Abstract
Objective: The study evaluates, qualitatively and quantitatively, the volatile oil profiles of the aerial parts of Jordanian garland Chrysanthemum coronarium L. (Asteraceae) and compares the findings with literature reports of garland of other sources, in terms of general composition and content of potentially active components.
Materials and methods: The chemical composition of the essential oils isolated by hydrodistillation from dried material composed of flowerheads (FH) and aerial parts except for flowerheads (AEF) was assessed by GC-FID and GC-MS.
Results and discussion: More than 60 components were identified in the studied oils, corresponding to about 99.6 and 99.7% of total oil constituents of FH and AEF, respectively. The oil was characterized by substantial levels of monoterpenes (76.9% in FH and 61.9% in AEF) and moderate levels of sesquiterpenes (15.7% in FH and 27.7% in AEF). The oil from FH was characterized by high levels of oxygenated monoterpenes (64.3%, compared to 15.3% in AEF) and moderate levels of both monoterpene-hydrocarbons (12.6%, compared to 46.6% in AEF as the major fraction) and sesquiterpene-hydrocarbons (14.7%, compared to 23.5% in AEF), while very low levels of oxygenated sesquiterpenes were observed in both oils (1.0% in FH and 4.2% in AEF). The principal oil component was camphor (17.5%) in FH and myrcene (36.7%) in AEF. Other major components identified in the FH oil were santolina triene (4.3%), neoiso-3-thujanol (5.6%), cis-chrysanthenyl acetate (10.8%), perilla aldehyde (11.7%), iso-italicene (4.7%), phenylpropyl butanoate (4.9%), and germacrene D (4.3%), while Z-β-ocimene (5.2%), isobornyl acetate (5.2%), E-β-farnesene (12.1%), and germacrene D (4.5%) were the major constituents of AEF oil.
Introduction
The genus Chrysanthemum (Asteraceae) is represented in Jordan by two species, namely: C. coronarium L. and C. segetum L. However, only C. coronarium is collected and used by locals. C. coronarium is commonly known as crown daisy or garland in English, and as “Besbas” or “Gassoum” in Arabic (Jordan). The plant is an annual herb, 30–70 cm long, showy, with many hairless and leafy branches. The leaves are dissected into small segments. Heads are 4–6 cm in diameter, with yellow-orange ray and tube flowers. In Jordan, the plant grows on roadsides and fields of warmer areas of the country such as Jordan Valley and Irbid (CitationAl-Eisawi, 1998). The plant is of Mediterranean origin, from where it spread to Europe, Western Africa, and Asia (CitationLarkcom, 1991). C. coronarium is an ornamental plant whose green leaves and shoots are consumed and appreciated as a Chinese vegetable. Its nutritional composition has been described by CitationWillis et al. (1984). Many other uses of the plant have been reported in the literature. It is used as a fodder crop and as a fish odor suppressant in prepared foods in Japan, where the leaves and stems, which are rich in β-carotene, minerals, and vitamins, are usually heated before consumption (CitationKasahura & Nishibori, 1995; CitationSulas & Caredda, 1997). From the plant aerial parts, several researchers have isolated insect antifeedant compounds (CitationTada & Chiba, 1984) and insect antijuvenile hormones (CitationBowers & Aregullin, 1987). In Jordan the stems of the plant are edible, while the flowers are used as plasters for dermal diseases and as a vermifuge (CitationOran & Al-Eisawi, 1998). The essential oil obtained from the aerial parts, particularly the flowerheads (FH), showed insecticidal properties against stored product pests (CitationPerez & Pascual-Villalobos, 1999). CitationAlvarez-Castellanos et al. (2001) also reported the antifungal activity of the oil against 12 agricultural pathogens.
Phytochemically, many compounds have been isolated and identified from different parts of the plant, some of which were also evaluated biologically; examples include the antioxidant quinic acid derivatives (CitationChuda et al., 1996, Citation1998; CitationTakenaka et al., 2000), polyacetylenic compounds (CitationSanz et al., 1990), sesquiterpene lactones (CitationEl-Masry et al., 1984), lyratol esters (CitationBohlmann & Fritz, 1979), coumarins, steroids (CitationAhmed et al., 1999), and miscellaneous compounds such as quercetin, emodin, chrysaphanol, and chrysazin (CitationGins et al., 2000).
