Abstract
Context: In traditional medicine propolis is widely used for the treatment of various ailments including ulcer and wound healing. The phytochemical screening of Indian propolis indicates the presence of biologically active ingredients in appreciable amounts. In the absence of systematic evaluation of wound healing properties of Indian propolis in the literature, the present study was undertaken.
Objective: The aim of this study was to evaluate the wound healing potential of Indian propolis on excision wounds induced in experimental rats.
Materials and methods: Excision wounds were created in male Wistar rats and were treated with Indian propolis ointment (nitrofurazone was used as a reference drug - widely used for wound healing) for a period of 14 days. Control rats were treated with petroleum jelly. The parameters analyzed include wound contraction, hydroxyproline, hexosamine, uronic acid, total protein, DNA, and RNA.
Results: Topical application of propolis ointment for 14 days significantly improved the wound contraction when compared to the control group of rats. The determination of hydroxyproline, hexosamine, uronic acid, DNA, RNA and protein levels in the wound matrix revealed the pro-healing effects of propolis. The results obtained were comparable with nitrofurazone.
Discussion and conclusion: It appears that the ethanol extract of Indian propolis possesses significant pro-healing activity by accelerating the healing process at various phases of tissue repair. The presence of biologically active ingredients such as flavonoids, phenolic acids, terpenes, benzoic acids, amino acids and vitamins, etc. in Indian propolis may readily account for the observed prophylactic action of propolis in wound healing.
Introduction
The wound may be defined as a loss or breaking of cellular and anatomic or functional continuity of living tissues. The wound is ischemic in nature. Wounds cause discomfort and are more prone to infection and other troublesome complications (CitationMeyer-Ingold, 1993). Impaired wound healing leads to significant patient morbidity and mortality. Wound healing, a fundamental response to tissue injury, is a complex process involving mechanisms such as coagulation, inflammation, matrix synthesis and deposition, angiogenesis, fibroplasia, epithelization, contraction and remodeling. The smooth progression of these events leads to early completion of wound closure. Successful wound healing requires coordination and precise signaling from various cells that produce an array of cytokines, growth factors, ground substances and collagen.
Wound healing is frequently a therapeutic challenge. Although there have been some advances in the treatment of wound healing, the best remains undecided. An ideal therapy should not only promote the rapid healing process but also act as an anti-scarring therapy. Scar formation and overproduction of extracellular matrix by connective tissue characterize a pathological process called “fibrosis” which occurs as a result of deranged healing in response to tissue damage. The molecular process leading to fibrosis is not different from normal formation of connective tissue and extracellular matrix in normal organs. The context, the environment and the overproduction make the difference (CitationFranklin, 1997). Many of the synthetic drugs currently used for the treatment of wounds are not only expensive but also pose problems such as allergy and drug resistance, and this situation has forced scientists to seek alternative drugs (CitationSubramanian et al., 2006). Hence, efforts are being made all over the world to discover an efficacious pro-healing agent that could obviate the prolonged treatment, cost and save the patient from severe secondary complications. A variety of natural products or their derivatives have been considered as potential candidates for wound healing as they provide a moist environment to encourage the establishment of suitable conditions for wound healing.
Propolis is a chemically very complex, resinous bee product collected by worker honeybees from parts of plants, buds, exudates and secretions in the neighborhood of their hive (CitationHausen et al., 1987). The bees pack them on their hind legs and bring them back to their hive to cover cracks and crevices and reduce the size of the hive entrance preventing the invasion of large insects like moths, butterflies, beetles, cicadas, etc. (CitationDaugsch et al., 2008). More importantly, it is also used as an “embalming” substance to cover hive invaders which bees have killed but cannot transport out of the hive (CitationBankova et al., 2000).
Propolis, a widely consumed folk medicine in the traditional medicinal system since ancient times, is a serious candidate to be added to topical formulations due to its outstanding biological properties (CitationAhn et al., 2004). As the most important “chemical weapon” of bees against pathogenic microorganisms and its lipophilic nature, propolis has been widely used as a remedy by humans, since ancient times. Use of propolis by humans has a long history predated only by the discovery of honey (CitationBurdock, 1998; CitationCastaldo & Capasso, 2002). It is claimed to be useful in cosmetics and prevention against hyperpigmentation (CitationShigemi, 2002). Propolis extracts were reported to have considerable biological attributes such as anticancer (CitationGrunberger et al., 1988), antioxidant (CitationRusso et al., 2002; CitationBhadauria et al., 2007), antimicrobial (CitationKoo et al., 2000), anti-inflammatory (CitationMirzoeva & Calder, 1996; CitationSforcin, 2007), antiviral (CitationKujumgiev et al., 1999), and hepatoprotective effects (CitationMahran et al., 1996; CitationMerino et al., 1996; CitationShukla et al., 2004).
