Abstract
Context: Although Colocasia esculenta Linn. (Araceae), commonly known as elephant ear (English), possesses diverse pharmacological activities in animals, little is known about its neuropharmacological activity.
Objective: The present study evaluated the neuropharmacological activities of hydroalcoholic extract of leaves of Colocasia esculenta (HECE) using several experimental models.
Materials and methods: Adult Wistar albino rats were subjected to behavior despair and elevated plus maze (EPM) tests. Thiopental-induced sedation and rotarod tests were conducted on Swiss albino mice.
Results and discussion: The effects of HECE on anxiety, depression, thiopental-induced sleeping time, and rotarod performance were evaluated. The anxiolytic activity of HECE (100, 200, and 400 mg/kg) per os (p.o.) was characterized by increased time spent and number of entries in open arms in the EPM paradigm as compared to control group (p < 0.001). The HECE (100, 200, and 400 mg/kg, p.o.) showed dose-dependent significant reduction in duration of immobility (p < 0.01) in the behavior despair test. The HECE at the doses 50 and 100 mg/kg, i.p. was found to produce a significant reduction in motor coordination (p < 0.001) and prolongation of thiopental-induced sleeping time (p < 0.001). The phytochemical screening revealed the presence of flavonoids, β-sitosterol, and steroids.
Conclusions: The results of the study for the first time show that the plant possesses neuropharmacological activity, confirming the traditional claims. Future research should focus on the identification and the neurobehavioral activity of the constituents from this plant.
Introduction
Medicinal herbs constitute the cornerstone of traditional medicinal practice worldwide. These herbs are relatively cheap, widely available, and their use depends on ancestral experience (CitationAmos et al., 2001). The medicinal plants represent a great untapped reservoir of drugs and the structural diversity of their component molecules makes them a valuable source of novel lead compounds (CitationNewman & Cragg, 2007). Colocasia esculenta Linn. (Araceae), commonly known as elephant ear in English, is a tall herb found throughout the hotter parts of India and Ceylon. It has been used traditionally as an expectorant, astringent, appetizer, otalgia, laxative, demulcent, and to treat internal hemorrhages (CitationCSIR, 2005). The plant is also useful as a nerve tonic, in cases of inflamed glands, piles, and diarrhea (CitationKirtikar & Basu, 2005). Cyanoglucoside from C. esculenta has been implicated as an aggravating factor in neurological disorders (CitationGrindley et al., 2002).
Although the plant is traditionally claimed to possess diverse pharmacological actions including neuropharmacological activity, only the anti-inflammatory (CitationShah et al., 2007) and anticancer activities (CitationBrown et al., 2005) have been studied. Considering the available information and folklore use of the plant, the present study was designed to evaluate the neuropharmacological profiles of the hydroalcoholic extract of leaves of C. esculenta (HECE) in various experimental models.
Methods
Collection of plant materials
Leaves of C. esculenta were purchased from a local market. The plant was identified and authenticated by P. Parmar, Botanical Survey of India, Jodhpur, India. A voucher specimen (SU/DPS/Herb/05) of the same has been deposited in the Department of Pharmaceutical Sciences, Saurashtra University, Rajkot for future reference.
Preparation of plant extract
Leaves were dried in shade, moderately ground by electric grinder and macerated with ethanol and water (50:50) for 7 days with intermittent shaking. On day 8, the macerate was filtered through muslin cloth and the solvent was completely removed under reduced pressure to give the hydroalcoholic extract (yield 9.8% w/w). The extract was stored in a refrigerator and prepared freshly in sodium carboxy methyl cellulose (SCMC) solution just before the experiments. The HECE was subjected to phytochemical investigations (CitationTrease & Evans, 2008).
Experimental animals
Male Wistar albino rats (250-300 g) were subjected to the elevated plus maze (EPM) and behavior despair tests (n = 5). Thiopental-induced sleeping time and rotarod tests were conducted using Swiss albino mice (25-30 g) (n = 5). All the animals were housed in groups in polypropylene cages and placed in a climate-controlled central animal house having temperature 22° ± 2°C, relative humidity 60% ± 5%, and a 12 h light/dark cycle (lights on at 08:00 h and off at 20:00 h). The animals were fed standard pellet diet (Amrut, Pranav Agro Industries, Baroda, Gujarat, India) and water ad libitum. All the protocols were approved (SU/DPS/IAEC/9003) by the Institutional Animal Ethics Committee (IAEC) of the Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA), Ministry of Environment and Forests, Government of India.