Several studies have reported the composition of the oil obtained from the FH of the plant. From the oil of FH of C. coronarium growing in Greece, 56 constituents were identified, of which oxygenated monoterpenes made the highest contribution to the total content (CitationBasta et al., 2007). The major compounds from two populations were camphor, trans-chrysanthenyl acetate, trans-chrysanthenyl isovalerate, and cis-chrysanthenyl acetate. A study from Chile (CitationSebastian et al., 2006) revealed the existence of a high content of oxygenated monoterpenes in one of the volatile fractions obtained from the flower heads of C. coronarium growing as an exotic plant in this country. The major identified components were camphor and trans-chrysanthenyl acetate. From two reports by CitationAlvarez-Castellanos et al. (2001) and CitationAlvarez-Castellanos and Pascual-Villalobos (2003), 12 monoterpenes (mainly camphor, lyratyl acetate, α- and β-pinenes) and three sesquiterpenes (mainly germacrene D) were identified in the oil obtained from a cultivar of C. coronarium from Murcia, Spain. Futhermore, in a study from Tuscany, Italy (CitationFlamini et al., 2003), the fragrances and essential oils from FH of a wild population of C. coronarium collected in Livorno were obtained by solid phase micro-extraction (SPME) and hydrodistillation, respectively. Analyses of these fractions showed the presence of 18 monoterpenes and five sesquiterpenes in the fragrance, and 16 monoterpenes and eight sesquiterpenes in the essential oils. Finally, the oils obtained from the aerial parts of C. coronarium growing wild in two different localities of Southern Italy (CitationSenatore et al., 2004) were analyzed, and revealed 68 and 43 constituents in both localities. trans-Tonghaosu, chrysanthenyl and lyratyl esters, and camphor were the main components of the latter oils.
In the present study, the essential oils hydrodistilled separately from the FH and from the aerial parts, except for FH, of C. coronarium growing wild in northern Jordan (Irbid) were fully analyzed by means of gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS) and compared. To our knowledge, this is the first report to document the analysis of the essential oil composition of Chrysanthemum spp. growing in Jordan and the neighboring countries south of the Mediterranean.
Materials and methods
Plant material: collection and identification
The whole aerial parts of wild growing C. coronarium were collected by the authors from Houfa, a village in the north of Jordan (Irbid governorate, Jordan) in mid-April 2008, during the flowering period. Two collections were performed on two consecutive days. The taxonomic identity of the plant species was confirmed by the assistance of a plant taxonomist (Dr. Mohammad Gharaibeh, Faculty of Agriculture, Jordan University of Science and Technology) and by the comparison of a collected voucher specimen with those of known identity in the herbarium of the Faculty of Agriculture, University of Jordan (UJ). A voucher specimen (ID: C.CorHud-04-08) of the collected plants was deposited in the research laboratory of the Department of Pharmaceutical Sciences, Faculty of Pharmacy, UJ.
Essential oil extraction
The aerial parts of the collected plant were further separated into two parts: aerial except for flowerheads (AEF) and flowerheads (FH), air-dried, and ground to about 0.5 mm particle size (30–35 mesh). To obtain the essential oil, about 500 g of the ground materials of each part, accurately weighed, was subject to hydrodistillation using a Clevenger-type apparatus (JSGW, India) for 4 h. The obtained oils were dried over anhydrous sodium sulfate, Na2SO4 (BDH Analar, England), and stored in dry, dark glass bottles at 4°C until analysis. The yield of oil was calculated as percentage volume per weight (% v/w) of the dried plant material. Of each oil sample, prepared as above, 5 μL was diluted up to 1 mL using GC-grade n-hexane (Scharlaue, Barcelona, Spain), and then 1 μL samples of the diluted oil were injected into GC-flame ionization detection (FID) and GC-MS systems for analysis. It should be noted that, for each plant part, the material collected on each day was separately extracted and analyzed.
