Abstract
Context: The leaf of sage Salvia officinalis L. (Lamiaceae) is reputed in the folk medicine of Arabia, and Jordan in particular, to relieve pain associated with gastrointestinal disturbance.
Objectives: Evaluation of the antinociceptive and anti-inflammatory activities of aqueous and butanol extracts of S. officinalis leaf.
Materials and methods: The analgesic effects of the aqueous extract (10, 31.6, 100, 316, 1000 mg/kg) and butanol extract (10, 31.6, 100, 316 mg/kg) were studied using the hot-plate test for mice and the formalin-induced paw licking in rats. The effects were compared to those of morphine and the influence of naloxone on these effects was also evaluated. The same concentrations of both extracts were used to evaluate their anti-inflammatory effects using the cotton pellet granuloma and carrageenan-induced paw edema in rats.
Results: The aqueous extract (10, 31.6, 100, 316, 1000 mg/kg) and butanol extract (10, 31.6, 100, 316 mg/kg) caused analgesic effect in the hot-plate latency assay as well as in early and late phases of formalin-induced paw licking in rats. These effects were reduced by the opioid receptor antagonist, naloxone (5 mg/kg). The same range of doses of both extracts caused dose-dependent inhibition of carrageenan-induced paw edema in rats as well as inhibition of cotton pellet granuloma.
Discussion and conclusion: These observations suggest that the sage leaf aqueous and butanol extracts have analgesic and anti-inflammatory effects, confirming the traditional use of this plant for pain alleviation.
Introduction
The sage Salvia officinalis L. (Lamiaceae), locally called meramiya, is a perennial sub-shrub native to the Mediterranean area but is known all over the world. The plant grows in various locations in Jordan and is used locally in folk medicine and in cosmetics. S. officinalis has been an important medicinal plant since earliest times (CitationPerry et al., 1999). This plant species is very rich in biologically active compounds and many studies have indicated its increasing medicinal importance; it is used for the treatment of various ailments, including the relief of menstrual problems such as cramp, as well as regulation of the menstrual cycle in amenorrhea due to its estrogenic substances (CitationDe Leo et al., 1998).
Experimental evidence already exists for a variety of bioactivities of different types of S. officinalis extracts. The extracts exhibited anti-inflammatory (CitationBaricevic et al., 2001), antioxidant (CitationFarag et al., 1989; CitationCuvelier et al., 1994; CitationWang et al., 1998; CitationSantos-Gomes et al., 2002), antibacterial, fungistatic (CitationBaricevic & Bartol, 2000), hypoglycemic (CitationAlarcon-Aguilar et al., 2002) and antimutagenic activities (CitationFilipic & Baricevic, 1997), as well as protective affects against lipid peroxidation (CitationHohmann et al., 1999; CitationZupko et al., 2001). Modern day clinical trials have shown that sage essential oil can improve the memory and has shown promise in the treatment of Alzheimer’s disease (CitationPerry et al., 1999; CitationSantos-Neto et al., 2006). The leaf extract also inhibits the pancreatic lipase activity and suppresses serum triglyceride (CitationNinomiya et al., 2004).
A bibliographic survey showed that much of the phytochemical investigations focused on the essential oil of this species. These studies resulted in the isolation and identification of about 40 compounds, with the principal components being 1,8-cineole, camphor, α-thujone, β-thujone, borneol, and viridiflorol (CitationRaal et al., 2007). Other components were also isolated, including luteolin-7-glucoside, rosmarinic acid, apianane terpenoids, salvin, carnosic acid, apigenin, luteolin, and luteolin glucuronides, vicenin-2, carnosol, and other phenolic glycosides (CitationLu & Yeap Foo, 2000; CitationDordevic et al., 2000; CitationMiura et al., 2001; CitationReuter et al., 2007). Some of these components have proven biological activities (CitationAizenman et al., 1982; CitationRau et al., 2006; CitationTalhouk et al., 2007; CitationHoriuchi et al., 2007).
There are suggestions in the literature that S. officinalis may reduce pain and have anti-inflammatory properties. Thus, in a randomized, double blind study on patients with pharyngitis, it was found that a 15% sage spray provided safe and convenient treatment for acute pharyngitis by reducing the throat pain intensity score (CitationHubbert et al., 2006). S. officinalis has also been reported to have antispasmodic effect, thus easing the tension in smooth muscles especially those of the gastrointestinal tract (CitationAbu-Irmaileh & Afifi; 2003). Whether this effect is accompanied by an analgesic effect or only limited to the relaxant effect of the sage extract is not known. A reduction in menstrual cramps has also been reported (CitationDe Leo et al., 1998). The present work was carried out to evaluate the potential antinociceptive and anti-inflammatory role of the leaf aqueous and butanol extracts of S. officinalis, and to investigate the scientific basis for the traditional use of this plant.
