Abstract
Context: Among strategies to combat malaria, the search for newer antimalarial compounds is a priority. Traditionally, Tagetes erecta Linn. (Compositae) has been used for the treatment of various diseases and ailments including malaria.
Objective: Five successive extracts (petroleum ether, chloroform, ethyl acetate, methanol and aqueous) of the roots of T. erecta and a new bithienyl compound, 2-hydroxymethyl-non-3-ynoic acid 2-[2,2']-bithiophenyl-5-ethyl ester from the roots of the plant, were evaluated for in vitro antiplasmodial activity against chloroquine sensitive and resistant strains of Plasmodium falciparum. The extracts were also tested for in vitro antimicrobial activity against seven microbial strains.
Materials and methods: The antiplasmodial screening was carried out using the schizont maturation inhibition assay. Preliminary antimicrobial screening was carried out using the agar well assay followed by determination of minimum inhibitory concentration (MIC) using two-fold serial dilutions.
Results: Among all the extracts tested, the ethyl acetate fraction exhibited significant antiplasmodial efficacy with the 50% inhibitory concentrations (IC50) of 0.02 and 0.07 mg/mL against the chloroquine sensitive and resistant strains of Plasmodium falciparum respectively. The new bithienyl compound also showed significant schizonticidal activity against both chloroquine sensitive and resistant strains of Plasmodium falciparum with the IC50 values of 0.01 and 0.02 mg/mL. Additionally, all extracts exhibited significant antimicrobial activity against three Gram-positive and two Gram-negative bacterial and two fungal strains with MIC values ranging between 12.5-100 µg/mL.
Discussion: The new bithienyl compound was profoundly able to arrest the ring stages of the malarial parasites thereby exerting its antiplasmodial effect.
Conclusion: The observations provide support for the ethnobotanical use of the plant.
Introduction
Malaria continues to be a disease of great concern as it causes morbidity and mortality on a large scale in tropical countries. The World Health Organization (WHO) estimates that there are around 300 to 500 million malaria cases annually, directly causing 1 million deaths and contributing to a further 1.7 million deaths (CitationWHO, 2002). India alone accounts for about 70% of the total malaria cases, out of 2.5 million reported cases in south-east Asia (CitationDash et al., 2008). In the state of West Bengal alone, malaria is responsible for around 28,500 clinical cases a year, of which more than 10% cases are Falciparum malaria (CitationRoy & Saha, 2005). Despite the discovery of new effective natural and synthetic antiplasmodial compounds, malaria remains a major endemic disease, as several factors (i.e., drug resistance, insecticide resistance, lack of knowledge, etc.) pose a serious challenge for complete eradication of the disease. Hence, to combat malaria there is a pressing need to eradicate mosquito vectors, and find newer antimalarial compounds and newer effective vaccines. However, the development of an effective vaccine has proven very difficult (CitationChattopadhyay & Kumar, 2009), and therefore the ethnobotanical investigations in traditional medicines may provide important sources of newer antimalarial compounds, e.g., the first antimalarial drug, quinine from Cinchona succirubra Pav. (Rubiaceae) bark and later artemisinin from Artemisia annua Linn. (Asteraceae). At present there has been a remarkable increase in the existence of potent antibacterial and antifungal agents that are used for therapy of a wide variety of microbial diseases. But because of the unprecedented increase in cases of antimicrobial drug resistance, mankind has been facing serious microbial threats. Therefore, there exists a need for newer agents, particularly from the traditional database for the control of such prevalent and recurring infectious diseases throughout the world.
Tagetes erecta Linn. (Compositae), commonly known as Aztec or African marigold, is a stout, branching herb extensively cultivated as a border annual in gardens all over India, branching herb extensively cultivated as a border annual in gardens all over India. Traditionally, the plant has been used for the treatment of a wide variety of diseases and ailments. Infusion of the plant has been used against rheumatism, cold and bronchitis, and juice of the leaves for earache (CitationCSIR, 1976). The florets are used for the treatment of eye diseases and ulcers, and extract of the roots has been credited as a laxative (CitationKirtikar & Basu, 1975). The essential oil from the aerial parts of T. erecta has shown antimicrobial efficacy against various strains of Gram-positive and Gram-negative bacteria and fungi (CitationGrover & Rao, 1978; CitationGarg & Dengre, 1986). The extracts of the aerial parts of T. erecta have also shown significant antimicrobial activity against several strains of bacteria and fungi (CitationNanir & Kadu, 1987; CitationPenna et al., 1994). The plant is also useful for other conditions such as hyperkeratotic plantar lesions (CitationKhan et al., 1996) and parakeratosis (CitationKhan & Evans, 1996). Phytochemical screening of various parts of the plant revealed the presence of flavonoids (CitationDas & Tripathi, 1997; CitationEl-Emary & Ali, 1983), phenols (CitationTripathy & Gupta, 1991), carotenoids (CitationHadden et al., 1999), 5-(but-1-ol-ynyl)-2,2'-bithienyl and methyl-3,5-dihydroxy-4-methoxy benzoate (CitationTripathy et al., 1992).
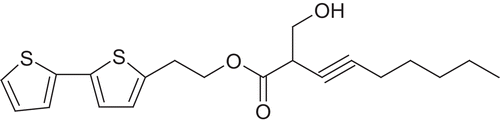
A decoction of the leaves of T. erecta has traditionally been used for the treatment of malaria in Madagascar and the thienyl compounds from the roots have been reported for their insecticidal and nematocidal activities (CitationRasoanaivo et al., 1992; CitationVasudevan et al., 1997). T. erecta is a widely cultivated potential ornamental plant, commonly known as marigold, native of Mexico and South America, naturalized elsewhere in the tropics and subtropics and introduced to India by the Portuguese (CitationJanakiram & Rao, 1996). Previous studies from our laboratory had isolated and reported a new bithienyl constituent, 2-hydroxymethyl-non-3-ynoic acid 2-[2,2']-bithiophenyl-5-ethyl ester () from the roots of T. erecta (CitationVasudeva et al., 2007). The present study investigates the in vitro antiplasmodial and antimicrobial potential of the newly reported bithienyl compound (compound 1) along with the five successive extracts, i.e., petroleum ether, chloroform, ethyl acetate, methanol and aqueous extracts of the roots of T. erecta.