Gas chromatography-flame ionization detection analysis
Quantitative analysis was done using a Hewlett-Packard HP-8590 gas chromatograph equipped with a split-splitless injector (split ratio 1:50) and an FID detector. The column was an Optima-5 (5% diphenyl, 95% dimethyl polysiloxane) fused silica capillary column (30 m × 0.25 mm, 0.25 film thickness). The oils were analyzed under linear temperature programming applied at 3°C/min from 60 to 250°C and held at 250°C for 5 min. The temperatures of the injector and detector were maintained at 250°C and 300°C, respectively. The oil hydrodistilled from each plant part was analyzed, and the relative peak area for each component of the oil was measured. Concentrations of oil components were calculated as percent content using their relative peak areas, assuming a unity response by all components. Each sample was analyzed twice.
Gas chromatography-mass spectrometry analysis
A Varian Chrompack CP-3800 GC/MS/MS-200 equipped with split-splitless injector and DB-5 GC column (5% diphenyl, 95% dimethyl polysiloxane) (30 m × 0.25 mm ID, 0.25 μm film thickness) was used. The injector temperature was set at 250°C with a split ratio of 1:10. Detector and transfer-line temperatures were 160°C and 230°C, respectively. A linear temperature program was used to separate the different oil components. Temperature programming was applied at 3°C/min heating rate starting from 60°C (initial temperature) to 250°C (final temperature) and held at 250°C for 5 min, with a total run time of 60 min. Each sample was analyzed twice. The mass detector was set to scan ions between 40 and 400 m/z using full scan mode and electron impact (EI, 70 eV). A hydrocarbon mixture of n-alkanes (C8–C20) was analyzed separately by GC-MS using the same column (DB-5) and under the same chromatographic conditions. The linear retention index (Kovat’s index) was calculated for each component (each peak) separated by GC-MS using the value of its retention time and the retention times of the reference n-alkanes, applying the Van den Dool equation (CitationVan den Dool & Kratz, 1963). Then, identification of the components of the oil was performed by matching their spectra with data bank mass spectra (Wiley, NIST, and Adams-2007 libraries) and also by comparing their linear retention indices (Kovat’s indices) with reported values in the literature, mainly the Adams library (CitationAdams, 2007). Identification of certain compounds (e.g. α-pinene, β-pinene, myrcene, δ-2-carene, limonene, 1,8-cineole, Z-β-ocimene, E-β-ocimene, linalool, camphor, borneol, isobornyl acetate, β-caryophyllene, germacrene D, and hexadecane) was further confirmed by co-chromatography of their authentic standards (purchased from Sigma-Aldrich) under the same chromatographic conditions as mentioned above.
Results and discussion
The simultaneous use of mass spectrum and retention (Kovat’s) index matching allowed for the unequivocal identification of more than 99% of the components of the oil, obtained from both parts of C. coronarium under study, as determined by the GC and GC-MS analyses. As shown in , the total number of identified compounds was 66 in the oil from FH and 61 in the oil from AEF, accounting for 99.6 and 99.7% of the total oil constituents from these parts, respectively. Moreover, and as expected, the oil yield was higher in FH (0.1% v/w of dried material) than in AEF (0.05%). Regarding oil composition, the oil obtained from Jordanian C. coronarium was notably rich in monoterpenoids (76.9% in FH and 61.9% in AEF), particularly oxygenated in FH (64.3%) and hydrocarbons in AEF (46.6%). A moderate level of sesquiterpenoids was observed in the oil from both parts (15.7% in FH and 27.7% in AEF), with hydrocarbons as the major fraction in both parts (14.7% in FH and 23.5% in AEF) and lower levels of oxygenated (1.0% in FH and 4.2% in AEF).
Table 1. Chemical composition of the essential oil hydrodistilled from the flowerheads (FH) and aerial parts except for flowerheads (AEF) of Chrysanthemum coronarium L. growing wild in Northern Jordan.