Materials and methods
Plant material
Fresh leaves of wild-growing Salvia officinalis were collected during April from Al-Fesaliya (Jordan) by one of us (EYQ). The plant material was identified and authenticated taxonomically at the Hashemite University herbarium. A voucher specimen was deposited under the number HU-153 at the Hashemite University herbarium, Zarka, Jordan, for future reference.
Preparation of aqueous extract
Aqueous extract was obtained by boiling 150 g of the ground, air dried leaf of S. officinalis in 1000 mL distilled water for 60 min with continuous stirring. The resultant solution was filtered through a Whatman filter paper. The filtrate was completely evaporated under reduced pressure at 50°C to give 7 g of gummy material. Solutions were prepared by dissolving weighed amounts of this material in physiological salt solution (PSS). PSS was prepared daily and had the following composition (mM): 118 NaCl; 4.7 KCl; 25 NaHCO3; 1 NaH2PO4 H2O; 0.5 Na2HPO4; 11.1 glucose; 0.5 MgCl2 6H2O and 2.5 CaCl2 2H2O. The pH of stock solution was adjusted to 7.4.
Preparation of the butanol extract
Powdered plant material (350 g) was soaked in 1000 mL of petroleum ether (60°–80°C) for 10 days at room temperature. The plant material was separated by filtration using cheesecloth. The plant material was extracted with 95% ethanol repeatedly (2 times; 10 days × 1 L each) at room temperature. Ethanol was evaporated under reduced pressure and the resulting crude extract (22 g) was mixed with water and chloroform (1:1; v/v). The aqueous layer was separated and extracted with butanol. The butanol layer was obtained and butanol was evaporated under reduced pressure to give 6 g of gummy material.
Phytochemical analysis
The aqueous and butanol extracts were subjected to preliminary screening for tannins, alkaloids, triterpenes and flavonoids using standard procedures (CitationTrease & Evans, 2001). The presence of these constituents was confirmed by thin layer chromatography.
Experimental animals
Non-fasting male Wistar rats (180–300 g), or mice (25–30 g), housed at 22°–25°C under a 12 h light/dark cycle and with access to food and water ad libitum, were used throughout the experiments. The experiments were carried out in accordance with the current guidelines for the care of laboratory animals at the Hashemite University.
Hot-plate test
The hot-plate test was assessed using groups of male mice, six animals per group. The temperature of the hot-plate was maintained at 50° ± 1°C. Latency to a discomfort reaction (licking paws) was determined in seconds before and 60 min after intraperitoneal administration of 10 mL/kg PSS (control), aqueous extract (10, 31.6, 100, 316, 1000 mg/kg), butanol extract (10, 31.6, 100, 316 mg/kg) or morphine (5 mg/kg; positive control). The largest doses were determined on the basis of preliminary LD50 experiments (for the aqueous extract) or on the basis of practical solubility (for butanol extract). Smaller doses were calculated to be located at approximately 0.5 log units from each other on a log scale. The cut-off time was 60 s. The prolongation of the latency times was compared to the values of the control and used for statistical comparison. Baseline was considered as the mean of three readings of the reaction time obtained before administration of aqueous extract, butanol extract or morphine and was defined as the normal reaction time of animals to this temperature. The increase over baseline (in %) was calculated by the formula: (A-B/B) × 100, where A is the mean of three readings of reaction time after treatment taken within 5-7 min; B is the mean of three readings of reaction time obtained before treatment. In this, and the following experiments, ED50 was determined from the plot of individual experiments by the best visual fit.
Formalin test
Rats were divided into groups (six rats each) and were injected intraperitoneally with either the physiological salt solution (PSS; control), five different concentrations of the aqueous extract of S. officinalis (10, 31.6, 100, 316, and 1000 mg/kg), four different concentrations of the butanol extract (10, 31.6, 100, and 316 mg/kg) or with 5 mg/kg morphine (positive control) respectively. Sixty min later, each rat received 50 µL of formalin (5%) subcutaneously (CitationTaylor et al., 2000) into the dorsal surface of the right hind paw using a microsyringe with a 27 gauge needle. Immediately after formalin injection, animals were placed individually in acrylic observation chambers (320 cm2 × 40 cm). Mirrors were arranged at angles to allow clear observation of the paws of the animals. Licking of the injected paw was defined as the nociceptive response. The total time of the response was measured during the periods of 0–5 min (early phase) and 15–40 min (late phase).
Carrageenan-induced paw edema
Edema was induced by injecting groups of rats with 0.1 mL of 1% (w/v) carrageenan suspension into the subplantar region of the right hind paw according to the method described by CitationHalici et al. (2007). The test groups of the rats were treated intraperitoneally with PSS (control), the aqueous extract (10, 31.6, 100, 316, 1000 mg/kg), butanol extract (10, 31.6, 100, 316 mg/kg), or 10 mg/kg indomethacin (positive control) 1 h before carrageenan injection. Measurement of paw size was carried out using a plethysmometer (Ugo Basile, Model 7140, Italy). Measurement was done immediately before and 1, 2, 3 and 5 h following carrageenan injection. Inflammation (%) was calculated as (paw final volume-initial volume/initial volume) × 100.
