Abstract
Rhinacanthus nasutus (L.) Kurz (Acanthaceae) has long been used in Thai traditional medicine for treatment of tinea versicolor, ringworm, pruritic rash, and abscess. The active constituents are known as a group of naphthoquinone esters, rhinacanthins. This work focused on establishment of R. nasutus root cultures and determination of rhinacanthin production. Induction of R. nasutus root formation was accomplished on solid Gamborg’s B5 (B5) medium, supplied with 0.1 mg/L indole-3-butyric acid (IBA) and 20 g/L sucrose. The effects of explants (whole leaf explants and four-side excised leaf explants), light and medium composition on root and rhinacanthin formation were investigated. The root formation from the whole leaf explants was 10 times higher than that from the four-side excised leaf explants. In addition, light possessed an inhibitory effect on the root and rhinacanthin formation of R. nasutus. Medium manipulation found that Murashige and Skoog (MS) medium supplied with 3 mg/L IBA and 30 g/L sucrose was the most suitable for induction of the root formation. Unfortunately, the obtained root cultures produced only rhinacanthin-C in very low amount, 0.026 mg/g dry weight (DW), when they were transferred into the same MS liquid medium. With semisolid medium (4 g/L agar) of the same MS composition, however, the root cultures appeared to produce higher content of rhinacanthin-C, -D and -N (3.45, 0.07 and 0.07 mg/g DW, respectively). Our finding suggests that culturing in semisolid medium is capable of improving of rhinacanthin production in R. nasutus root cultures.
Introduction
Rhinacanthin-C, -D and -N () are natural naphthoquinone esters accumulated in Rhinacanthus nasutus (L.) Kurz, a small shrub of the Acanthaceae family. These compounds have been reported as antifungal (CitationPanichayupakaranant et al., 2009), antiviral (CitationSendl et al., 1996), anti-inflammatory, anti-allergy (CitationTewtrakul et al., 2009a, Citation2009b), anticancer (CitationWu et al., 1998; CitationGotoh et al., 2004) and antiplatelet aggregation (CitationWu et al., 1998) agents. Nowadays, many of plants containing high-value compounds are difficult to cultivate or are becoming endangered because of over-harvesting (CitationRates, 2001). Plant cell culture methods and biotechnological approaches are attractive alternative sources to the whole plant for the production of high-value secondary metabolites and improve the productivity of plant cell culture. When compared to traditional agricultural growth, medicinal plant tissue cultures offer a number of year-round, continuous productions of plant medicinal compounds under highly controlled conditions. In addition, in vivo production of secondary metabolites by the plant can be highly influenced by plant growth environment factors such as climactic and soil conditions, pathogen attack, and herbivores (CitationWink, 2003).
It has been reported that harvesting periods affect the rhinacanthin content or quality of R. nasutus raw materials (CitationPanichayupakaranant et al., 2006). Recently, the study on rhinacanthin production by R. nasutus in vitro cultures is rarely reported. There is only one report on micropropagation of R. nasutus (CitationJohnson et al., 2002). Our preliminary study on the establishment of R. nasutus cell suspension cultures was achieved using B5 liquid medium supplied with various plant regulator compositions, e.g., IBA (0.1, 0.5, 1 and 2 mg/L) and 6-benzylaminopurine (BA) (0.1, 0.5, 1 and 2 mg/L). Unfortunately, neither of them produced rhinacanthins. There are several reports on naphthoquinone production by the root cultures of medicinal plants, e.g., Lithospermum canescens (Michx.) Lehm. (Boraginaceae) (CitationPietrosiuk et al., 2006), L. erythrorhizon Sieb. et Zucc. (CitationKöhle et al., 2002), Impatiens balsamina L. (Balsaminaceae) (CitationPanichayupakaranant & De-Eknamkul, 1992), Plumbago rosea L. (Plumbaginaceae) (CitationPanichayupakaranant & Tewtrakul, 2002; CitationGangopadhyay et al., 2008) and Sesamum indicum L. (Pedaliaceae) (CitationOgasawara et al., 1993), etc. This work was therefore focused on establishment of R. nasutus root cultures and determination of rhinacanthin production. The effects of light and cultured medium on root formation and rhinacanthin production of R. nasutus are reported herein.
