Abstract
Context: Costus spicatus Swartz (Costaceae), commonly called “cana-do-brejo’” in Brazil’s northeast, is a medicinal plant found in wet coastal forests. In folk medicine an infusion of the aerial parts is taken to treat inflammation and pain.
Objective: The methanol extract obtained from the leaves of Costus spicatus (MECs) was evaluated for antinociceptive and anti-inflammatory activities.
Methods: Analgesic and anti-inflammatory activities were studied by measuring nociception through acetic acid, formalin, and hot-plate tests, while inflammation was induced by carrageenan. All experiments were conducted with experimental animals.
Results and discussion: Following oral administration, MECs (100, 200, and 400 mg/kg) significantly reduced the number of writhes (52.8, 43.1, and 55.3%, respectively) in the writhing test and the number of paw licks during phase 1 (61.9, 54.1, and 92.1%) and phase 2 (62.5, 82.9, and 98.1%, all doses) during the formalin test when compared to the control group animals. The reaction time during the hot-plate test was increased significantly and was dose-dependent, whereas pretreatment with naloxone rigorously reduced the analgesic potential of MECs, which suggested participation of the opioid system in the modulation of pain induced by MECs. Such results were unlikely to be provoked by motor abnormality, as MECs-treated mice did not exhibit any performance alteration during the Rota-rod test. The administration of 200 and 400 mg/ kg (i.p.) of MECs exhibited an anti-inflammatory effect during the carrageenan test, which was based on interference with inflammatory mediator synthesis.
Conclusion: We conclude that MECs has antinociceptive and anti-inflammatory activities in rodents.
Introduction
Medicinal plants, considered to possess therapeutic properties, have been used since the beginning of human civilization to treat different diseases, and the use of this effective strategy for the promotion of human health has significantly increased in recent years (CitationCalixto et al., 2000). This fact is related to several factors, including the safety, effectiveness, and better quality control of phytomedicines available on the market today (CitationDe Souza et al., 2009). Although in recent years notable progress has been made concerning the development of natural therapies, there is an urgent need to discover effective and potent analgesic agents (CitationCalixto et al., 2000; CitationBarbosa-Filho et al., 2006; CitationMelo et al., 2008).
Costus spicatus Swartz (Costaceae), popularly known as “cana-do-brejo” in Brazil’s northeast, is a medicinal plant found in wet coastal forests. The rhizomes of this plant are used in folk medicine as a diuretic, a hypoglycemic, for the treatment of complaints of the bladder and urethra, and to expel kidney stones (CitationManfred, 1947; CitationGasparri, 2005). An infusion of the aerial parts of this plant is taken to treat colds, sore throats, dysentery, and diarrhea (CitationCruz, 1965). Flavonol glycosides have been isolated from the leaves and have demonstrated an inhibitory activity on nitric oxide production by activated macrophages (CitationDa Silva et al., 2000). Additionally, the same group showed that polysaccharides isolated from the fresh stems of C. spicatus inhibited capillary permeability and demonstrated phagocytosis-stimulating properties in rodents (CitationDa Silva & Parente, 2003). It has been reported that some flavonol glycosides have an anti-inflammatory effect through the inhibition of some macrophage functions involved in the inflammatory process, such as nitric oxide production (CitationFang et al., 2005). Other reports show that the plant extract, rich in flavonoids, has antinociceptive properties due to a possible interaction with the opioid system (CitationGhannadi et al., 2005; CitationMaleki-Dizaji et al., 2007).
To date, no data exist about the possible antinociceptive and anti-inflammatory activities of C. spicatus in experimental animals. Thus, the purpose of the present study was to evaluate the antinociceptive and anti-inflammatory effects of the methanol extract obtained from the leaves of C. spicatus on rodents.
Materials and methods
Plant material
The leaves of C. spicatus were collected in November 2008 in São Cristóvão, Sergipe, Brazil. A voucher specimen (ASE 11453) is preserved in the Herbarium of the Departamento de Biologia at the Universidade Federal de Sergipe (UFS), Sergipe, Brazil.
Preparation of plant extract
The dried leaves of the plant (2.100 kg) were powdered and extracted exhaustively at room temperature with methanol in successive phases. After evaporation of the solvent under reduced pressure, 260 g of the methanol extract of C. spicatus (MECs) was obtained.