Cotton pellet granuloma in rats
Rats were divided into groups, six animals per group. The groups were treated orally daily for four days with either PSS (control), 10 mg/kg indomethacin (positive control), aqueous extract of S. officinalis (10, 31.6, 100, 316, and 1000 mg/kg), or butanol extract (10, 31.6, 100, and 316 mg/kg). Sterilized cotton pellets weighing 30 mg were introduced subcutaneously into the groin region of the rats according to the method of CitationMossa et al. (1995). The animals were sacrificed on the fifth day with an overdose of ether. The pellets surrounded by granuloma tissue were dissected out carefully and dried in an oven at 60°C to a constant weight. Mean weight of the granuloma tissue formed in each group was obtained and the percentage of inhibition caused by the different treatments was expressed by comparison with the control group.
Evaluation of the mechanism of action of S. officinalis leaf extracts
To evaluate the mechanism of action of Salvia officinalis extracts animals were pre-treated with the opioid antagonist naloxone (5 mg/kg). Naloxone was administered i.p. 15 min before administration of PSS, the ED50 of Salvia aqueous extract and butanol extract, or morphine (5 mg/kg). Using the hot-plate test and the formalin test as described above, licking was calculated after 60 min of extracts or morphine administration.
Statistical analysis
The values were expressed as the mean ± SEM. Data were analyzed by one-way analysis of variance (ANOVA) followed by Duncan’s test for multiple comparisons. Differences were considered significant when P <0.05.
Results
Antinociceptive activity
Hot-plate test
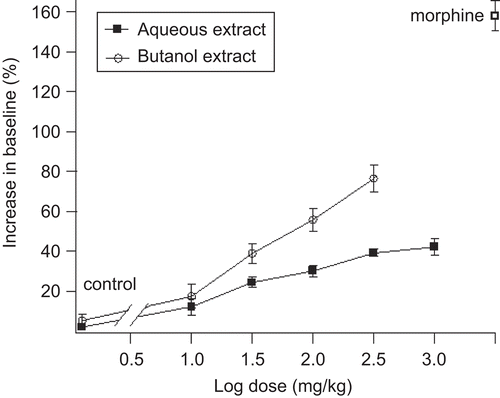
shows that the S. officinalis leaf aqueous (10, 31.6, 100, 316, 1000 mg/kg) and butanol (10, 31.6, 100, 316 mg/kg) extracts increased, in a dose-dependent manner, the latency time (expressed as percentage increase in baseline) taken by the animals to start licking their hind paw when subjected to the hot-plate. ED50 values for aqueous and butanol extracts were 28.2 ± 1.9 mg/kg and 31.5 ± 2.6 mg/kg, respectively.
Formalin test
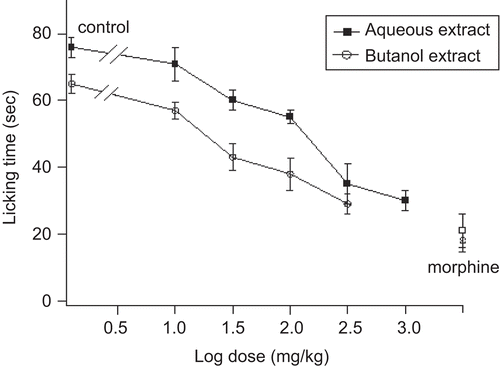
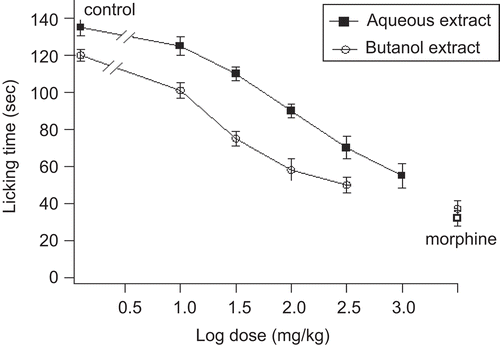
S. officinalis leaf aqueous and butanol extracts decreased the licking time in both early and late phases after formalin injection ( and ). The decrease in licking time in both phases was dose-dependent. For the early phase, the ED50 values for aqueous and butanol extracts were 28.5 ± 3.4 and 20.2 ± 2.5 mg/kg, respectively, whereas for the late phase the ED50 values for both extracts were 100 ± 4.5 and 26.5 ± 3 mg/kg, respectively.