Drugs and chemicals
Imipramine was obtained as a gift sample from Torrent Research Centre, Ahmedabad, Gujarat, India. Diazepam and thiopental were purchased from Sigma (St. Louis, MO). The solvents used were of analytical grade.
Administration of drugs
Imipramine and thiopental were dissolved in distilled water, while diazepam and HECE were prepared as suspension in distilled water using 0.5% SCMC as the suspending agent. Animals were assigned to different treatment groups (n = 5). The control group received the vehicle (0.5% SCMC, 1 mL/kg) per os (p.o.), whereas different treatment groups received HECE, imipramine or diazepam. All drugs and HECE were prepared just before experimentation. All the doses of HECE were administered orally, whereas standard drugs were administered intraperitoneally (i.p.).
Acute toxicity study
The acute toxicity study was performed as per the method described by CitationLitchfield and Wilcoxon (1949) and LD50 was calculated accordingly. Briefly, the HECE in the dose range of 10–1600 mg/kg was administered intraperitoneally to different groups of mice (n = 10). The animals were examined every 30 min up to a period of 3 h and then, occasionally for an additional period of 4 h; finally, overnight mortality was recorded. All tests on rats were performed at three dose levels 100, 200, and 400 mg/kg, p.o. body weight corresponding to 10, 20, and 40% of LD50 value (1000 mg/kg, i.p.), respectively. The dose selected for thiopental-induced sleeping time and rotarod test in mice was 50 and 100 mg/kg, i.p.
Elevated plus maze test
The method of CitationPellow et al. (1985) was followed. Briefly, the EPM apparatus comprised two open arms (50 cm × 10 cm) and two closed arms (50 cm × 10 cm × 50 cm) that extended from a common central platform (10 cm × 10 cm). The floor and the walls of each arm were wooden and painted black. The entire maze was elevated to a height of 25 cm above floor level. Testing was conducted in a quiet room that was illuminated only by a dim light. Rats were individually placed on the centre of the maze facing an open arm. The number of entries and the time spent in open arms were recorded over a period of 5 min. Arm entries were recorded when rat enters all its four paws into an arm. The percentage of open arm entries (100 × open/total entries) was calculated for each animal. The experimental animals were pretreated with diazepam (5 mg/ kg, i.p.) or HECE (100, 200, and 400 mg/kg, p.o.) and 0.5% SCMC (control group) treatments were given 60 min prior to the test. The standard group animals received diazepam (5 mg/ kg, i.p.) 30 min before the commencement of experiment. Diazepam (5 mg/kg, i.p.) was used as standard anxiolytic drug. Between each trial, the maze was wiped clean with a damp sponge and dried with paper towels to ensure uniform results (CitationEmamghoreishi et al., 2005).
Behavior despair test
The procedure described by CitationPorsolt et al. (1978a) was followed with slight modification of the deep water level suggested by CitationDetke et al. (1995) to ensure that rats could not support themselves by touching the bottom with their feet. Male rats were used for this test (CitationPadovan & Guimaraes, 2004; CitationCalil & Marcondes, 2006). Swimming sessions were conducted by placing rats in an individual glass cylinder (35 cm × 25 cm) containing water (25° ± 1°C) 27 cm deep. Two swimming sessions were conducted between 9:00–16:00 h. All the rats were subjected to an initial 15-min pretest followed 25 h later by a 5-min test. Drugs were administered three times during the period between these two sessions, the first immediately after the pretest session and then, after 6 and 23 h of the first dose. Following both swimming sessions, the rats were removed from the cylinder, dried with paper towels, placed in the cages under a heating source (15 min), and returned to their home cages. The immobility period was measured in seconds in each test session of 5 min. The water in the cylinder was changed after every other trial. Imipramine (12.5 mg/kg, i.p.) served as standard drug in this model (CitationBhattamisra et al., 2008).