The composition of propolis varies according to the plant sources, local flora, regional vegetation and season of collection by the bees (CitationBanskota et al., 2001; CitationBankova, 2005a). So far, more than 300 constituents have been identified in different propolis samples, with more than 150 being present in any given sample, including flavonoids, cinnamic acid derivatives, benzoic acids, amino acids, phenolic acids, phenolic aldehydes, polyphenols, steroids, terpenes and inorganic compounds (CitationSalatino et al., 2005; CitationKhalil, 2006). However, most of the pharmacological properties of propolis are principally attributed to the presence of flavonoids (CitationMirzoeva & Calder, 1996), which constitute more than 50% of its total weight. However, the plant origin and region of collection bear great significance with respect to biological activity of propolis (CitationChristov et al., 2006; CitationMani et al., 2006). The biological properties of propolis are not only beneficial to bees but have general pharmacological value as a natural mixture (CitationTeixeira et al., 2005). The distinct chemistry of propolis from different origins leads to the expectation that the biological properties of different propolis types will be dissimilar. However, in most cases, this is not true (CitationBankova, 2005b). Propolis samples from Brazil, Peru, the Netherlands and China showed similar biological activities (CitationBanskota et al., 2000).
In India, there has been a growing interest in the potential of natural products obtained from plants and animals for development of drugs with wound healing properties as taught in a popular form of Indian medicine known as Ayurveda (CitationBiswas & Mukherjee, 2003). Propolis was selected for the present study because it is widely used and easily available throughout the subcontinent, and is also cost effective. Further, literature survey revealed that no systematic scientific investigation has been made with regard to the wound healing activity of Indian propolis.
Hence, the present work was aimed to evaluate the wound healing potential of propolis on excision wounds induced in Wistar rat models. The extract in the form of an ointment was topically applied and the efficacy was compared with a standard drug formulation.
Methods and materials
Preparation of ethanol extract of propolis
The raw propolis samples were collected in September 2008 in Mudivaithanendal, Tuticorin, Tamil Nadu, using propolis traps to minimize their contamination with foreign substances from hives. They were kept desiccated and in the dark until their processing. Propolis powder (100 g) was extracted in 500 mL ethanol (95% v/v) by stirring overnight and centrifuged at 27,000 g for 15 min (CitationGekker et al., 2005). The supernatant was then concentrated in a rotary evaporator under reduced pressure 450 mmHg at 40°C and the residue was stored in the dark at room temperature until use. The yield of the ethanol extract was 16.5% w/w. The dried extract was used for the preparation of propolis ointment.
Qualitative phytochemical analysis of propolis
The ethanol extract of propolis was subjected to qualitative phytochemical analysis as described by CitationHarborne (1998) and CitationKokate (2001) for the presence of flavonoids, phenolic compounds, terpenes, steroids, proteins, amino acids and glycosides.
Determination of total polyphenol content
Total polyphenol contents in ethanol extract of propolis were determined according to the Folin-Ciocalteu colorimetric method (CitationSingleton et al., 1999; CitationKumazawa et al., 2002). A standard curve was built with gallic acid reference solutions. Aliquots ranging from 2 to 10 mL of standard aqueous gallic acid solution (100 μg/mL) were pipetted in to 100 mL volumetric flasks containing 70 mL of distilled water. Folin-Ciocalteu reagent (5 mL) and 10 mL of saturated sodium carbonate solution were added, and the volume was made up to 100 mL with distilled water. The solution was thoroughly mixed. The blank was prepared in the same manner, but without gallic acid. After 1 h of incubation at room temperature, the absorbance was measured at 760 nm. For determination of the total phenolic content of propolis, aqueous solutions at the final concentration of 20 μg/mL were used, proceeding in the same manner described for the reference solutions and the total polyphenolic content was expressed as mg per g of gallic acid equivalents.
Determination of total flavonoid content
Total flavonoid contents in the ethanol extract of propolis were determined according to the method of CitationWoisky and Salatino (1998) with minor modifications. A standard curve was built with quercetin reference solutions. Aliquots ranging from 2 to 8 mL of standard quercetin ethanol extract solution (50 μg/mL) were pipetted in to 25 mL volumetric flasks containing 1 mL aqueous aluminum chloride solution at 2.5% (w/v) and the volume was made up with ethanol. The blank was prepared by diluting 1 mL of aluminum chloride solution in a 25 mL volumetric flask with ethanol. After 1 h at room temperature, the absorbance was measured at 420 nm. Propolis samples were evaluated at a final concentration of 20 μg/mL, proceeding in the same manner described for the reference solutions and the total flavonoid content was calculated as quercetin equivalents (mg/g) from a calibration curve.
Preparation of propolis ointment
The ethanol extract of propolis was prepared as an ointment using petroleum jelly (melting point 60-65°C) at a concentration of 10% (w/w) and the ointment was kept in sterile glass container, properly sealed and preserved at 4°C and used for topical application.
Animals
Six-week old, Male Wistar rats weighing 150-170 g were used in the study. They were individually housed and maintained in a laboratory environment in a 12 h dark–light cycle. Animals were fed with standard pellet diet and water ad libitum. The experiments were conducted under the protocols approved by the Institutional Animal Ethics Committee (IAEC No. 01/031/08). The Organization for Economic Co-operation and Development (OECD) guidelines for the testing of chemicals such as available information on the test substance, selection of animal species, housing and feeding conditions, number of animals, dosage fixation, route of administration, toxicological parameters, data and reporting, etc. were followed prior to conduct the experiments.