Effects of naloxone
In an attempt to elucidate the mechanism by which S. officinalis leaf aqueous and butanol extracts induced antinociceptive effect, animals were pre-treated with the opioid antagonist, naloxone. shows that 5 mg/kg naloxone had insignificant effect on the control animals that received PSS, but reduced the antinociceptive activity of S. officinalis leaf extracts, although it did not block it totally. The doses of S. officinalis extracts used were the ones that used to cause ED50. The effect was more pronounced on the formalin test than the hot-plate test.
Table 1. Effect of naloxone on S. officinalis leaf extracts- or morphine- induced antinociceptive activitya.
Anti-inflammatory activity
Carrageenan-induced paw edema
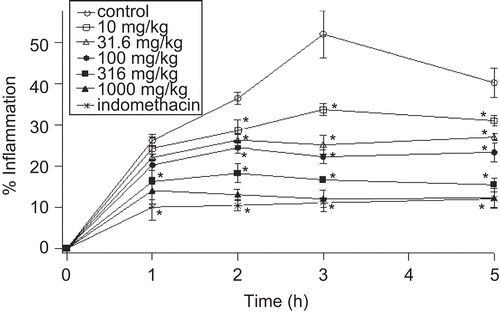
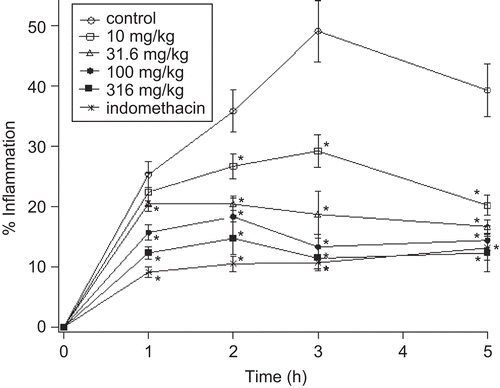
Preliminary experiments showed that the degree of swelling of the carrageenan-injected paws was maximal at 3 h after injection of carrageenan. and show the effects of S. officinalis leaf aqueous extract, butanol extract and indomethacin on the carrageenan-induced edema test. Statistical analysis shows that edema inhibition by leaf extracts was dose-dependent and was significantly different from the control group. The results showed that the aqueous and butanol extracts at the doses of 1000 and 316 mg/kg, respectively, had comparable anti-inflammatory effects to those produced by 10 mg/kg of indomethacin.
Cotton pellet granuloma
Intraperitoneal doses of S. officinalis leaf aqueous and butanol extracts caused a dose-dependent reduction in the granuloma tissue formation (). The ED50 values for aqueous and butanol extracts were 90 ± 5 mg/kg and 29 ± 4 mg/kg, respectively.
Table 2. Effects of the aqueous and butanol extracts of S. officinalis leaf on cotton pellet granuloma in ratsa.
Discussion
The present study is an attempt to establish a scientific basis for the folkloric ethnomedical uses of medicinal plants and an effort in the search for alternative medicines. The plant S. officinalis is commonly used in the Mediterranean region and in many cases it is added as a herb to different foods and drinks. The plant, therefore, is apparently safe; using the arithmetic method of Karbar (CitationSaidu et al., 2007), preliminary evaluation of its LD50 values in our laboratory for mice showed that LD50 was >1000 mg for both the aqueous and the butanol extracts since no deaths were observed at such doses (unpublished observations).
The present experiments demonstrate that the aqueous and butanol extracts of S. officinalis have both antinociceptive as well as anti-inflammatory effects in mice and rats. The hot-plate test is a useful model to evaluate the centrally acting analgesic drugs (CitationFranca et al., 2001). Experimental evidence shows that both extracts delayed, in a dose-dependent manner, the reaction times of mice subjected to the hot-plate test. This is demonstrated as an increase in the baseline of latency to show nociception in animals treated with either extract when compared to control animals treated with PSS. The same test also delayed the reaction time when morphine was used in a dose that is known to have potent analgesic effect (CitationAsongalem et al., 2004). The efficacy for either extract, however, was much lower than morphine notwithstanding the much higher concentrations of extracts used (1000 and 316 mg for aqueous and butanol extracts, respectively). These observations suggest that both extracts inhibit centrally located pain receptors.
The aqueous and butanol extracts of S. officinalis also reduced the licking time in response to pain induced by formalin during the first and second phases of the formalin test. The formalin test was chosen to evaluate the potential analgesic effects of extracts because of its advantages over other models of pain (CitationMcNamara et al., 2007). Injection of formalin into the hind paw induces a biphasic response; the first phase results from direct activation of primary afferent neurons (C-fibers) and lasts up to 10 min after injection, whereas the second phase reflects both afferent inputs and central sensitization in the dorsal horn and lasts 30-60 min after a transient decline in pain behavior (CitationTjolsen et al., 1992; CitationLee & Jeong, 2002; CitationMcNamara et al., 2007). This latter phase involves stimulation of nociceptors by inflammatory mediators and/or by first phase-induced spinal cord hyperexcitability (CitationTaylor et al., 2000).