Materials and methods
Plant material and chemicals
Rhinacanthus nasutus plants were grown in the botanical garden of the Faculty of Pharmaceutical Sciences, Prince of Songkla University. The voucher specimen (specimen no. 001 18 14) was identified by Pharkphoom Panichayupakaranant and deposited at the herbarium of the Faculty of Pharmaceutical Sciences, Prince of Songkla University, Thailand. Rhinacanthin-C, -D and -N were previously purified (CitationPanichayupakaranant et al., 2009). All chemicals used were analytical grade and were purchased from Sigma (St. Louis, MO) or Merck (Darmstadt, Germany).
Effect of explants and light on the root formation
R. nasutus root cultures were either initiated from the whole leaf explants or four-side excised leaf explants of R. nasutus on solid B5 supplied with 0.1 mg/L IBA. The cultures were incubated at 25° ± 2°C under light or dark conditions. The amounts of roots per explant were recorded after 4 weeks.
Effect of cultured media on root formation
The whole leaf explants of R. nasutus were initiated on various solid media including B5, MS and woody plant medium (WPM) supplied with various concentrations of IBA (1, 2 and 3 mg/L). After optimization of the basal medium and IBA concentration, manipulation of auxin was performed using IBA, 3-indoleacetic acid (IAA), α-naphthalene acetic acid (NAA) and 2,4-dichlorophenoxyacetic acid (2,4-D) at 3 mg/L. Subsequently, effects of kinetin and sucrose concentrations on root formation were examined using MS medium supplied with a combination of 3 mg/L IBA and various concentrations of kinetin (0, 0.5, 1 and 2 mg/L) or sucrose (30, 60, 90 and 120 g/L). The cultures were incubated at 25° ± 2°C under dark conditions. The amounts of roots per explant were recorded after 4 weeks.
Effect of light on rhinacanthin production in the root cultures
The root cultures of R. nasutus were maintained in B5 liquid medium supplied with 0.1 mg/L IBA. The cultures were incubated on a shaker (80 rpm), at 25° ± 2°C under light or dark conditions. After three successive subcultures, the root cultures (4 weeks old) were harvested and subjected to HPLC determination of rhinacanthin production.
Determination of rhinacanthin production in the root cultures
The root cultures (15 days old) were harvested by vacuum filtration. The harvested biomasses were dried in a hot air oven at 50°C and ground. The powdered samples (20 mg) were extracted with ethyl acetate 20 mL (×2) with the aid of ultrasonication for an hour. After the filtrates were evaporated to dryness the obtained residues were dissolved in methanol (1 mL). These sample preparations were then subjected to analysis of rhinacanthin content using the HPLC method as described below.
HPLC analysis was carried out using an Agilent 1100 series equipped with photodiode-array detector and autosampler (Palo Alto, CA). Separation was achieved at 25°C on a 150 mm × 4.6 mm TSK-gel ODS-80Tm column (Tosho Bioscience, Tokyo, Japan). The mobile phase consisted of methanol and 5% aqueous acetic acid (gradient elution as follow 0–10 min; 85:15, 17–30 min; 90:10) and was pumped at a flow rate of 0.4 mL/min. The injection volume was 20 µL. The quantification wavelength was set at 254 nm. The calibration curves were established from the standards rhinacanthin-C, -D and -N at the concentrations between 3.6–57.5 µg/mL, 0.24–7.69 µg/mL and 0.16–10 µg/mL, respectively. The linear equations of Y = 106504X + 21.141 (r2 = 0.9999), Y = 178576X + 1.5311 (r2 = 0.9999) and Y = 276858X + 8.2617 (r2 = 0.9999) correspond to rhinacanthin-C, -D and -N, respectively.
Statistical analysis
Statistical analyses – mean ± standard error (SE) – were carried out using one-way ANOVA. By convention, results are considered statistically significant when P <0.05.