Wound creation
The animals were fasted overnight and anesthetized with 1 mL intravenous thiopentone sodium (40 mg/kg b.w.) (CitationPerez Gutierrez & Vargas, 2006). A wide area of the dorsum of each rat was depilated using toothed forceps, sterile pointed scissors and a scalpel blade. The area was then cleaned with 70% ethanol to maintain aseptic conditions. An excision wound was created according to the CitationMorton and Malone method (1972). A full thickness of the excision wound of circular area 300 mm and 2 mm depth was inflicted on either side of the depilated dorsum of each rat. Excess bleeding was mopped using sterile gauze. The entire wound was left open (CitationDiwan et al., 1982). Animals were closely observed for any infection, and those that showed signs of infection were separated and excluded from the study. Animals were euthanized after completion of the study.
Experimental design
The animals were divided into three groups each comprising of a minimum of six rats as follows.
Group 1: Excision wound-induced rats were treated with petroleum jelly and considered as the control;
Group 2: Excision wound-induced rats treated with propolis ointment (10% w/w) for 14 days;
Group 3: Excision wound-induced rats treated with standard drug ointment (0.2% w/w nitrofurazone ointment) for 14 days (CitationHarish et al., 2008).
The treatment schedule was twice daily with topical application of the formulated ointment as well as the standard drug ointment, while the control group was dressed with ointment base containing same quantity of petroleum gel. Sterile cotton swabs were used for uniform application of ointments.
Wounds were traced on 1 mm2 graph paper on the day of wounding and subsequently on alternate days until healing was complete. Changes in wound area were calculated, giving an indication of the rate of wound contraction. The period of epithelization was calculated as the number of days required for falling off the dead tissue remnants without any residual raw wound. The percentage reduction in wound size was calculated using the following equation:
where A0 and At are initial wound area and wound area after time interval “t”. The distance from the right wound margin to the left wound margin was measured. The length of new generated epithelium across the surface of the wound was determined as the sum of the new epidermis growing from the right and left margin of the wound (CitationBalakrishnan et al., 2006).
Biochemical estimations
On postoperative days 7 and 14, an appreciable amount of granulation tissue formed on the wound, which was excised and its weight recorded. The tissues were dried in an oven at 60°C for 72 h and the dry weight was again noted. The dried tissue was added with 5 mL of 6 N HCl and kept at 110°C for 24 h in sealed tubes. The hydrolysate was neutralized to pH 7. The neutralized acid hydrolysate of the dry tissue was used for the determination of collagen by the estimation of hydroxyproline as described by CitationWoessner (1961). The collagen content can be calculated by multiplying the hydroxyproline content by the factor 7.46 (CitationNeuman & Logan, 1950). Hexosamine content was estimated according to the method of CitationAdamsons (1964). Uronic acid content was estimated by the method of CitationBitter and Muir (1962). The DNA and RNA contents were assayed by the methods of CitationBurton (1966) and CitationAlmog and Shirey (1978), respectively. The protein content in the tissue extract was estimated by CitationLowry et al. (1951).
Statistical analysis
Data analysis was done by an investigator who was “blinded” to the treatment. Results, expressed as mean ± SEM, were evaluated by one-way analysis of variance (ANOVA) using SPSS (version 15.0) program followed by LSD. Values were considered statistically significant when P < 0.05.
Results
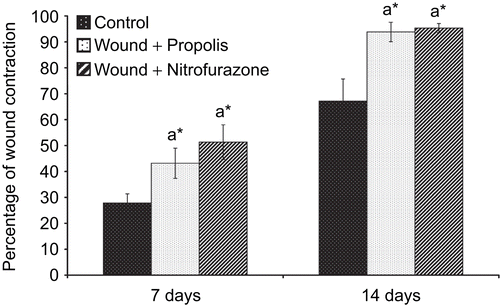
Qualitative analysis of propolis revealed the presence of flavonoids, polyphenols, steroids, triterpenes, phenolic compounds, glycosides, vitamins and amino acids. The amount of total polyphenol and flavonoid contents in the ethanol extract of Indian propolis were found to be 238 ± 5.4 mg/g and 168 ± 6.5 mg/g, respectively. The wounds treated with propolis as well as the standard drug formulation exhibited marked dryness of wound edges with a regeneration of healing tissue. depicts the percentage wound contraction in excision wounds of control and experimental groups of rats. The topical application of propolis as well as the standard drug formulation significantly (P < 0.05) improved the rate of wound contraction throughout the experimental period. The treated wounds were found to contract much faster than control wounds.
Figure 1. Effect of Indian propolis on the level of percentage wound contraction in the excision wound model. Values are mean ± SEM; n = 6 in each group. *Significant at p < 0.05 as compared with the control group of rats.