The mechanism by which formalin triggers C-fibers activation remained unknown for a relatively long time. Recently, however, CitationMcNamara et al. (2007) demonstrated that formalin activates primary afferent neurons through a specific and direct action on TRPA1, a member of the transient receptor potential family of cation channels, expressed by a subset of C-fiber nociceptors, and this effect is accompanied by increased influx of Ca2+ ions. TRPA1 cation channels at primary sensory terminals were also reported to mediate noxious mechanical stimuli (CitationKerstein et al., 2009).These experiments suggest that Ca2+ mobilization through TRPA1 cation channels is concomitant with noxious chemical (i.e. formalin) and mechanical stimuli as they produce their algesic action. On the other hand, morphine as an opioid agonist, as well as cannabinoids CB1 receptor agonists, have antihyperalgesic effects which were accompanied by inhibition of the increase in intracellular Ca2+ concentration evoked by depolarization (CitationKhasabova et al., 2004). If this is the case it is likely that the observed inhibitory effects of S. officinalis extract on the responses to pain are due to similar effects, thus inhibiting the increase in intracellular Ca2+ through TRPA1, presumably evoked by formalin. The leaf extracts of S. officinalis may contain substances that affect the metabolism of Ca2+. Preliminary phytochemical analysis of aqueous and butanol extracts showed the presence of tannins, triterpenes, alkaloids and flavonoids (data not shown). Flavonoids, for example, have been found to suppress the intracellular Ca2+ ion elevation in a dose-dependent manner, as well as the release of proinflammatory mediators such as TNFα (CitationKempuraj et al., 2005).
In the present experiments, the highest concentrations used of both aqueous and butanol extracts reduced the measured pain behavioral parameter in both phase 1 and phase 2 to a degree that was almost similar to that caused by morphine, although the concentrations used were 60-200 times higher than that of morphine. Morphine has been shown to produce dose-dependent inhibition of both phases in the formalin test with ID50 values of 0.66 and 4.1 nmol, respectively (CitationPrzesmycki et al., 1997).
The second phase of formalin hyperalgesia has an inflammatory component since it has been shown that IL-1β and TNF-α are involved in this second phase and that non-selective COX inhibitors are effective in reducing the pain in the formalin test (CitationGranados-Soto et al., 2001). Since the aqueous and butanol extracts of S. officinalis inhibited phase 2 in the formalin test, it is likely that they exert their antinociceptive effects by inhibiting the synthesis or production of inflammatory cytokines and mediators such as prostaglandins, histamine and kinins. This suggestion is supported by the observation that aqueous and butanol extracts of S. officinalis cause dose-dependent inhibition of carrageenan-induced edema.
Inflammation is characterized by the production of a whole host of chemical mediators including prostaglandins, leukotrienes, histamine, bradykinins, platelet-activating factor, cytokines such as IL-1 and TNF- α, and release of chemicals from tissues and migrating cells, as well as reactive oxygen species (CitationCuzzocrea et al., 1999; CitationBeloeil et al., 2005). Carrageenan-induced inflammation in the paw has been reported to cause the production of histamine and cytokines such as TNF-α which have the capacity to activate neutrophils for the various functions (CitationMaruyama et al., 2006) and to cause significant induction of COX-2 and PGE2 (CitationNantel et al., 1999). The observation that aqueous and butanol extracts reduced, in a dose-dependent manner, the carrageenan-induced paw edema suggests that these extracts may inhibit the synthesis or the production of inflammatory mediators. Although significant, this reduction, however, has not reached the same efficacy as indomethacin, the non-selective inhibitor of COX used as a reference drug, even though the doses of extracts were much higher than that of indomethacin used in the present experiment.
The aqueous and butanol extracts of S. officinalis also significantly reduced the mass of the cotton-pellet granuloma. The highest concentrations of extracts reduced the mass to values that are comparable to those achieved with the known anti-inflammatory drug indomethacin. This observation suggests that S. officinalis extracts have inhibitory effects on subacute inflammation (CitationNandal et al., 2009), and further suggests that the extracts’ inhibitory action is not peculiar to edema only but to inflammation in general (CitationTheobald et al., 1983). This observation suggests that the extracts may have the capability to reduce the synthesis of mucopolysaccharides, collagen and the number of fibroblasts, the natural proliferative events in the granulation in tissue formation.