Results and discussion
Effect of the leaf explants and light on root formation of R. nasutus
Establishment of R. nasutus root cultures using either four-side excised leaves or the whole leaves as the starting materials on the solid B5 medium and cultured under either light or dark conditions showed that only the explants that initiated under the dark conditions were capable of producing the root cultures. In addition, the whole leaf explants produced higher amount of roots than the four-side excised leaf explants (). The root formation on the whole leaf explants was 10 times higher than that on four-side excised leaf explants. Our observation also found that the root formation had usually taken place at the middle vein on the leaf base. This may be the reason why the four-side excised leaf explants produced fewer amounts of roots. Our finding indicates that the root formation of R. nasutus is inhibited by light. The negative effect of light on root formation seems to agree with previous reports (CitationFuernkranz et al., 1990; CitationVan der Krieken et al., 1992). However, the effect of light on promotion of root formation has also been reported (CitationLovell & Moore, 1969).
Table 1. Effect of the leaf explants and light on the root formation of R. nasutus.
Effect of light on rhinacanthin production in root cultures
After 8 weeks, the roots cultures were transferred to the same B5 liquid medium and cultured either under light or dark conditions. After several subcultures, the 4-week old cultured roots were subjected to quantitative determination of rhinacanthin production. On the basis of HPLC analysis, the cultured root in both conditions produced only rhinacanthin-C as the major naphthoquinone. However, the root cultures in dark conditions produced higher biomass and amount of rhinacanthin-C (2.80 ± 0.009 mg/g DW) than those in light conditions (0.68 ± 0.011 mg/g DW). This result agrees with the previous report on the accumulation of rhinacanthins, which accumulated higher in the roots of the intact plant than the aerial parts that were exposed to light (CitationPanichayupakaranant et al., 2006). In addition, it has been reported that light played a critical role in the negative regulation of shikonin biosynthesis in L. erythrorhizon cell cultures (CitationYazaki et al., 1999, Citation2001; CitationYazaki, 2001). These results suggest that both growth and rhinacanthin-C formation of R. nasutus root cultures are inhibited by light.
Effect of cultured media on root formation
Although the root cultures were established on solid B5 medium supplied with 0.1 mg/L IBA and 20 g/L sucrose, their growth rate was very slow. Medium manipulation was therefore examined in order to improve growth of R. nasutus root cultures. In this study, a number of root formations per explant were used as growth parameter. Medium manipulation was performed by variation of the basal media (B5, MS and WPM) as well as IBA concentrations (1, 2, and 3 mg/L). The result showed that MS medium supplied with 3 mg/L IBA was the most appropriate cultured medium for induction of the root formation in R. nasutus (). This may be due to the effect of a high concentration of NH4NO3 in the MS medium as well as high concentration of the auxin. To determine the effect of auxin type on the root formation, medium manipulation was further performed by variation of auxin (IBA, IAA, NAA and 2,4-D) at 3 mg/L in MS medium. It was found that the most appropriate auxin for root formation of R. nasutus was IBA (). In contrast, high concentration of 2,4-D completely inhibited the root formation. It has been reported that kinetin also plays an important role on root formation of I. balsamina (CitationPanichayupakaranant & De-Eknamkul, 1992). The effect of kinetin concentration (0, 0.5, 1, and 2 mg/L) in MS medium supplied with 3 mg/L IBA on the root formation of R. nasutus was therefore determined. The result indicated an increase in kinetin concentration in the cultured medium and a decrease of root formation (). Moreover, an increase in kinetin concentration resulted in de-differentiation of the root cultures. Callus formation was observed at high concentration of kinetin. This suggests that kinetin inhibits root formation of R. nasutus.
Table 2. Effect of basal medium and IBA concentration on the root formation of R. nasutus.
Table 3. Effect of plant growth regulators and sucrose composition on the root formation of R. nasutus.
Sucrose is one of the most important nutrients for plant in vitro culture. It is known as a carbon source that is essential for plant cell growth. In this study, variation of sucrose concentrations (30, 60, 90, and 120 g/L) in the cultured medium was also examined to determine the effect on the root formation of R. nasutus. The results showed that increasing of sucrose concentration in the cultured medium led to decrease of root formation (). An increase of sucrose concentration results in an increase of the osmotic pressure of the cultured medium. Higher osmotic pressure may inhibit nutrient and plant growth regulator absorption of plant cells. The root formation was therefore decreased when there was an increase in the sucrose concentration. These medium manipulation studies suggest that MS medium supplied with 3 mg/L IBA and 30 g/L is the most appropriate medium for root formation of R. nasutus.