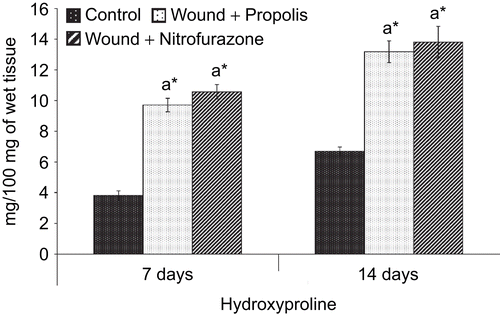
The hydroxyproline content in the granulation tissue of control and experimental groups of rats is presented in . The hydroxyproline level in the granulation tissue was significantly (P < 0.05) increased throughout the experimental period in both propolis-treated as well as nitrofurazone-treated groups of rats when compared with control rats.
Figure 2. Effect of Indian propolis on the level of hydroxyproline in the excision wound model. Values are mean ± SEM; n = 6 in each group. *Significant at p < 0.05 as compared with the control group of rats.
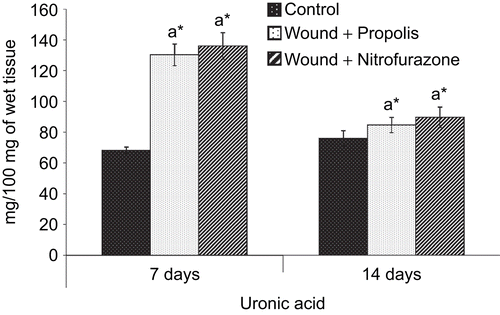
and show the levels of hexosamine and uronic acid in the granulation tissue of control and experimental groups of rats, respectively. The levels of hexosamine and uronic acid were significantly (P < 0.05) increased during the early stages of wound healing, i.e., day 7 and subsequently, the degree of elevation was decreased on postoperative day 14 in both control and experimental groups of rats.
Figure 3. Effect of Indian propolis on the level of hexosamine in the excision wound model. Values are mean ± SEM; n = 6 in each group. *Significant at p < 0.05 as compared with the control group of rats.
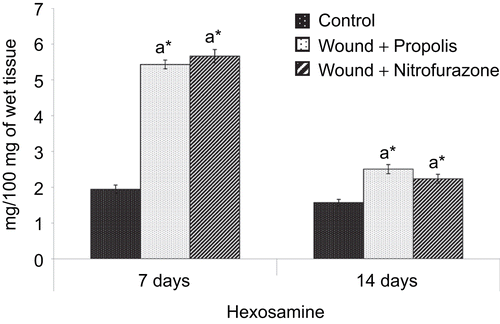
Figure 4. Effect of Indian propolis on the level of uronic acid in excision wound model. Values are mean ± SEM; n = 6 in each group. *Significant at p < 0.05 as compared with the control group of rats.
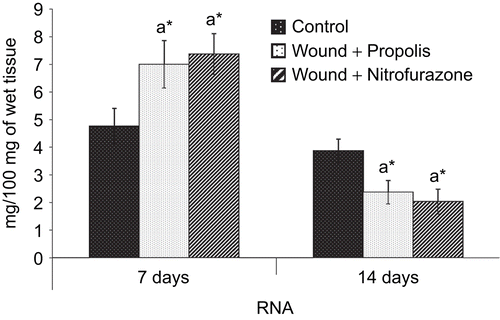
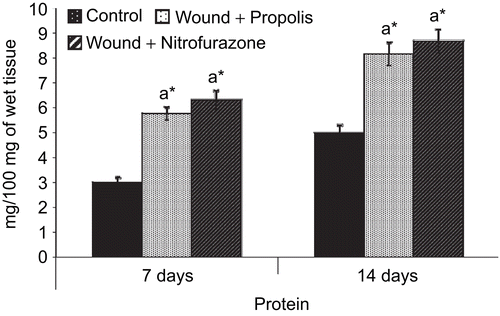
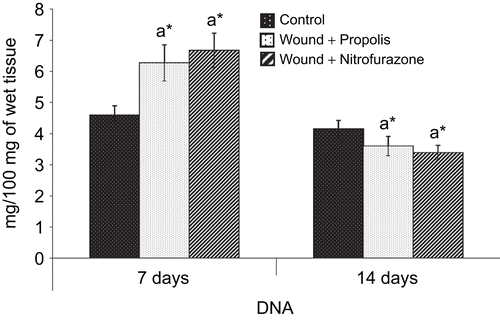
The amount of DNA, RNA and protein in the granulation tissue of control and experimental groups of rats are shown in , and , respectively. There was a significant (P < 0.05) increase in the levels of DNA, RNA and protein content on postoperative day 7 in both propolis-treated as well as standard drug-treated groups when compared to control group of rats. However, the levels of DNA and RNA were decreased and the total protein content was increased thereafter on day 14.
Figure 5. Effect of Indian propolis on the level of DNA in excision wound model. Values are mean ± SEM; n = 6 in each group. *Significant at p < 0.05 as compared with the control group of rats.
Discussion
Wound healing is the physiological response to the tissue injury that results in the replacement of destroyed tissue by living tissue and thus restoration of tissue integrity. It involves a highly coordinated cascade of cellular responses encompassing the interaction of many cell types over long periods of time. Wound contracture occurs throughout the healing process and it mainly depends on the extent of tissue damage, repairing ability and general state of the health of the tissue.