Drugs
Acetic acid, carrageenan, diazepam, and polyoxyethylene-sorbitan monolate (Tween 80) were purchased from Sigma (USA). Morphine (MOR), naloxone (NAL), indomethacin (INDO), and aspirin (acetylsalicylic acid) were purchased from União Química Farmacêutica Nacional (Brazil). The MECs was administered per os (p.o.) in volumes of 0.1 mL/10 g (mice) and 0.1 mL/100 g (rats), and the doses (100, 200, and 400 mg/kg) were adjusted for these respective volumes.
Animals
All experiments were performed on male Swiss mice (28–33 g) and male Wistar rats (180–200 g), housed in numbers of 20 or five to each cage, respectively, under a 12 h light/12 h dark cycle (lights on at 6:00 h) in a temperature controlled room (22 ± 2°C) with free access to laboratory chow and tap water. Mice were acclimatized to the laboratory conditions for at least 1 h before tests, which were carried out between 9:00 and 17:00 h. All experiments were conducted under the ethical guidelines of the International Association for the Study of Pain (IASP) (CitationZimmermann, 1983). Besides this, all experimental procedures were previously approved by the Committee on the Ethical Use of Animals at the Federal University of Sergipe (CEPA/UFS # 58/07), where the study was conducted. Animals were used only once throughout the experiments. The number of animals and intensities of noxious stimuli used were the minimum necessary to demonstrate the consistent effects of drug treatments.
Acetic acid-induced writhing
The acetic acid test was done using the method described by CitationKoster et al. (1959) and modified by CitationBroadbear et al. (1994). Initially, the mice were divided into five groups (n = 8). Subsequently, MECs (100, 200, and 400 mg/kg), vehicle (saline/Tween 80 0.2%, as control group), and aspirin (200 mg/kg) were administered p.o. 60 min before an injection of 0.25 mL per animal of acetic acid (0.85%). Each animal was isolated in an individual observation chamber, and 15 min after acetic acid injection the cumulative number of writhing responses was recorded for 15 min.
Formalin-induced pain
The formalin test was carried out as described by CitationHunskaar and Hole (1987). The animals were divided into six groups (n = 8) and treated with vehicle (control, p.o.), MECs (100, 200, and 400 mg/kg, p.o.), MOR (5 mg/ kg, s.c.), or aspirin (200 mg/kg; p.o.). After 60 min, 20 µL of a 2.5% formalin solution (0.92% formaldehyde) in a phosphate buffer (pH 7.2) was injected into the dorsal surface of the left hindpaw using a microsyringe with a 26-gauge needle. The duration of paw licking was measured from 0 to 5 min (first phase) and 15 to 30 min (second phase) after formalin administration.
Hot-plate test
The hot-plate test was carried out as described by CitationEddy and Leimbach (1953). In this test, the reaction of mice to painful stimulus was measured. Mice were placed individually on a metal plate heated to 52 ± 0.5°C and covered with a glass cylinder (25 cm high, 15 cm in diameter). The time (in seconds) elapsing to the first pain response (licking of the forepaws or jumping) was determined by a stop-watch and then recorded. The experiments were conducted 60 min following the p.o. administration of MECs (100, 200, and 400 mg/kg). The effect of pretreatment with NAL (1.5 mg/kg, i.p.) on the antinociception produced by MECs (400 mg/kg) and morphine (5 mg/kg, s.c.) was determined.
Rota-rod test
Initially, mice able to remain on the Rota-rod apparatus (AVS®, Brazil) longer than 180 s (at 9 rpm) were selected 24 h before the test (CitationRosland et al., 1990). Then, the selected animals were divided into five groups (n = 8) and treated with vehicle (control, p.o.), MECs (100, 200, and 400 mg/kg, p.o.), and diazepam (3 mg/kg, i.p.). Sixty minutes later, each animal was tested on the Rota-rod and the time (in seconds) that they remained on the bar, for up to 180 s, was recorded 60 min after treatment.
Carrageenan-induced edema test
Acute hindpaw edema was produced by injecting 0.1 mL of carrageenan (1%, prepared as a suspension in distilled water plus Tween 80 at 0.2%) locally into the subplantar aponeurosis of the right hindpaw of rats (CitationWinter et al., 1962). Animals were divided into five groups, six rats per group. MECs was administered p.o. at different doses (100, 200, and 400 mg/kg) in the absence and presence of the standard drug indomethacin (INDO, 10 mg/kg, p.o.), and vehicle (p.o.) was given to a control group. MECs and INDO were administered 1 h prior to injection of carrageenan (CitationAmresh et al., 2007). Paw volume was measured by dislocation of the water column of a plethysmograph (LE 7500; PanLab, Spain) immediately after carrageenan application (time zero) and at 1, 2, 3, and 4 h after its administration.