Rhinacanthin production in R. nasutus root cultures
The root cultures of R. nasutus were then maintained in B5 liquid medium supplied with 3 mg/L IBA and 30 g/L sucrose. After several subcultures, the cultured roots (4-week old) were subjected to quantitative determination of rhinacanthin production. On the basis of HPLC analysis, the root cultures produced only rhinacanthin-C (). In addition, accumulation of rhinacanthin in the root cultures was very low (). The rhinacanthin production was lower than that of the former root cultures in B5 medium supplied with 0.1 mg/L IBA. This may be due to a better growth of the root cultures in MS medium supplied with 3 mg/L IBA which affects rhinacanthin production. An improving of rhinacanthin production in the root cultures was therefore examined by mimic immobilization technique. Immobilization technique with calcium alginate gel has successfully increased naphthoquinone production in Plumbago rosea (CitationKomaraiah et al., 2003). In this study the cultured roots were transferred into the MS medium containing 4 g/L agar and maintained as semisolid culture on the shaker (80 rmp). After several subcultures, the root cultures were harvested and subjected to determination of rhinacanthin production by HPLC. The semisolid conditions were capable of increasing rhinacanthin-C, -D and -N production by the root cultures (). However, the content of rhinacanthin-C, -D and -N were still less than those in the intact leaves as previously reported (CitationPanichayupakaranant et al., 2009). Improvements of rhinacanthin production in R. nasutus root cultures by other techniques, such as elicitation and transformed hairy root culture, are needed.
Figure 2. HPLC chromatograms of the standard rhinacanthin (A) and R. nasutus root culture extracts; the root culture in MS liquid medium (B) and in MS semisolid medium (C).
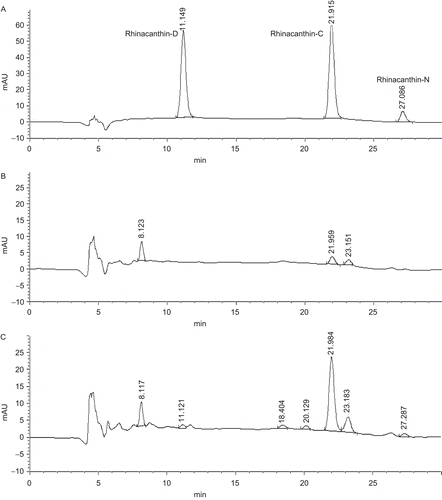
Table 4. Rhinacanthin production in R. nasutus roots cultured in liquid and semisolid MS media.
Conclusions
Our findings suggest that the whole leaf explant is an appropriate initiating material for root induction of R. nasutus on MS medium supplied with 3 mg/L IBA and 30 g/L sucrose. In addition, the root formation is inhibited by light. Culturing in semisolid medium may be a strategy for improving of secondary metabolite production in plant tissue cultures.
Declaration of interest
The authors wish to thank Prince of Songkla University and Faculty of Pharmaceutical Sciences for a financial support in this research.
References
- Fuernkranz HA, Nowak CA, Maynard CA (1990): Light effects on in vitro adventitious root formation in axillary shoots of mature Prunus serotina. Physiol Plant 80: 337–341.
- Gangopadhyay M, Sircar D, Mitra A, Bhattacharya S (2008): Hairy root culture of Plumbago indica as a potential source for plumbagin. Biologia Plantarum 52: 533–537.
- Gotoh A, Sakaeda T, Kimura T, Shirakawa T, Wada Y, Wada A, Kimachi T, Takemoto Y, Iida A, Iwakawa S, Hirai M, Tomita H, Okamura N, Nakamura T, Okumura K (2004): Antiproliferative activity of Rhinacanthus nasutus (L.) Kurz extracts and the active moiety, rhinacanthin C. Biol Pharm Bull 27: 1070–1074.
- Johnson M, Vallinayagam S, Manickam VS, Seenp S (2002): Micropropagation of Rhinacanthus nasutus (L.) Kurz.: A medicinally important plant. Phytomorphology 52: 331–336.