Thiopental-induced sleeping time
The procedure of CitationSukma et al. (2002) was followed. Briefly, 30 min after the administration of HECE (50 and 100 mg/kg, i.p.) or SCMC (0.5%, i.p.), mice received thiopental (50 mg/kg, i.p.). The time elapsed from the thiopental injection to loss of the righting reflex was taken as sleeping latency. The time elapsed between the loss and voluntary recovery from the righting reflex was considered as the total sleeping time.
Test for motor coordination (rotarod performance)
The test was conducted as per the method described by CitationIbarrola et al. (2006). Briefly, mice were placed on a rotarod apparatus the whole instrument (including rod) is divided into six equal compartments consisting of a horizontal rotating rod having 2.5 cm diameter with rotating speed 12 rpm and divided in six equal compartments. The animals remaining on the rod for two or more min in two successive trials were selected for the test. Animals were divided into four groups: control, diazepam-treated (0.5 mg/kg, i.p.), and HECE-treated (50 and 100 mg/kg, i.p.) groups. After 30 min of various treatments, they were placed on the spinning bar of the rotarod apparatus for 1 min. The time spent (in sec) on the rotating rod was recorded.
Statistical analysis
All the data were expressed as mean ± SEM from five animals. The data obtained was analyzed using the one-way ANOVA followed by Student-Newman-Keuls test for determining the level of significance and p < 0.05 was considered statistically significant.
Results
Acute toxicity studies
The acute toxicity studies showed that the LD50 of the HECE in mice was 1000 mg/kg by i.p. route. Preliminary phytochemical tests indicated the presence of flavonoids, β-sitosterol, and steroids in the plant. The HECE did not show the presence of alkaloids.
Elevated plus maze
As shown in , the control animals showed more preference for the closed (dark) arms and exhibited anxiety-like symptoms characterized by immobility, freezing, and defecation on entering the open arms. As compared to the control group, the HECE-treated (100, 200, and 400 mg/kg, p.o.) animals showed significant increase in total number of entries (p < 0.01) and the time spent in the open arms. Moreover, HECE reduced anxiety-like symptoms in a dose-dependent manner. Diazepam (5 mg/kg, i.p.), a standard drug, significantly increased the number of entries as well as time spent in the open arms (p < 0.001), indicating anxiolytic activity. The higher dose of HECE (400 mg/kg, p.o.) produced a peak anxiolytic effect that is well comparable to diazepam (p < 0.001).
Table 1. Effect of hydroalcoholic extract of C. esculenta leaves in the elevated plus maze testa.
Behavior despair test
shows the antidepressant effect of HECE and imipramine in the experimental animals. The control animals remained immobile for most of the time during the test session. HECE (100, 200, and 400 mg/kg, p.o.) induced a dose-dependent significant reduction in the immobility time of rats (p < 0.01) as compared to the control group. In the same experimental conditions, the antidepressant activity of the reference drug imipramine (12.5 mg/kg, i.p.) was clearly evident (p < 0.01). The antidepressant effect produced by HECE (400 mg/ kg, p.o.) was comparable to that of imipramine.
Table 2. Effect of hydroalcoholic extract of C. esculenta leaves on the immobility period during behavior despair testa.
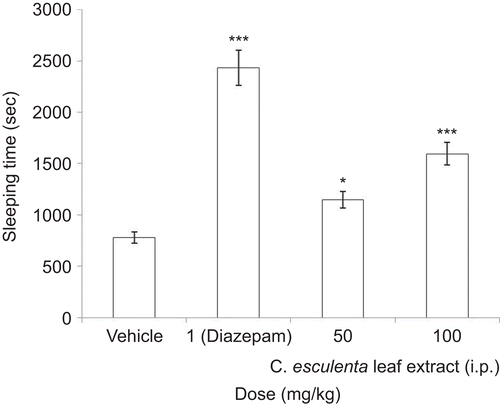
Thiopental-induced sleeping time
As shown in , HECE (50 and 100 mg/kg, i.p.) showed dose-dependent prolongation of thiopental-induced sleeping time as compared to the control group. Prior treatment of diazepam (1 mg/kg, i.p.) potentiated thiopental-provoked sleep.