Statistical analysis
All data are presented as mean ± standard error of the mean (SEM) and the differences between control and treated groups were evaluated by one-way analysis of variance (ANOVA) followed by Dunnett’s or Fisher’s test. In all cases differences were considered significant if p < 0.05.
The percentage of inhibition by an antinociceptive agent was determined for the acetic acid-induced writhing and formalin tests using the following formula (CitationReanmongkol et al., 1994): inhibition % = 100 × (control – experiment)/control. The percentage of inhibition of edema volume between treated and control groups was calculated using the following formula: inhibition % = 100 × (Vc – Vt)/Vc, where Vc and Vt represent the mean increases in paw volume in the control and treated groups, respectively.
Results
In control group mice, the number of writhes during the 15 min test period was 12.3 ± 1.5 (n = 8). The treatment of the animals with MECs (100, 200, and 400 mg/kg, p.o.) produced a significant and dose-dependent inhibition of the number of writhes when compared to the control (). The inhibition induced by 400 mg/kg MECs was similar to that produced by 200 mg/kg aspirin (55.3 and 68.3%, respectively).
Table 1. Effect of methanol extract of C. spicatus (MECs) or aspirin on writhing induced by acetic acid (n = 8).
In this work, MECs demonstrated analgesic effects on both the first (0–5 min) and second phases (15–30 min) of formalin-induced pain. These phases correspond to neurogenic and inflammatory pain, respectively. Neurogenic-induced pain was blocked only at 400 mg/kg (92.1%, p < 0.001), whereas all doses of MECs significantly blocked inflammatory pain. Aspirin (200 mg/kg, p.o.) was significantly active (58.6%, p < 0.001) in the second phase, i.e. inflammatory pain ().
Table 2. Effect of methanol extract of C. spicatus (MECs) or aspirin on formalin-induced pain (n = 8).
As demonstrated in , MECs administered at doses of 200 and 400 mg/kg caused a significant increase in hot-plate test response latency when compared to control group animals. Naloxone (1.5 mg/kg, i.p.) partially reversed the antinociceptive effect of the MECs.
Table 3. Effect of methanol extract of C. spicatus (MECs) or morphine (MOR) on the hot-plate test in the absence and presence of naloxone (NAL) in mice (n = 8).
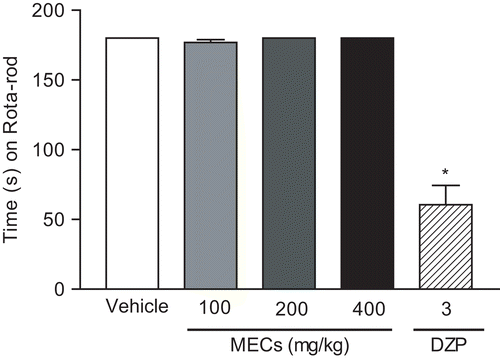
In the Rota-rod test, MECs-treated mice did not show any significant motor performance alterations with doses of 100, 200, or 400 mg/kg (). As might be expected, the central nervous system (CNS) depressant diazepam (3 mg/kg) reduced the time of treated animals on the Rota-rod apparatus.
Figure 1. Time (s) on the Rota-rod apparatus observed in mice after p.o. treatment with vehicle (control), MECs (100, 200 and 400 mg/kg), or diazepam (DZP, 3 mg/kg, i.p.). The motor response was recorded for 180 s following drug treatment. Statistical differences vs. control group were calculated using ANOVA, followed by Dunnett’s test (n = 8). *p < 0.01.
MECs presented a dose-dependent anti-inflammatory activity at all concentrations tested, with a significant difference at the concentration of 100 mg/kg, 4 h after carrageenan injection ().
Table 4. Effect of methanol extract of C. spicatus (MECs) or indomethacin (INDO) on carrageenan-induced hindpaw edema in rats (n = 6).
Discussion
In this study we evaluated the antinociceptive and anti-inflammatory effects of the methanol extract obtained from the leaves of Costus spicatus Swartz (MECs) using acetic acid-induced writhing, formalin, hot-plate, and carrageenan-induced edema tests in rodents.
MECs, at all doses, was able to inhibit acetic acid-induced writhing in mice; hence, it can be suggested that the analgesic effect of the extract is peripherally mediated (CitationMelo et al., 2008). This method is not only simple and reliable, but also affords rapid evaluation of peripheral type analgesic action. In acetic acid-induced abdominal writhing, pain is elicited by the injection of an irritant such as acetic acid into the peritoneal cavity, which produces episodes of characteristic stretching (writhing) movements, and the inhibition of the number of episodes by analgesics is easily quantifiable (CitationDuarte et al., 1988). Furthermore, these results support the hypothesis of MECs participation in the inhibition of prostaglandin synthesis, since the nociceptive mechanism of abdominal writhing induced by acetic acid involves the process of release of arachidonic acid metabolites via COX (cyclooxygenase) and prostaglandin biosynthesis (CitationBasbaum & Julius, 2001).
