 54: 21–30.
- Jensen SR, Kirk O, Nielsen BJ, Norrestam R (1987): 9-Hydroxy substituted iridiods from Gelsemium sempervirens. Phytochemistry. 26: 1725–1731.
- Kogure N, Nishiya C, Kitajima M, Takayama H (2005): Six new indole alkaloids from G. sempervirens Ait. Tetrahedron Lett. 46: 5857–5861.
- Kulkarni SK, Reddy DS (1996): Animal behavioural models for testing antianxiety agents. Method Findings Exp Clin Pharmacol. 18: 219–230.
- Kumar S, Sharma A (2006): Apigenin – the anxiolytic constituent of Turnera aphrodisiaca Ward. Pharm Biol. 44: 84–90.
- Lister RG (1987): The use of a plus maze to measure anxiety in the mouse. Psychopharmacology. 92: 180–185.
- Mitte K, Noack P, Steil R, Hautzinger M (2005): A meta-analytic review of the efficacy of drug treatment in generalized anxiety disorder. J Clin Psychopharmacol. 25: 141–150.
- Nebera SA, Nebera OA, Pakhomova NN (2003): Homoeopathic preparation for treating myopia. RU Patent No. 2203674. Chem Abst. 139: 219298.
- OECD (1996). Test No. 423: Acute oral toxicity – acute toxic class method. In: OECD Guidelines for the Testing of Chemicals. Paris, OECD.
- Pellow S, Chopin P, File SE, Briley M (1985): Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 14: 149–176.
- Peng W, Hsieh M, Lee Y, Lin Y, Liao J (2000): Anxiolytic effect of seed of Ziziphus jujube in mouse models of anxiety. J Ethnopharmacol. 72: 435–441.
- Rabbani M, Sajjadi SE, Jafarian A, Vaseghi G (2005): Anxiolytic effect of Salvia reuterana Bioss on the elevated plus maze model of anxiety in mice. J Ethnopharmacol. 101: 100–103.
- Raupp IM, Sereniki A, Virtuoso S, Ghislandi C, Cavalcanti e Silva EL, Trebien HA, Miguel OG, Andreatini R (2008): Anxiolytic-like effects of chronic treatments with Erythrina velutina extract in the elevated plus-maze test. J Ethnopharmacol. 118: 295–299.
- Reynolds EH (2003): Brain and mind: A challenge for WHO. Lancet. 361: 1924–1925.
- Rodgers RJ (1997): Animal model of anxiety: Where next? Behav Pharmacol 8: 477–496.
- Voorhees J, Nachman L (2002): Herbal composition for treating symptoms of influenza. United States Patent No. 64550703. Chem Abstr. 137: 159364.
- Wenkert E, Chang CJ, Corchan DW, Polliccari R (1972): 13C nuclear magnetic resonance spectroscopy of naturally occurring substances, VIII. Structure of gelsevirine. Experientia. 28: 377–379.
- WHO (2001): The world health report 2001 – Mental health: New understanding, new hope. Geneva, WHO.