Test for motor coordination (rotarod performance)
As shown in , diazepam (0.5 mg/kg, i.p.) and HECE (50 and 100 mg/kg, i.p.) exhibited a marked reduction in motor coordination (p < 0.001) test. Diazepam and HECE-treated mice were unable to hold onto the rotating rod.
Table 3. Effect of hydroalcoholic extract of C. esculenta leaves on muscle relaxant activity in rotarod performancea.
Discussion
C. esculenta is traditionally used for the treatment of anxiety, depression, and other CNS disorders. Scientific data on these properties of the plant are not available. Therefore, we investigated the effects of different doses of HECE using several neuropharmacological models. EPM is one of the most widely used models of animal anxiety (CitationHogg, 1996; CitationRodgers et al., 1997). The anxiety produced in this model is due to natural stimuli, i.e., the fear of a new, brightly-lit open space and of balancing on a relatively narrow raised platform (CitationDawson & Tricklebank, 1995). The frequency and time spent in the open arms is the major index of the anxiety in rodents (CitationPellow & File, 1986; CitationEmamghoreishi et al., 2005).
In the present study, HECE significantly increased number of entries and time spent in the open arms in dose-dependent manner indicating anxiolytic activity. It has been shown that GABAergic neurotransmission plays an important role in stress and anxiety associated with elevated plus maze test (CitationZwanzger & Rupprecht, 2005). It is likely that HECE may have modulated the benzodiazepine or other sites of GABA receptors to produce the anxiolytic effect. Several studies report that flavonoids isolated from Passiflora coerulea (CitationWolfman et al., 1994) and Tilia americana (CitationHerrera-Ruiz et al., 2008) possess anxiolytic activity. Consistent with these studies, the flavonoids apigenin and luteolin found in C. esculenta leaves (CitationIwashina et al., 1999) are likely to exhibit the anxiolytic effect.
The behavior despair test has been validated as a suitable tool to evaluate drugs with putative antidepressant effects (CitationPorsolt et al., 1978a; CitationAnisman & Matheson, 2005; CitationMatthews et al., 2005). In this model, when rodents are forced to swim in a confined space, they tend to become immobile after vigorous activity (struggling). This inescapable stressful situation leads to depression (CitationPorsolt et al., 1978b). In the present study, administration of HECE significantly reduced total immobility time and enhanced struggling behavior in a dose-dependent manner, suggesting an antidepressant effect. It is reported that GABA, an inhibitory neurotransmitter is involved in the pathophysiology of depression. Moreover, neurochemical research has revealed that the monoamines (5-HT, NA, and dopamine) have a crucial role in the development of the depression syndrome (CitationNaughton et al., 2000). The antidepressant effect of the HECE may be attributed to the modulation of one or more of these neurotransmitters. It has been found that flavonoids isolated from plant species such as Hypericum perforatum (CitationButterweck et al., 2000) showed antidepressant activity. Thus, it is likely that flavonoids present in HECE may be responsible for the observed antidepressant effect.
The anxiolytic and antidepressant effects shown by HECE suggest etiological similarity in the development of anxiety and depression. Several hypotheses have been proposed to explain this aspect. The serotonergic theory postulates excessive functioning of the serotonergic neurotransmission as the cause of depression and anxiety (CitationDeakin, 1983). Another theory proposes involvement of GABAergic neurotransmission, which forms the basis of action of anxiolytic activity of many drugs, may also be involved in the antidepressant activity (CitationLloyd et al., 1989). It can be hypothesized that HECE may have acted by modulating one or more of the above mentioned neurotransmitters.
In the thiopental-induced sleep test, HECE potentiated the effect of thiopental. The prolongation of thiopental-induced sleeping time may be attributed to an inhibition of thiopental metabolism or to an action on the central mechanism involved in the regulation of sleep (CitationN’Gouemo et al., 1994). Thus, suggesting HECE as a mild neurosedative drug (CitationCapasso et al., 1996). HECE significantly decreased motor activity in the rotarod performance test. Reduced motor coordination indicates mild sedative action (CitationMasur et al., 1971), supporting results obtained from thiopental-induced sleep test.