Although there have been some advances in the wound healing processes, the duration could not be shortened. The present study was aimed to evaluate the wound healing potential of ethanol extract of propolis at the wound site of excision wounds in experimental rats. Alcoholic extracts of propolis are assumed to contain almost all biologically active propolis constituents and for this reason are commonly used in practice (CitationGhisalberti, 1979; CitationHayashi et al., 1999). The extract in the form of an ointment was topically applied and the efficacy was compared with a standard drug ointment. The parameters analyzed include percentage wound contraction, hydroxyproline (collagen), hexosamine, uronic acid, DNA, RNA, and protein.
Studies on acute wounds in animal models shows that the wound healing process can be broadly categorized into three stages: inflammatory phase comprising establishment of hemostasis and inflammation, proliferative phase consisting of granulation, contraction and epithelization, and finally the remodeling phase which ultimately determines the strength and the appearance of the healed tissue (CitationPeacock, 1984). The normal healing cascade begins with an orderly process of hemostasis, which leads to an inflammatory cell cascade (CitationBroughton et al., 2006). Hemostasis occurs within minutes of the initial injury unless there are underlying clotting disorders. The platelets seals off the damaged blood vessels by secreting vasoconstrictive substances. They also secrete factors which interact with and stimulate the intrinsic clotting cascade through the production of thrombin, which in turn initiates the formation of fibrin from fibrinogen. The fibrin mesh strengthens the platelet aggregate into a stable hemostatic plug. Finally, platelets also secrete cytokines such as platelet-derived growth factor (PDGF) which is recognized as one of the first factors secreted in initiating subsequent steps.
The inflammatory cells invade the wound site within a few hours after injury. Neutrophils arrive first, followed by monocytes, macrophages, fibroblasts and lymphocytes (CitationLi et al., 2007). This stage usually lasts up to 4 days post injury. The inflammatory response causes the blood vessels to become leaky releasing plasma and polymorphonucleocytes into the surrounding tissue. The neutrophils, with the aid of mast cells, phagocytize debris and microorganisms and provide the first line of defense against infection. Macrophages are able to phagocytize bacteria and provide a second line of defense. They also secrete a variety of chemotactic and growth factors such as fibroblast growth factor (FGF), epidermal growth factor (EGF), transforming growth factor beta (TGF-β) and interleukins-I (IL-1) which initiates the formation of granulation tissue (CitationBroughton et al., 2006).
The granulation phase is characterized clinically by the presence of pebbled red tissue in the wound base and involves replacement of dermal tissues and sometimes sub-dermal tissues in deeper wounds as well as contraction of the wound. As the granulation phase progresses, the predominant cells in the wound site are reparatory cells such as fibroblasts, endothelial cells, pericytes, keratinocytes, which are responsible for the formation of new matrix needed for structure and function repair of injured tissue (CitationDiegelmann & Evans, 2004; CitationWhitney, 2005). In the remodeling or maturation phase, the fibroblasts promote tensile strength which sometimes may take up to two years after wounding. The data obtained in the present study are in line with the above findings.
The time required for complete epithelization of the excision wound is an important parameter to assess the wound healing process. The pro-healing activity of propolis was conspicuous as the healing parameters analyzed were significantly altered. By comparison, the propolis as well as standard drug ointment-treated wounds were clean with healthy granulation tissue. In control wounds, epithelial reorganization was very slow. The results of the study have shown that propolis accelerated wound closure from the initial stage and wound contraction was nearly 90% for both ointments in 14 days. Thus, topical application of propolis extract at the wound site elicited significant wound healing activity which may be due to its angiogenic and mitogenic potential.
Wound contraction begins almost concurrently with collagen synthesis. The rate of contraction depends on the degree of tissue laxity and shape of the wound. Humans have tight skin, and this difference makes comparison with loose skinned animals such as rats difficult. Although there are inherent drawbacks in using rats for comparisons with human skin wound healing, there are also advantages in the use of rats as a research model, such as the availability of a broad knowledge based on rat wound healing gained from years for previous research (CitationCross et al., 1995).
The quantitative measurement of hydroxyproline, an amino acid found only in collagen, is directly proportional to the formation of collagen and its estimation helps clinically to understand the progress rate at which healing process ensues in the wound tissue (CitationGardner, 1967). It is known that collagen accumulation is the sum of synthesis and destruction and both occur simultaneously during the wound healing process (CitationMinor, 1980). Significantly (P < 0.05) elevated levels of hydroxyproline observed in both propolis-treated as well as standard drug ointment-treated groups of rats indicate the wound healing potential of propolis (). Higher concentration of hydroxyproline observed in the propolis-treated group of rats indicates faster rate of wound healing and the results are comparable with the standard drug formulation-treated group of rats. This observation is also consistent with the rate of wound contraction. Collagen not only confers strength and integrity to the tissue matrix but also plays an important role in homeostasis and epithelization which take part in the latter phases of wound healing. Thus, the observed increase in collagen synthesis in the propolis-treated rats may significantly contribute to healing and also provide strength to the repaired tissue.