The results show that MECs, at the doses of 100, 200, and 400 mg/kg (p.o.), produced significant anti-nociception during the hot-plate and formalin tests. Substances and drugs that produce strong inhibitory effects in the hot-plate test can inhibit centrally induced pain and act as strong analgesics (CitationParkhouse & Pleuvry, 1979; CitationPrado et al., 1990). This central analgesic action was confirmed by the blocking effect of naloxone (CitationBelvisi et al., 1998). On the other hand, the formalin paw-licking test is a model of tonic pain and resembles human clinical pain conditions (CitationTjolsen et al., 1992). The observed activities of the extract in the hot-plate and formalin tests therefore suggest that MECs has strong analgesic activities. These findings support the use in folk medicine of the plant decoction for the treatment of headache.
Lack of motor coordination in the Rota-rod test is characteristic of drugs that reduce CNS activity, such as neuroleptics, anxiolytics, sedatives, and hypnotics (CitationSen & Chaudhuri, 1992). The animals treated with MECs did not present significant alterations in performance time on the Rota-rod apparatus, therefore not interfering with the motor coordination of the animals and/or discarding the muscular relaxant effect or even common neurotoxicity as some drugs with a depressant profile on the CNS.
The initial phase of carrageenan paw edema is mediated by histamine and serotonin, while the mediators in the later phase are suggested to be arachidonate metabolites (prostaglandins and leukotrienes) producing an edema dependent on the mobilization of neutrophils (CitationVinegar et al., 1987; CitationHwang et al., 1996). In our experiments, the edematous response was significantly suppressed in rats pretreated with MECs in the first phase of edema, suggesting an inhibitory effect on the release of histamine and/or serotonin. MECs showed a significant inhibition of edema in the second and third phases, suggesting inhibition of 5-lipoxygenase and/or cyclooxygenase, both enzymes involved in the formation of prostaglandins and leukotrienes. This edematous response was also significantly reduced in rats pretreated with indomethacin, a compound known to be a cyclooxygenase inhibitor.
The ability of the MECs, in this study, to suppress abdominal writhes, increase the latency of reaction time in the hot-plate test, and inhibit both phases of formalin-induced pain, as well as suppress carrageenan-induced inflammation, confirm the analgesic and anti-inflammatory activities of MECs.
Conclusion
Taken together, all data presented in this work lead to the conclusion that the methanol extract obtained from the leaves of C. spicatus possesses analgesic and anti-inflammatory properties, which are probably mediated via the inhibition of prostaglandin synthesis as well as central inhibitory mechanisms. Therefore, the extract will be of potential benefit in the management of pain and inflammatory disorders.
Acknowledgements
We thank Mr Osvaldo Andrade Santos for technical support. This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq, Fundação de Apoio à Pesquisa e à Inovação Tecnológica do Estado de Sergipe – FAPITEC-SE, CAPES, Ministério da Saúde, and Secretaria Estadual de Saúde – SES/SE, Brazil.
Declaration of interest
Grant Edital 06/2007, to one of the authors (Márcio R.V. Santos), Project Coordinator.
References
- Amresh G, Reddy GD, Rao CV, Singh PN (2007): Evaluation of anti-inflammatory activity of Cissampelos pareira root in rats. J Ethnopharmacol 110: 526–531.
- Barbosa-Filho JM, Medeiros KCP, Diniz MFFM, Batista LM, Athayde-Filho PF, Silva MS, Cunha EVL, Almeida JRGS, Quintans-Júnior LJ (2006): Natural products inhibitors of the enzyme acetylcholinesterase. Rev Bras Farmacogn 16: 258–285.
- Basbaum AI, Julius D (2001): Molecular mechanisms of nociception. Nature 413: 203–210.
- Belvisi MG, Chung DM, Barnes PJ (1998): Opioid modulation of non-cholinergic neural bronchoconstriction in guinea-pig in vivo. Br J Pharmacol 95: 413–418.
- Broadbear JH, Negus SS, Butelman ER, Costa BR, Woods JH (1994): Differential effects of systemically administered nor-binaltorphimine (nor-BNI) on κ-opioid agonists in mouse writhing assay. Psychopharmacology 15: 311–319.