Phytochemical screening revealed presence of flavonoids, steroids, and β-sitosterol. It can be recalled that several constituents from plants are known to possess neurobehavioral effects in experimental animals. For instance, steroids have been reported as potent sedative agents that inhibited spontaneous motor activity in mice (CitationDubois et al., 1986). Similarly, flavonoids are reported to possess anxiolytic effect (CitationWolfman et al., 1994; CitationHerrera-Ruiz et al., 2008) and antidepressant effect (CitationButterweck et al., 2000). Therefore, it is likely that flavonoids and steroidal content of this extract might be contributing in part to the observed neuropharmacological activity.
Conclusions
The present study for the first time provides evidence for the neuropharmacological activity of HECE in experimental animals. The presence of flavonoids, β-sitosterol, and steroids in HECE could be responsible for these activities. The need of the hour is to identify and isolate the phytoconstituents responsible for the observed central effects in animals and to understand their molecular mechanisms.
Acknowledgements
We are grateful to the Head, Department of Pharma-ceutical Sciences, Saurashtra University, Rajkot, Gujarat, India for providing the facilities during the course of this study. Special thanks to Professor P. Parmar, Botanical Survey of India for identification and authentication of the plant. A gift sample of imipramine by Torrent, Gandhinagar, India is gratefully acknowledged.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Amos S, Adzu B, Binda L, Wambebe C, Gamaniel K. (2001). Neuropharmacological effect of the aqueous extract of Sphaeranthus senegalensis in mice. J Ethnopharmacol, 78, 33–37.
- Anisman H, Matheson K. (2005). Stress, depression, and anhedonia: Caveats concerning animal models. Neurosci Biobehav Rev, 29, 525–546.
- Bhattamisra SK, Khanna VK, Agrawal AK, Singh PN, Singh SK. (2008). Antidepressant activity of standardised extract of Marsiela minuta Linn. J Ethnopharmacol, 117, 51–57.
- Brown AC, Reitzenstein JE, Liu J, Jadus MR. (2005). The anti-cancer effects of poi (Colocasia esculenta) on colonic adenocarcinoma cells in vitro. Phytother Res, 19, 767–771.
- Butterweck V, Jurgenliemk G, Nahrstedt A, Winterhoff H. (2000). Flavonoids from Hypericum perforatum show antidepressant activity in the forced swimming test. Planta Med, 66, 3–6.
- Calil CM, Marcondes FK. (2006). The comparison of immobility time in experimental rat swimming models. Life Sci, 79, 1712–1719.
- Capasso A, De Feo V, De Simone F, Sorrentino L. (1996). Pharma-cological effects of aqueous extract from Valeriana adscendeus. Phytother Res, 10, 309–312.
- CSIR (Council for Scientific and Industrial Research) (2005). The Wealth of India (Raw Materials). New Delhi, India: NISCAIR Press, 225.
- Dawson GR, Tricklebank MD. (1995). Use of the elevated plus maze in the search for novel anxiolytic agents. Trends Pharmacol Sci, 16, 33–36.
- Deakin JFW. (1983). Role of serotogenic system in escape, avoidance and other behaviors. In: Cooper SJ, ed. Theory in Psychopharmacology. London: Academic Press, 149–193.
- Detke MJ, Lucki I, Rickels M. (1995). Active behaviours in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology, 121, 66–72.
- Dubois MA, Ilyas M, Wagner H. (1986). Cussonosides A and B, two triterpenes-saponins from Cussonia barteri. Planta Med, 52, 80–83.
- Emamghoreishi M, Khasaki M, Aazam MF. (2005). Coriandrum sativum: Evaluation of its anxiolytic effect in the elevated plus-maze. J Ethnopharmacol, 96, 365–370.
- Grindley BA, Omoruyi F, Asemota HN, Morrisona A. (2002). Carbohydrate digestion and intestinal ATPases in streptozotocin-induced diabetic rats fed extract of yam (Dioscorea cayenensis) or dasheen (Colocasia esculenta). Nutr Res, 22, 333–341.
- Herrera-Ruiz M, Roman-Ramos R, Zamilpa A, Tortoriello J, Jimenez-Ferrer JE. (2008). Flavonoids from Tilia americana with anxiolytic activity in plus-maze test. J Ethnopharmacol, 118, 312–317.