- Köhle A, Sommer S, Yazaki K, Ferrer A, Boronat A, Li SM, Heide L (2002): High level expression of chorismate pyruvate-lyase (UbiC) and HMG-CoA reductase in hairy root cultures of Lithospermum erythrorhizon. Plant Cell Physiol 43: 894–902.
- Komaraiah P, Ramakrishna SV, Reddanna P, Kavi Kishor PB (2003): Enhanced production of plumbagin in immobilized cells of Plumbago rosea by elicitation and in situ adsorption. J Biotechnol 101: 181–187.
- Lovell P, Moore K (1969): The effects of light and cotyledon age on growth and root formation in excised cotyledons of Sinapis alba L. Planta 85: 351–358.
- Ogasawara T, Chiba K, Tada M (1993): Production in high-yield of a naphthoquinone by a hairy root culture of Sesamum indicum. Phytochemistry 33: 1095–1098.
- Panichayupakaranant P, Charoonratana T, Sirikatitham A (2009): RP-HPLC analysis of rhinacanthins in Rhinacanthus nasutus: validation and application for the preparation of rhinacanthin high-yielding extract. J Chromatogr Sci 47: 705–708.
- Panichayupakaranant P, Chatkrapunt U, Supavita T (2006): Pharmacognostic and chemical studies on the leaves of Rhinacanthus nasutus. Nigerian J Nat Prod Med 10: 1–5.
- Panichayupakaranant P, De-Eknamkul W (1992): Study on naphthoquinone formation in in vitro cultures of Impatiens balsamina L. Thai J Pharm Sci 16: 29–37.
- Panichayupakaranant P, Tewtrakul S (2002): Plumbagin production by root cultures of Plumbago rosea. Electron J Biotechnol 5: 228–232.
- Pietrosiuk A, Sykłowska-Baranek K, Wiedenfeld H, Wolinowska R, Furmanowa M, Jaroszyk E (2006): The shikonin derivatives and pyrrolizidine alkaloids in hairy root cultures of Lithospermum canescens (Michx.) Lehm. Plant Cell Rep 25: 1052–1058.
- Rates SMK (2001): Plants as sources of drugs. Toxicon 39: 603–613.
- Sendl A, Chen JL, Jolad SD, Stoddart C, Rozhon E, Kernan M (1996): Two new naphthoquinones with antiviral activity from Rhinacanthus nasutus. J Nat Prod 59: 808–811.
- Tewtrakul S, Tansakul P, Panichayupakaranant P (2009a): Effects of rhinacanthins from Rhinacanthus nasutus on nitric oxide, prostaglandin E2 and tumor necrosis factor-alpha releases using RAW264.7 macrophage cells. Phytomedicine 16: 581–585.
- Tewtrakul S, Tansakul P, Panichayupakaranant P (2009b): Anti-allergic principles of Rhinacanthus nasutus leaves. Phytomedicine 16: 929–934.
- Van der Krieken WM, Breteler H, Visser MHM, Jordi W (1992): Effect of light and riboflavin on indolebutyric acid-induced root formation on apple in vitro. Physiol Plant 85: 589–594.
- Wink M (2003): Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 64: 3–19.
- Wu TS, Hsu HC, Wu PL, Teng CM, Wu CY (1998): Rhinacanthin Q, A naphthoquinone from Rhinacanthus nasutus and its biological activity. Phytochemistry 40: 2001–2003.
- Yazaki K, Matsuoka H, Shimomura K, Bechthold A, Sato F (2001): A novel dark-inducible protein, LeDI-2, and its involvement in root-specific secondary metabolism in Lithospermum erythrorhizon. Plant Physiol 125: 1831–1841.
- Yazaki K, Matsuoka H, Ujihara T, Sato F (1999): Shikonin biosynthesis in Lithospermum erythrorhizon: Light-induced negative regulation of secondary metabolism. Plant Biotechnol 16: 335–342.
- Yazaki K (2001): Root-specific production of secondary metabolites: Regulation of shikonin biosynthesis by light in Lithospermum erythrorhizon. Nat Med 55: 49–54.