The observation that the anti-inflammatory activity of the aqueous and butanol extracts of S. officinalis was reduced by pretreatment with naloxone, the antagonist of opioid receptors, in a relatively large dose (CitationGerrits et al., 1995; CitationAmaral et al., 2007) suggests that the action of these extracts was only partially mediated by the opioid receptors, and therefore the remaining fraction of this activity may be mediated by other mechanisms. On the one hand it has been shown that the cannabinoid system modulates pain in various types of pain models, since CB2 receptor agonists produce antinociceptive effects in models of inflammatory and nociceptive pain (CitationHohmann & Suplita, 2006; CitationAnand et al., 2009). These findings suggest that S. officinalis extracts may stimulate the release of endocannabinoids, affect the cannabinoids degrading enzymes or may contain exogenous cannabinoid-like substances that activate cannabinoid receptors. Furthermore, this potential antinociceptive effect of cannabinoids may involve activation of the opioid system (CitationIbrahim et al., 2005) as it has been shown that CB receptor agonists enhance the effect of μ-opioid receptor agonists in a variety of models of analgesia (CitationCox et al., 2007). On the other hand, some of this activity may be mediated by inhibition of release of chemical mediators of inflammation. These possibilities remain only speculative and await further testing, but since naloxone had only a minimal effect on control animals we may conclude that the small inhibition of the antinociceptive effect of the plant extract observed in the presence of naloxone is due to interaction with opioid receptors.
It is noteworthy that the butanol extract of S. officinalis was more potent than the aqueous extract in the late phase of formalin test, the carrageenan-induced paw edema and in the cotton-pellet granuloma but was equipotent or only slightly more potent in the hot-plate test and the early phase of formalin tests, respectively. This observation suggests that the active ingredients of both extracts are more effective in inhibiting the release of mediators peripherally. Although the nature of the chemical compounds responsible for the antinociceptive and anti-inflammatory effects of the aqueous and the butanol extracts of S. officinalis remains speculative, preliminary phytochemical analysis of aqueous and butanol extracts showed the presence of tannins, triterpenes, alkaloids, and flavonoids (data not shown).
Conclusion
The aqueous and butanol extracts of S. officinalis possess acute and subacute anti-inflammatory effects exerted both centrally and peripherally as demonstrated by the hot-plate test, the formalin test, the carrageenan-induced paw edema and the cotton-pellet granuloma. These effects seem to be only partially mediated by the opioid receptor, whether this mediation is direct or indirect (i.e., activation by released CB receptor agonists). Other likely possibilities have also been discussed.
Nevertheless, the present observations lend pharmacological support to the folkloric uses of S. officinalis in the treatments of inflammatory and nociceptive conditions.
Declaration of interest
This work was supported by a grant from the Deanship for Scientific Research, Hashemite University
References
- Abu-Irmaileh B, Afifi F (2003): Herbal medicine in Jordan with special emphasis on commonly used herbs. J Ethnopharmacol 89: 193–197.
- Aizenman B, Derbentseva N, Zelepukha S, Negrash A, Volosovets P (1982): Salvin, an antibiotic from Salvia officinalis. Mikrobiol Zhurnal 44: 69–72.
- Alarcon-Aguilar FJ, Roman-Ramos R, Flores-Saenz JL, Aguirre-Garcia F (2002): Investigation on the hypoglycemic effects of extracts of four Mexican medicinal plants in normal and alloxan-diabetic mice. Phytother Res 16: 383–386.
- Amaral JF, Silva MIG, Neto MRA, Neto PFT, Moura BA, Melo CTV, Araujo FLO, Sousa DP, Vasconcelos PF, Vasconcelos SMN, Sousa FCF (2007): Antinociceptive effect of the monoterpene R- (+)-limonene in mice. Biol Pharm Bull 30: 1217–1220.
- Anand P, Whiteside G, Fowker CJ, Hohmann AG. (2009).Targeting CB2 receptors and the endocannabinoid system for the treatment of pain. Brain Res Rev 60: 255–266.
- Asongalem EA, Foyet HS, Ngogang J, Folefoc GN, Dimo T, Kamtchouing P (2004): Analgesic and antiinflammatory activities of Erigeron floribundus. J Ethnopharmacol 91: 301–308.
- Baricevic D, Sosa S, Della Loggia R, Tubaro A, Simonovska B, Krasna A and Zupancic A (2001): Topical anti-inflammatory activity of Salvia officinalis L. leaf: The relevance of ursolic acid. J Ethnopharmacol 75: 125–132.
- Baricevic D, Bartol T. (2000). The biological/pharmacological activity of the Salvia genus, in: Kintzios SE, ed. Sage: The Genus Salvia. Amsterdam, Harwood Academic Publishers, pp. 143–184.
- Beloeil H, Asehnoune K, Moine P, Benhamou D, Mazoit J-X (2005): Bupivacaine’s action on the carrageenan-induced inflammatory response in mice: Cytokine production by leukocytes after ex-vivo stimulation. Anesth Analges 100: 1081–1086.