Hexosamine and uronic acid, the matrix molecules, which act as ground substratum for the synthesis of new extracellular matrix, are increased during the early stages of wound healing and the degree of synthesis is decreased thereafter. Collagen content of the wound increases rapidly following the lag period and as the collagen content increases the hexosamine content of the tissue declines (CitationAlitalo et al., 1980). The decrease in hexosamine and the uronic acid content was associated with concomitant increase in collagen content (CitationDunphy & Udupa, 1955). Uronic acid in the wound attracts fibroblasts and stimulates collagen synthesis by providing more fluid that facilitates greater cell mobility, early remodeling and helps the wound to heal without scar formation (CitationHu et al., 2003; CitationTomlinson & Ferguson, 2003).
The increase in DNA, RNA and protein contents of treated wounds indicates hyperplasia of cells. The elevated levels of DNA, RNA and protein contents of the granulation tissue of propolis as well as the standard drug formulation group of rats indicate their prophylactic action on protein synthesis. CitationNayak et al. (2006) reported that the increase in the weight of the granulation tissue during wound healing is due to the presence of a higher content of protein.
Exposed subcutaneous tissues often provide a favorable substratum for a wide variety of microorganisms to contaminate and colonize. Wound contaminants are likely to originate from three main sources, namely the environment, the surrounding skin and endogenous sources involving mucous membranes (CitationDuerden, 1994). CitationBonvehí et al. (1994) reported that the minimum inhibitory concentration of propolis is about 400 times higher than that reported for tetracyclines against E. coli and about 53 times higher against B. subtilis and S. aureus.
In recent years oxidative stress has been implicated in a variety of degenerative processes and diseases. These mainly include acute and chronic inflammatory conditions like wound healing (CitationMaier & Chan, 2002). Propolis has been shown to possess antioxidant properties (CitationAhn et al., 2004). The flavonoids which are responsible for the free radical scavenging activity are believed to be one of the important components involved in wound healing. Phytochemical screening revealed the presence of appreciable amounts of flavonoids in propolis (CitationVolpi & Bergonzini, 2006) and this could be the reason for its pro-healing activity.
Flavonoids are known to reduce lipid peroxidation not only by preventing or slowing the onset of cell necrosis but also by improving vascularity. Hence any drug that inhibits lipid peroxidation is believed to increase the viability of collagen fibrils by increasing the strength of collagen fibrils, increasing the circulation, preventing the cell damage and by promoting DNA synthesis (CitationGetie et al., 2002). Flavonoids (CitationTsuchiya et al., 1996) and triterpenoids (CitationScortichini & Pia Rossi, 1991) are also known to promote the wound healing process mainly due to their astringent and antimicrobial properties which seem to be responsible for wound contraction and increased rate of epithelization.
The complete isolation and identification of the active ingredients in the Indian propolis extract falls beyond the scope of the study. However, quantitative estimation of flavonoids and polyphenols along with the qualitative analysis of other phytochemicals suggest that these are responsible for the observed wound healing properties of Indian propolis. Further studies are in progress to isolate and quantify the individual active principle(s) responsible for the wound healing properties of Indian propolis.
The results in the healing and sealing of wounds makes Indian propolis as an important natural product for assistance in the process of wound healing which may be due to the synergistic actions of biologically active ingredients present in the propolis ointment. In conclusion, the present study provides a rational basis for the beneficial usage of Indian propolis as folk medicine since ancient times and also confirms that propolis or its constituents can be considered as suitable, powerful, natural wound healing medicine, perhaps in overcoming defects associated with healing failure in chronic wounds.
Declaration of interest
The authors report no conflicts of interst.
References
- Adamsons RJ, Musco F, Enquist IF (1964): The relationship of collagen content to wound strength in normal and scorbutic animals. Surg Gynecol Obstet 119: 323–329.
- Ahn MR, Kumazawa S, Hamasaka T, Bang KS, Nakayama T (2004): Antioxidant activity and constituents of propolis collected in various areas of Korea. J Agric Food Chem 52: 7286–7292.
- Alitalo K, Hovi T, Vaheri A (1980): Fibronectin is produced by human macrophages. J Exp Med 151: 602–613.
- Almog R, Shirey TL (1978): A modified orcinol test for the specific determination of RNA. Anal Biochem 91: 130–137.
- Balakrishnan B, Mohanty M, Fernandez AC, Mohanan PV, Jayakrishnan A (2006): Evaluation of the effect of incorporation of dibutyryl cyclic adenosine monophosphate in an in situ-forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials 27: 1355–1361.
- Bankova V (2005a): Recent trends and important developments in propolis research. Evid Based Complement Altern Med 2: 29–32.
- Bankova V (2005b): Chemical diversity of propolis and the problem of standardization. J Ethnopharmacol 100: 114–117.
- Bankova VS, De Castro SL, Marcucci MC (2000): Propolis: Recent advances in chemistry and plant origin. Apidologie 31: 3–15.
- Banskota AH, Tezuka Y, Adnyana IK, Midorikawa K, Matsushige K, Message D, Huertas AA, Kadota S (2000): Cytotoxic, hepatoprotective and free radical scavenging effects of propolis from Brazil, Peru, the Netherlands and China. J Ethnopharmacol 72: 239–246.
- Banskota AH, Tezuka Y, Kadota S (2001): Recent progress in pharmacological research of propolis. Phytother Res 15: 561–571.