When comparing the composition profiles of the oils obtained from both plant parts, the observed differences were mainly quantitative and to a lesser extent qualitative. Here, in addition to the general quantitative differences in the main terpenoid classes as mentioned above, the differences were also notable in the principal component and other major components. In the oil from FH the principal component was camphor (17.5%), which is an oxygenated monoterpene (ketone), and other major components (>4.0%) were santolina triene (4.3%), neoiso-3-thujanol (5.6%), cis-chrysanthenyl acetate (10.8%), iso-italicene (4.7%), phenylpropyl butanoate (4.9%), and germacrene D (4.3%). In the AEF, the principal component was myrcene (36.7%), which is a hydrocarbon monoterpene, and the other major components (>4.0%) included Z-β-ocimene (5.2%), isobornyl acetate (5.2%), E-β-farnesene (12.1%), and germacrene D (4.5%). Moreover, one of the main quantitative variations observed between both parts was in the levels of the principal and some of the major components, as shown in and . More notable, however, were the differences in myrcene, camphor, cis-chrysanthenyl acetate, and perilla aldehyde. On the other hand, qualitative differences included mainly those components detected only in one part and not in the other (). Examples included tricyclene, dimethoxy-Z-citral, and β-sesquiphellandrene, which were only detected in FH oil, and α-zingiberene, caryophyllene oxide, longiborneol, E-eugenol acetate, and E-nerolidyl isobutyrate, which were observed only in AEF oil.
Figure 1. Differences in percent contents of principal and major components identified in oils hydrodistilled from flowerheads (FH) and aerial parts except for flowerheads (AEF) of Chrysanthemum coronarium L. growing wild in Jordan.
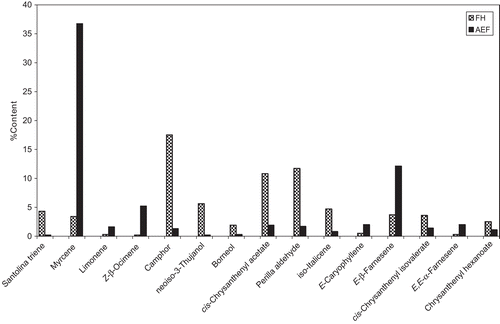
The oil obtained from the FH of C. coronarium has been previously studied in some Mediterranean countries including Italy, Spain, and Greece. Compared to the Jordanian plant oil, some qualitative and quantitative differences were evident. Analysis of the oil hydrodistilled from the FH of the plant growing wild in Southern Italy (CitationSenatore et al., 2004) revealed the identification of 68 and 43 constituents in the oils of plants growing in Lascari and Palinuro, respectively. The major identified components were the trans-spiroketal-enol ether: 2-(2,4-hexadiynylidene)-1,6-dioxaspiro[4,4]non-3-ene (17.8–12.4%), known as trans-tonghaosu, chrysanthenyl acetates (trans-: 6.7–5.5% and cis-: 5.4–14.5%), lyratyl propanoates (Z-: 0.2–6.6% and E-: 0.7–3.6%), and camphor (5.8–7.5%). In the present study all these latter compounds were detected, although in relatively different amounts ( and ), except for lyratyl esters, which were not detected in the present study.