- Hogg S. (1996). A review of the validity and variability of the elevated plus maze as an animal model of anxiety. Pharmacol Biochem Behav, 54, 21–30.
- Ibarrola MC, Ibarrola DA, Montalbetti Y, Kennedy ML, Heinichen O, Campuzano M, Tortoriello J, Wasowski C, Marder M, De Lima TC, Mora S. (2006). The anxiolytic-like effects of Aloysia polystachya (Griseb.) Moldenke (Verbenaceae) in mice. J Ethnopharmacol, 105, 400–408.
- Iwashina T, Konishi T, Takayama A, Fukada M, Ootani S. (1999). Isolation and identification of the flavonoids in the leaves of taro. Ann Tsukuba Bot Gard, 18, 71–74.
- Kirtikar KR, Basu BD. (2005). Indian Medicinal Plants. Vol. 4, Dehradun: International Book Distributors, 2615.
- Litchfield JT, Wilcoxon F. (1949). A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther, 96, 99–113.
- Lloyd KG, Zivkovic B, Scatton B, Morselli PL, Bartholoni G. (1989). The GABAergic hypothesis of depression. Prog Neuropsycho-pharmacol Biol Psychiatry, 13, 341–351.
- Masur J, Martz RMW, Carlini EA. (1971). Effects of acute and chronic administration of Cannabis sativa and (−) delta9-trans-tetrahydrocannabinol on the behavior of rats in an open-field arena. Psychopharmacology, 19, 388–397.
- Matthews K, Christmas D, Swan J, Sorrell E. (2005). Animal models of depression: Navigating through the clinical fog. Neurosci Biobehav Rev, 29, 503–513.
- N’Gouemo P, Nguemby-Bina C, Baldy-Moulinier M. (1994). Some neuropharmacological effects of an ethanolic extract of Maprounea africana in rodents. J Ethnopharmacol, 43, 161–166.
- Naughton M, Mulrooney JB, Leonard BE. (2000). A review of the role of serotonin receptors in psychiatric disorders. Hum Psychopharmacol, 15, 397–415.
- Newman DJ, Cragg GM. (2007). Natural products as sources of new drugs over the last 25 years. J Nat Prod, 70, 461–477.
- Padovan CM, Guimaraes FS. (2004). Antidepressant-like effects of NMDA-receptors antagonist injected into the dorsal hippocampus of rats. Pharmacol Biochem Behav, 77, 15–19.
- Pellow S, Chopin P, File SE, Briley M. (1985). Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods, 14, 149–167.
- Pellow S, File SE. (1986). Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol Biochem Behav, 24, 525–529.
- Porsolt RD, Anton G, Blavet N, Jalfre M. (1978a). Behavioral despair in rats: A new model sensitive to antidepressant treatments. Eur J Pharmacol, 47, 379–391.
- Porsolt RD, Bertin A, Jalfre M. (1978b). “Behavioral despair” in rats and mice: Strain differences and the effects of imipramine. Eur J Pharmacol, 51, 291–294.
- Rodgers RJ, Cao BJ, Dalvi A, Holmes A. (1997). Animal models of anxiety: An ethological perspective. Braz J Med Biol Res, 30, 289–304.
- Shah BN, Nayak BS, Bhatt SP, Jalalpure SS, Seth AK. (2007). The anti-inflammatory activity of the leaves of Colocasia esculenta. Saudi Pharm J, 15, 228–232.
- Sukma M, Chaiyo C, Murakami Y, Tohda M, Matsumoto K, Watanabe H. (2002). CNS inhibitory effects of barakol, a constituent of Cassia siamia Lamk. J Ethnopharmacol, 83, 87–94.
- Trease EG, Evans WC. (2008). Textbook of Pharmacognosy. 15th edn, New Delhi, India: Alden Press, 539–541.
- Wolfman C, Viola H, Paladini A, Dajas F, Medina JH. (1994). Possible anxiolytic effects of chrysin, a central benzodiazepine receptor ligand isolated from Passiflora coerulea. Pharmacol Biochem Behav, 47, 1–4.
- Zwanzger P, Rupprecht R. (2005). Selective GABAergic treatment for panic? Investigations in experimental panic induction and panic disorder. J Psychiatry Neurosci, 30, 167–175.