- Calixto JB, Beirith A, Ferreira J, Santos AR, Cechinel Filho V, Yunes RA (2000): Naturally occurring antinociceptive substances from plants. Phytother Res 14: 401–418.
- Cruz GL (1965): Livro Verde das Plantas Medicinais e Industriais do Brasil. Belo Horizonte, Velloso AS, p. 46.
- Da Silva BP, Bernardo RR, Parente JP (2000): Flavonol glycosides from Costus spicatus. Phytochemistry 53: 87–92.
- Da Silva BP, Parente JP (2003): Bioactive polysaccharides from Costus spicatus. Carbohydr Polym 51: 239–242.
- De Souza MM, Pereira MA, Ardenghi JV, Mora TC, Bresciani LF, Yunes RA, Delle Monache F, Cechinel-Filho V (2009): Filicene obtained from Adiantum cuneatum interacts with the cholinergic, dopaminergic, glutamatergic, GABAergic, and tachykinergic systems to exert antinociceptive effect in mice. Pharmacol Biochem Behav 93: 40–46.
- Duarte IDG, Nakamura M, Ferreira SH (1988): Participation of the sympathetic system in acetic acid-induced writhing in mice. Braz J Med Biol Res 21: 341–343.
- Eddy NB, Leimbach D (1953): Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther 107: 385–393.
- Fang SH, Rao YK, Tzeng YM (2005): Inhibitory effects of flavonol glycosides from Cinnamomum osmophloeum on inflammatory mediators in LPS/IFN-gamma-activated murine macrophages. Bioorg Med Chem 13: 2381–2388.
- Gasparri S (2005): Estudo das atividades antioxidante e mutagênica/antimutagênica induzidas pelo extrato vegetal da Costus spicatus. Dissertação de Mestrado. Canoas, Universidade Luterana do Brasil.
- Ghannadi A, Hajhashemi V, Jafarabadi H (2005): An investigation of the analgesic and anti-inflammatory effects of Nigella sativa seed polyphenols. J Med Food 8: 488–493.
- Hwang S, Lam M, Li C, Shen T (1996): Release of platelet activating factor and its involvement in the first phase of carrageenin rat foot edema. Eur J Pharmacol 120: 33–41.
- Hunskaar S, Hole K (1987): The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain 30: 103–104.
- Koster R, Anderson M, Beer EJ (1959): Acetic acid for analgesic screening. Fed Proc 18: 412–416.
- Maleki-Dizaji N, Fathiazad F, Garjani A (2007): Antinociceptive properties of extracts and two flavonoids isolated from leaves of Danae racemosa. Arch Pharm Res 30: 1536–1542.
- Manfred L (1947): 7000 Recetas Botánicas a Base de 1300 Plantas Medicinales Americanas. Buenos Aires, Editorial Kier.
- Melo MGD, Araújo AAS, Rocha CPL, Almeida EMSA, Siqueira RS, Bonjardim LR, Quintans LJ Jr (2008): Purification, physicochemical properties, thermal analysis and antinociceptive effect of atranorin extracted from Cladina kalbii. Biol Pharm Bull 31: 1977–1980.
- Parkhouse J, Pleuvry BJ (1979): Analgesic Drugs. Oxford, Blackwell, pp. 1–5.
- Prado WA, Tonussi CR, Rego EM, Corrado AP (1990): Antinociception induced by intraperitoneal injection of gentamicin in rats and mice. Pain 41: 365–371.
- Reanmongkol W, Matsumoto K, Watanabe H, Subhadhirasakul S, Sakai SI (1994): Antinociceptive and antipyretic effects of alkaloids extracted from the stem bark of Hunteria zeylanica. Biol Pharm Bull 17: 1345–1350.
- Rosland JH, Tjolsen A, Maehle B, Hole K (1990): The formalin test in mice: effect of formalin concentration. Pain 42: 235–242.
- Sen T, Chaudhuri KN (1992): Studies on the neuropharmacological aspects of Pluchea indica root extract. Phytother Res 6: 175–179.
- Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K (1992): The formalin test: an evaluation of the method. Pain 51: 5–17.
- Vinegar R, Truax JF, Selph JL, Johnston PR, Venable AL, McKenzie KK (1987): Pathway to carrageenan-induced inflammation in the hind limb of the rat. Fed Proc 46: 118–126.
- Winter CA, Riseley EA, Nuss GW (1962): Carrageenan-induced edema in the hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med 111: 544–547.
- Zimmermann M (1983): Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16: 109–110.