- Cox ML, Heller VL, Welch SP (2007): Synergy between Δ9-tetrahydrocannabinol and morphine in the arthritic rat. Eur J Pharmacol 567: 125–130.
- Cuvelier M, Besret C, Richard H (1994): Antioxidant constituents in sage (Salvia officinalis). J Agric Food Chem 42: 665–669.
- Cuzzocrea S, Sautebin L, De Sarro G, Costantino G, Rombola L, Mazzon M, Ialenti A, De Sarro A, Ciliberto G, Di Rosa M, Caputi AP, Thiemermann, C (1999): Role of IL-6 in the pleurisy and lung injury caused by carrageenan. J Immunol 163: 5094–5104.
- De Leo V, Lanzetta D, Cazzavacca R, Morgante G (1998): Treatment of neurovegetative menopausal symptoms with a phytotherapeutic agent. Minerva Ginecol 50: 207–11.
- Dordevic S, Cakie M, Amr S (2000) The extraction of apigenin and luteolin from the sage Salvia officinalis L. from Jordan. Working Living Environ Protection 1: 87–93.
- Farag RS, Badei AZMA, Hewedi FM, El-Baroty, GSA (1989): Antioxidant activity of some spice essential oils on linoleic acid oxidation in aqueous media. J Am Oil Chem Soc 66: 792–799.
- Filipic M, Baricevic D (1997): Antimutagenic activity of Salvia officinalis extracts against UV-induced mutations in E. coli strains. Mutation Res 379: S182.
- Franca DS, Souza ALS, Almeida KR, Dolabella SS, Matinelli C, Coelho MM (2001): B vitamins induce an antinociceptive effect in acetic acid and formaldehyde models of nociception in mice. Eur J Pharmacol 421: 157–164.
- Gerrits MA, Patkina N, Zvartau EE, van Ree JM (1995): Opioid blockade attenuates acquisition and expression of cocaine-induced place preference conditioning in rats. Psychopharmacology (Berlin) 119: 92–98.
- Granados-Soto V, Alonso-Lopez R, Asomoza-Espenoza R, Rufino, M, Gomes-Lopez, LD, Feriera SH (2001): Participation of Cox, IL-1β and TNFα in formalin induced inflammatory pain. Proc West Pharmacol Soc 44: 15–17.
- Halici Z, Dengiz GO, Odabasoglu F, Suleyman H, Cadirci E, Halici M (2007): Amiodarone has anti-inflammatory and anti-oxidative properties: An experimental studying rats with carrageenan-induced paw edema. Eur J Pharmacol 566: 215–221.
- Hohmann AG, Suplita RL, Jr. (2006): Endocannabinoid mechanisms of pain modulation. AAPS J 8: E693–E708.
- Hohmann J, Zupko I, Redei D, Csanyi M, Falkay G, Mathe I, Janicsak G (1999): Protective effects of the aerial parts of Salvia officinalis, Melissa officinalis and Lavandula angustifolia and their constituents against enzyme-dependent and enzyme-independent lipid peroxidation. Planta Med 65: 576–578.
- Horiuchi K, Shiota S, Hatano T, Yoshida T, Kuroda T, Tsuchiya T (2007): Antimicrobial activity of oleanolic acid from Salvia officinalis and related compounds on enterococci (VRE). Biol Pharm Bull 30: 1147–1149.
- Hubbert M, Sievers H, Lehnfeld R, Kehrl W (2006): Efficacy and tolerability of a spray with Salvia officinalis in the treatment of acute pharyngitis – A randomized, double blind, placebo-controlled study with adaptive design and interim analysis. Eur J Med Res 11: 20–26.
- Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, Davar G, Makriyannis A, Vanderah TW, Mata HP, Malan TP (2005): CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci USA 102: 3093–3098.
- Kempuraj D, Madhappan B, Kristodoulou S, Boucher W, Cao J, Papadopoulou N, Cetrulo C, Theoharides T (2005): Flavonols inhibit proinflammatory mediators, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br J Pharmacol 145: 934–944.
- Kerstein, PC; Camino, DD; Morgan, MM; Stucky, CL (2009): Pharmacological blockade of TRPA1 inhibits mechanical firing in nociceptors. Molecular Pain 5: 19–25.
- Khasabova IA, Harding-Rose C, Simone DA, Seybold VS (2004): Differential effects of CB1 and opioid agonists on two populations of adult rat dorsal root ganglion neurons. J Neurosci 24: 1744–1753.
- Lee I-O, Jeong Y-S (2002): Effects of different concentrations of formalin on paw edema and pain behaviors in rats. J Korean Med Sci 17: 81–85.
- Lu Y, Yeap Foo, L (2000): Flavonoid and phenolic glycosides from Salvia officinalis. Phytochemistry 55: 263–267.