- Bhadauria M, Nirala SK, Shukla S (2007): Propolis protects CYP 2E1 enzymatic activity and oxidative stress induced by carbon tetrachloride. Mol Cell Biochem 302: 215–224.
- Biswas TK, Mukherjee B (2003): Plant medicines of Indian origin for wound healing activity: A review. Int J Low Extrem Wounds 2: 25–39.
- Bitter T, Muir HM (1962) A modified uronic acid carbazole reaction. Anal Biochem 4: 330–334.
- Bonvehí JS, Coll FV, Jordà RE (1994): The composition, active components and bacteriostatic activity of propolis in dietetics. J Am Oil Chem Soc 71: 529–532.
- Broughton G Jr, Janis JE, Attinger CE (2006): The basic science of wound healing. Plast Reconstr Surg 117: 12S–34S.
- Burdock GA (1998): Review of the biological properties and toxicity of bee propolis (propolis). Food Chem Toxicol 36: 347–363.
- Burton K (1966): Isolation of certain oligonucleotides obtained by degradation of deoxyribonucleic acid. Biochem J 98: 68–69.
- Castaldo S, Capasso F (2002): Propolis, an old remedy used in modern medicine. Fitoterapia 73: S1–S6.
- Christov R, Trusheva B, Popova M, Bankova V, Bertrand M (2006): Chemical composition of propolis from Canada, its antiradical activity and plant origin. Nat Prod Res 20: 531–536.
- Cross SE, Naylor IL, Coleman RA, Teo TC (1995): An experimental model to investigate the dynamics of wound contraction. Br J Plast Surg 48: 189–197.
- Daugsch A, Moraes CS, Fort P, Park YK (2008): Brazilian red propolis – Chemical composition and botanical origin. Evid Based Complement Altern Med 5: 435–441.
- Diegelmann RF, Evans MC (2004): Wound healing: An overview of acute, fibrotic and delayed healing. Front Biosci 9: 283–289.
- Diwan PV, Tilloo LD, Kulkarni DR (1982): Influence of Tridax procumbens on wound healing. Indian J Med Res 75: 460–464.
- Duerden BI (1994): Virulence factors in anaerobes. Clin Infect Dis 18: S253–S259.
- Dunphy JE, Udupa KN (1955): Chemical and histochemical sequences in the normal healing of wounds. N Engl J Med 253: 847–851.
- Franklin TJ (1997): Therapeutic approaches to organ fibrosis. Int J Biochem Cell Biol 29: 79–89.
- Gardner DG (1967): The relationship of hexosamine and hydroxyproline to connective tissue generation. A review of the literature. J Oral Ther Pharmacol 3: 473–484.
- Gekker G, Hu S, Spivak M, Lokensgard JR, Peterson PK (2005): Anti-HIV-1 activity of propolis in CD4(+) lymphocyte and microglial cell cultures. J Ethnopharmacol 102: 158–163.
- Getie M, Gebre-Mariam T, Rietz R, Neubert RH (2002): Evaluation of the release profiles of flavonoids from topical formulations of the crude extract of the leaves of Dodonea viscosa (Sapindaceae). Pharmazie 57: 320–322.
- Ghisalberti EL (1979): Propolis: A review. Bee World 60: 59–84.
- Grunberger D, Banerjee R, Eisinger K, Oltz EM, Efros L, Caldwell M, Estevez V, Nakanishi K (1988): Preferential cytotoxicity on tumor cells by caffeic acid phenethyl ester isolated from propolis. Experientia 44: 230–232.
- Harborne JB (1998): Phytochemical Methods. A Guide to Modern Techniques of Plant Analysis. New York, Chapman and Hall.
- Harish BG, Krishna V, Santosh Kumar HS, Khadeer Ahamed BM, Sharath R, Kumara Swamy HM (2008): Wound healing activity and docking of glycogen-synthase-kinase-3-beta-protein with isolated triterpenoid lupeol in rats. Phytomedicine 15: 763–767.
- Hausen BM, Wollenweber E, Senff H, Post B (1987): Propolis allergy. (I). Origin, properties, usage and literature review. Contact Dermatitis 17: 163–170.
- Hu M, Sabelman EE, Cao Y, Chang J, Hentz VR (2003): Three-dimensional hyaluronic acid grafts promote healing and reduce scar formation in skin incision wounds. J Biomed Mater Res B Appl Biomater 67: 586–592.
- Hayashi K, Komura S, Isaji N, Ohishi N, Yagi K (1999): Isolation of antioxidative compounds from Brazilian propolis: 3,4-Dihydroxy-5-prenylcinnamic acid, a novel potent antioxidant. Chem Pharm Bull 47: 1521–1524.
- Khalil ML (2006): Biological activity of bee propolis in health and disease. Asian Pac J Cancer Prev 7: 22–31.
- Kokate CK (2001): Pharmacognosy. Mumbai, Nirali Prakasham.
- Koo H, Gomes BP, Rosalen PL, Ambrosano GM, Park YK, Cury JA (2000) In vitro antimicrobial activity of propolis and Arnica montana against oral pathogens. Arch Oral Biol 45: 141–148.