In another study from Italy (CitationFlamini et al., 2003), both the oil hydrodistilled from the FH and the fragrances obtained from different parts of C. coronarium growing in the Tuscany region were studied. The major components of the oil were camphor (22.1%), which was the principal, cis-chrysanthenyl acetate (19.9%), santolina triene (9.3%), germacrene D (8.5%), (E)-β-farnesene (6.4%), and myrcene (5.0%). Analysis of the headspace-obtained fragrances of the different plant parts (pollen, whole FH, ligulate and tubular florets, flower buds, involucral bracts and leaves) of the above population showed different profiles between these parts and generally revealed, as major components, camphor and cis-chrysanthenyl acetate, which were found mainly in ligulate (38.1 and 8.8%, respectively) and tubular (34.4 and 15.9%, respectively) florets. All these components, detected in the oil of FH, were also identified in the Jordanian garland oil obtained from the same parts, with almost comparable levels, particularly for camphor and cis-chrysanthenyl acetate. However, perilla aldehyde, a component identified in an appreciable amount (11.7%) in Jordanian oil, was only detected in the fragrances of most parts but not in the oils of FH. In a study from Spain (CitationAlvarez-Castellanos et al., 2001), the FH oil obtained from C. coronarium plants collected from an experimental plot in Murcia showed camphor (29.2%), α- and β-pinene (14.8 and 9.5%, respectively), and lyratyl acetate (9.8%) as the major components. Moreover, another study on plants cultivated in the same region (CitationAlvarez-Castellanos & Pascual-Villalobos, 2003) showed camphor (17.66, 20.45%), lyratyl acetate (10.67, 10.47%), and α-pinene (6.44, 8.27%) as the major components of the FH oil obtained from fertilized and un-fertilized populations, respectively. On the other hand, in the oils of the FH of C. coronarium collected from two different locations, Diminio and Porto Rafti, in Greece (CitationBasta et al., 2007), 56 constituents were identified, of which oxygenated monoterpenes made the highest contribution. The major constituents in the oil from Diminio plants were trans-chrysanthenyl acetate (13.2%), trans-chrysanthenyl isovalerate (10.2%), and cis-chrysanthenyl acetate (9.9%), while those of Porto Rafti plants were camphor (15.7%), cis-chrysanthenyl acetate (9.1%), and trans-chrysanthenyl acetate (7.8%). Finally, a study from Chile (CitationSebastian et al., 2006) revealed the existence of a high content of oxygenated monoterpenes in one of the volatile fractions obtained from the FH of C. coronarium growing as an exotic plant in this country. The major identified components were camphor (25.1%), trans-chrysanthenyl acetate (25.9%), and bornyl acetate (13.8%).
More or less, the findings of these previous studies concerning C. coronarium oil do not differ markedly from the oil under study, except in the relative quantities of the principal and major components as mentioned above. However, the characteristic levels of the phenolic fraction (4.9% in FH and 3.4% in AEF) in the Jordanian garland oil, particularly phenylpropyl butanoate, would be distinctive to the present oil, where the level of myrcene in AEF oil (36.7%) was the highest reported of all. Moreover, lyratyl acetates, reported to occur in some of the oils discussed above, were completely absent in the Jordanian garland oil. In conclusion, it is obvious that the oils obtained from both plant parts are different in their composition. Particularly, the oil obtained from the FH was found to contain higher quantities of known active constituents such as phenolic and oxygenated compounds (), (CitationBruneton, 1999; CitationEvans, 2002; CitationSenatore et al., 2004). These latter considerations are so important when collecting the oil for nutritional, pharmaceutical, and/or medical purposes.
Acknowledgements
The authors would like to thank Mr. Ismaeel Abaza, Faculty of Pharmacy at UJ, for his help in plant preparation and oil distillation, and his technical assistance in GC-MS operation.
Declaration of interest
The authors report no conflict of interest.
References
- Adams RP (2007): Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. Carol Stream, IL, Allured Publishing Corporation.
- Ahmed KM, Khattab AM, El-Khrisy EAM, Abdel-Amid AZ (1999): Constituents and molluscicidal activity of the aerial parts of Acacia saligna, Chrysanthemum coronarium and Chrysanthemum parthenium. Bull Natl Res Centre (Egypt) 24: 13–25.
- Al-Eisawi DM (1998): Field Guide to Wild Flowers of Jordan and Neighbouring Countries. Amman, Jordan, Jordan Press Foundation Al Rai.
- Alvarez-Castellanos PP, Bishop CD, Pascual-Villalobos MJ (2001): Antifungal activity of the essential oil of flowerheads of garland chrysanthemum (Chrysanthemum coronarium) against agricultural pathogens. Phytochemistry 57: 99–102.
- Alvarez-Castellanos PP, Pascual-Villalobos MJ (2003): Effect of fertilizer on yield and composition of flowerhead essential oil of Chrysanthemum coronarium (Asteraceae) cultivated in Spain. Ind Crops Prod 17: 77–81.
- Basta A, Pavlovi M, Couladis M, Tzakou O (2007): Essential oil composition of the flowerheads of Chrysanthemum coronarium L. from Greece. Flavour Fragr J 22: 197–200.