- Maruyama N, Ishibashi H, Hu W, Morofuji S, Inouye S, Yamaguchi H, Abe S (2006): Suppression of carrageenan- and collagen II-induced inflammation in mice by geranium oil. Mediators Inflamm 2006: 625–637.
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM (2007): TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA 104: 13525–13530.
- Miura K, Kikuzaki H, Nakatani N (2001): Apianane terpenoids from Salvia officinalis. Phytochemistry 58: 1171–1175.
- Mossa JS, Rafatullah S, Galal AM, Al-Yahya MA (1995): Pharmacological studies of Rhus retinorrhaea. Int J Pharmacog 33: 242–246.
- Nandal S, Dhir A, Kuhad A, Sharma S, Shopra K (2009): Curcumin potentiates the anti-inflammatory activity of cyclooxygenase inhibitors in the cotton pellet granuloma pouch model. Meth Find Exp Clin Pharmacol 3: 89–93.
- Nantel F, Denis D, Gordon R, Northey A, Cirino M, Metters KM, Chan CC (1999): Distribution and regulation of cyclooygenase-2 in carrageenan-induced inflammation. Br J Pharmacol 128: 853–859.
- Ninomiya K, Matsuda H, Shimoda h, Nishida N, Kasajima N,Yoshino T, Morikawa T, Yoshikawa M (2004): Carnosic acid, a new class of lipid absorption inhibitor from sage. Biorg Med Chem Lett 14: 1943–1946.
- Perry EK, Pickering AT, Wang WW, Houghton PJ, Perry NSL (1999): Medicinal plants and Alzheimer’s disease: From ethnobotany to phytotherapy. J Pharm Pharmacol 51: 527–534.
- Przesmycki K, Dzieciuch JA, Czuczwar SJ, Kleinrok Z (1997): Isobolographic analysis of interaction between intrathecal morphine and clonidine in the formalin test in rats. Eur J Pharmacol 337: 11–17.
- Raal A, Orav A, Arak E (2007): Compostion of the essential oil of Salvia officinalis L. from various European countries. Nat Prod Res 21: 406–411.
- Rau O, Wurglics M, Paulke A, Zitzkowski J, Meindl N, Bock A, Dinger-Man T, Abdel-Tawab, M, Schubert-Zsilavecz M (2006): Carnosic acid and carnosol, phenolic diterpene compounds of the labiate herbs rosemary and sage, are activators of the human peroxisome proliferator activated receptor gamma. Planta Med 72: 881–887.
- Reuter J, Jocher A, Hornstein S, Monting JS, Schemmp CM (2007): Sage extract rich in phenolic diterpenes inhibits ultraviolet-induced erythema in vivo. Planta Med 73: 1190–1191.
- Saidu Y, Bilbis LS, Lawal, M, Isezuo SA, Hassan SW, Abbas, AY (2007): Acute and subchronic toxicity studies of crude aqueous extract of Albizzia chevalieri harns (Leguminosae). Asian J Biochem 2: 224–236.
- Santos-Gomes PC, Seabra RM, Andrade PB, Fernandes-Ferrerira M (2002): Phenolic antioxidant compounds produced by in vitro shoots of sage (Salvia officinalis L.) Plant Sci 162: 981–987.
- Santos-Neto LL, Toledo MAV, Medeiros-Souza P, Souza GA (2006): The use of herbal medicine in Alzheimer’s disease – A systematic review. Compl Alter Med 3: 441–445.
- Talhouk R, Karam C, Fostok S, El-Jouni W, Barbour EK. (2007). Anti-inflammatory bioactivities in plant extracts. J Med Food 10: 1–10.
- Taylor BK, Peterson MA, Roderick RE, Tate J, Green PG, Levine JO, Basbaum AI (2000): Opioid inhibition of formalin-induced changes in plasma extravasation and local blood flow in rats. Pain 84: 263–270.
- Theobald HM, Moore RW, Katz LB, Pieper RO, Peterson RE (1983): Enhancement of carrageenan and dextran-induced edemas by 2,3,7,8-tetrachlorodibenzo-p-dioxin and related compounds. Pharmacol Exptl Ther 225: 576–583.
- Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K (1992): The formalin test: An evaluation of the method. Pain 51: 5–17.
- Trease G, Evans M (2001): Pharmacopoea and related drugs of biological origin, in: A Textbook of Pharmacognosy, fifteenth edition. London, WB Saunders.
- Zupko I, Hohmann J, Redei D, Falkay G, Mathe I (2001): Antioxidant activity of leaf of Salvia species in enzyme dependent and enzyme independent system of lipid peroxidation and their phenolic constituents. Planta Med 67: 366–368.
- Wang MF, Li JG, Rangarajan M, Shao Y, LaVoie EJ, Huang T-C, Ho, C-T (1998): Antioxidative phenolic compounds from sage (Salvia officinalis L.) J Agric Food Chem 46: 4869–4873.