- Kujumgiev A, Tsvetkova I, Serkedjieva Y, Bankova V, Christov R, Popov S (1999): Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J Ethnopharmacol 64: 235–240.
- Kumazawa S, Taniguchi M, Suzuki Y, Shimura M, Kwon MS, Nakayama T (2002): Antioxidant activity of polyphenols in carob pods. J Agric Food Chem 50: 373–377.
- Li J, Chen J, Kirsner R (2007): Pathophysiology of acute wound healing. Clin Dermatol 25: 9–18.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951): Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275.
- Mahran LG, el-Khatib AS, Agha AM, Khayyal MT (1996): The protective effect of aqueous propolis extract on isolated rat hepatocytes against carbon tetrachloride toxicity. Drugs Exp Clin Res 22: 309–316.
- Maier CM, Chan PH (2002): Role of superoxide dismutases in oxidative damage and neurodegenerative disorders. Neuroscientist 8: 323–334.
- Mani F, Damasceno HC, Novelli EL, Martins EA, Sforcin JM (2006): Propolis: Effect of different concentrations, extracts and intake period on seric biochemical variables. J Ethnopharmacol 105: 95–98.
- Merino N, González R, González A, Remirez D (1996): Histopathological evaluation on the effect of red propolis on liver damage induced by CCl4 in rats. Arch Med Res 27: 285–289.
- Meyer-Ingold W (1993): Wound therapy: Growth factors as agents to promote healing. Trends Biotechnol 11: 387–392.
- Minor RR (1980): Collagen metabolism: A comparison of diseases of collagen and diseases affecting collagen. Am J Pathol 98: 225–280.
- Mirzoeva OK, Calder PC (1996): The effect of propolis and its components on eicosanoid production during the inflammatory response. Prostaglandins Leukot Essent Fatty Acids 55: 441–449.
- Morton JJ, Malone MH (1972): Evaluation of vulneray activity by an open wound procedure in rats. Arch Int Pharmacodyn Ther 196: 117–126.
- Nayak S, Nalabothu P, Sandiford S, Bhogadi V, Adogwa A (2006): Evaluation of wound healing activity of Allamanda cathartica. L. and Laurus nobilis. L. extracts on rats. BMC Complement Altern Med 6: 12.
- Neuman RE, Logan MA (1950): The determination of collagen and elastin in tissues. J Biol Chem 186: 549–556.
- Peacock EE (1984): Contraction. In Peacock EE, ed. Wound Repair. Philadelphia,WB Saunders, 39–55.
- Perez Gutierrez RM, Vargas S R (2006): Evaluation of the wound healing properties of Acalypha langiana in diabetic rats. Fitoterapia 77: 286–289.
- Russo A, Longo R, Vanella A (2002): Antioxidant activity of propolis: Role of caffeic acid phenethyl ester and galangin. Fitoterapia 73: S21–S29.
- Salatino A, Teixeira EW, Negri G, Message D (2005): Origin and chemical variation of Brazilian propolis. Evid Based Complement Altern Med 2: 33–38.
- Scortichini M, Pia Rossi M (1991): Preliminary in vitro evaluation of the antimicrobial activity of terpenes and terpenoids towards Erwinia amylovora (Burrill). J Appl Bacteriol 71: 109–112.
- Sforcin JM (2007): Propolis and the immune system: A review. J Ethnopharmacol 113: 1–14.
- Shigemi T (2002): Application of the material of honeybee origin. Analysis of the constituents, chemical evaluation and tyrosinase inhibition of propolis. Fragr J 30: 25–32.
- Shukla S, Bhadauria M, Jadon A (2004): Effect of propolis extract on acute carbon tetrachloride induced hepatotoxicity. Indian J Exp Biol 42: 993–997.
- Singleton VL, Orthofer R, Lamuela-Raventos RM (1999): Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol 299: 152–178.
- Subramanian S, Sathish Kumar D, Arulselvan P (2006): Wound healing potential of Aloe vera leaf gel studied in experimental rabbits. Asian J Biochem 1: 178–185.
- Teixeira EW, Negri G, Meira RM, Message D, Salatino A (2005): Plant origin of green propolis: Bee behavior, plant anatomy and chemistry. Evid Based Complement Altern Med 2: 85–92.
- Tomlinson A, Ferguson MW (2003): Wound healing: A model of dermal wound repair. Methods Mol Biol 225: 249–260.
- Tsuchiya H, Sato M, Miyazaki T, Fujiwara S, Tanigaki S, Ohyama M, Tanaka T, Iinuma M (1996): Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J Ethnopharmacol 50:27–34.
- Volpi N, Bergonzini G (2006): Analysis of flavonoids from propolis by on-line HPLC-electrospray mass spectrometry. J Pharm Biomed Anal 42: 354–361.
- Whitney JD (2005): Overview: Acute and chronic wounds. Nurs Clin North Am 40: 191–205.
- Woessner JF Jr (1961): The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 93: 440–447.
- Woisky RG, Salatino A (1998): Analysis of propolis: Some parameters and procedures for chemical quality control. J Agric Res 37: 99–105.