- Bohlmann F, Fritz U (1979): Naturally occurring terpene derivatives. 230. New lyratol esters from Chrysanthemum coranarium. Phytochemistry 18: 1888–1889.
- Bowers W, Aregullin M (1987): Discovery of an antijuvenile hormone from Chrysanthemum coronarium. Mem Inst Oswaldo Cruz (Rio de Janeiro) 82(III): 51–54.
- Bruneton J (1999): Pharmacognosy, Phytochemistry, Medicinal Plants. Paris, Technique & Documentation.
- Chuda Y, Ono H, Ohnishi-Kameyama M, Nagata T, Tsushida T (1996): Structural identification of two antioxidant quinic acid derivatives from garland (Chrysanthemum coronarium L.). J Agric Food Chem 44: 2037–2039.
- Chuda Y, Suzuki M, Nagata T, Tsushida T (1998): Contents and cooking loss of three quinic acid derivatives from garland (Chrysanthemum coronarium L.). J Agric Food Chem 46: 1437–1439.
- El-Masry S, Abou-Dania AHA, Darwish FA, Abou-Karam MA, Grenz M, Bohlmann F (1984): Sesquiterpene lactones from Chrysanthemum coronarium. Phytochemistry 23: 2953–2954.
- Evans WC (2002): Trease and Evans Pharmacognosy. London, Saunders.
- Flamini G, Cioni PL, Morelli I (2003): Differences in the fragrances of pollen, leaves, and floral parts of garland (Chrysanthemum coronarium) and composition of the essential oils from flowerheads and leaves. J Agric Food Chem 51: 2267–2270.
- Gins VK, Kolesnikov MP, Kononkov PF, Trishin ME, Gins MS (2000): Hydroxyanthroquinones and flavonoids of garland chrysanthemum. Prikl Biokhim Mikrobiol 36: 344–353.
- Kasahura K, Nishibori K (1995): The suppressing effect of garland chrysanthemum on the odor of niboshi soup stock. Fish Sci 61: 672–674.
- Larkcom J (1991): Oriental Vegetables. London, John Murray.
- Oran SA, Al-Eisawi DM (1998): Check-list of medicinal plants in Jordan. Dirasat Med Biol Sci 25: 84–112.
- Perez MP, Pascual-Villalobos MJ (1999): Efectos del aceite esencial de inflorescencias de Chrysanthemum coronarium L. en mosca blanca y plagas de almacen. Investig Agraria Prod Prot Vegetal 14: 249–258.
- Sanz JF, Falco E, Marco JA (1990): New acetylenes from Chrysanthemum coronarium L. Liebigs Ann Chem 3: 303–305.
- Sebastian B, Urzua AM, Vines M (2006): Analysis of surface and volatile compounds of flower heads of introduced plants of Chrysanthemum coronarium L. growing wild in Chile. Flavour Fragr J 21: 783–785.
- Senatore F, Rigano D, De Fusco R, Bruno M (2004): Composition of the essential oil from flowerheads of Chrysanthemum coronarium L. (Asteraceae) growing wild in Southern Italy. Flavour Fragr J 19: 149–152.
- Sulas L, Caredda S (1997): Introduzione in coltura di nuove speci foraggere: produttività e composizione bromatologica di Chrysanthemum coronarium L. sottoposto a pascolamento simulato. Riv Agron 4: 1021–1028.
- Tada M, Chiba K (1984): Novel plant growth inhibitors and insect antifeedants from Chrysanthemum coronarium (Japanese name: shungiku). Agric Biol Chem 48: 1367–1369.
- Takenaka M, Nagata T, Yoshida M (2000): Stability and bioavailability of antioxidants in garland (Chrysanthemum coronarium L.). Biosci Biotechnol Biochem 64: 2689–2691.
- Van den Dool H, Kratz PD (1963): A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J Chromatogr 11: 463–471.
- Willis RBH, Womg AWK, Scriben FM, Grenfield H (1984): Nutrient composition of Chinese vegetables. J Agric Food Chem 32: 413–416.