Methods
Plant material and extraction
The roots (4 kg) of T. erecta were collected in April 2005 from the Hisar district of Haryana, India. The plant was authenticated by H.B. Singh, taxonomist at National Institute of Science Communication and Information Resources (NISCAIR), New Delhi, and a voucher specimen (no. GJU/HS-509) has been retained at the Department of Pharmacognosy, Faculty of Pharmaceutical Sciences, Guru Jambheshwar University of Science and Technology, Hisar, Haryana. The roots were washed with water and air-dried at room temperature (30–40°C) and thereafter ground to pass through a 1-mm sieve. The dried powdered root (1 kg) was then successively percolated in petroleum ether (5 L), chloroform (5 L), ethyl acetate (5 L), methanol (5 L) and distilled water (5 L), for 7 days, with each solvent. The extracts were filtered and concentrated to dryness using a rotary evaporator. The extracts were stored at 4°C until analysis.
Parasite strains and in vitro culture
Chloroquine sensitive (MRC-pf-2) and chloroquine resistant (MRC-pf-56) strains of Plasmodium falciparum were obtained from the Malaria Parasite Bank maintained by the National Institute of Malaria Research (Indian Council of Medical Research), New Delhi, India. The cultures were maintained in the laboratory using the candle jar method (CitationTrager & Jensen, 1976) in human red blood cells (blood type O+) at a 5% hematocrit in RPMI-1640 medium (Sigma) with 25 mM HEPES, 0.2% sodium bicarbonate and 15% human AB+ serum.
Micro-organism strains and in vitro culture
Staphylococcus aureus NCIM2901, Bacillus subtilis NCIM2063, Micrococcus luteus MTCC1541, Escherichia coli NCIM2065, Pseudomonas aeruginosa ATCC27853, Candida albicans ATCC10231 and Aspergillus niger NCIM590 were obtained from the National Chemical Laboratory (NCL), Pune, India. The bacterial cultures were maintained on nutrient agar (HiMedia, India) by subculturing them on fresh slants after every four weeks and incubating them at 37° ± 1°C for 24 h. The cultures of Candida albicans and Aspergillus niger were maintained on Sabouraud dextrose agar and potato dextrose agar (Hi-media) by sub-culturing them on fresh slants and incubating them at 28 ± 1°C, for 2 days and 7 days, respectively. All stock cultures were stored at 4°C.
In vitro antiplasmodial test
The antiplasmodial screening of the successive extracts was carried out using the in vitro schizont maturation inhibition assay (CitationValecha et al., 1994) at the National Institute of Malaria Research, Delhi, India. The parasite cultures, prior to experimentation, were synchronized by treatment with 5% d-sorbitol (CitationLambros & Vanderburg, 1979). Synchronized cultures containing ring-staged parasites were suspended in equal volumes of human serum. Stock solutions were prepared separately by weighing 1 mg of dried herbal extracts of the plant on a Shimadzu balance having sensitivity of 0.1 mg and dissolving them in a minimum volume (10 µL) of 100% dimethylsulfoxide (DMSO) and finally diluting with serum free medium to a concentration of 1 mg/mL. Serial double dilutions of each set of plant extracts were made in triplicates in 96-well microtiter plates (Axygen, Germany) with concentration ranging from 0.5–0.01 mg/mL against a negative control containing the serum-free medium with same concentration of DMSO. In each well 100 µL of the diluted extract, 10 µL parasitized blood (4–5% rings) in 100 µL incomplete medium (without serum) and 5% hematocrit were added. Chloroquine (0.5 mg/mL to 0.15 µg/mL) was used as a positive control and four wells of the last row were set as general controls to check the normal growth of the parasites.
Schizont maturation time was calculated from the growth of the parasites cultured in general control wells. Accordingly, thin smears were drawn (approximately 24-28 h of incubation) from each of the experimental and control wells on properly labeled slides. The blood smears were air dried and fixed in methanol. Dried slides were stained using Jaswant Singh Bhattacherji (JSB)-stain and observed with oil immersion under microscope (100 ×) for the study of parasitemia, particularly the inhibition of schizont maturation. Numbers of schizonts were counted per 200 asexual stage parasites. The values were compared between test and control wells. The percentage of inhibition was calculated as: 100 - a, where ‘a’ is the percentage of schizonts in the test wells determined as a = z/m × 100 (m and z are the mean numbers of schizonts per 200 asexual parasites in control and test wells, respectively). The 50% inhibitory concentration (IC50) of all extracts were estimated from the graph drawn on the inhibition (%) data.
In vitro antimicrobial activity
The successive extracts were screened for their in vitro antimicrobial activities against three Gram-positive bacteria (Staphylococcus aureus, Bacillus subtilis and Micrococcus luteus), two Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa) and two fungal strains (Candida albicans and Aspergillus niger) by the agar well assay (CitationBritish Standards Institution, 1968). Minimum inhibitory concentrations (MIC) of individual extracts were determined by the tube dilution method (CitationCappucino & Sherman, 1999) using cephalexin (Ranbaxy, Haryana, India) and clotrimazole (Glenmark, Mumbai, India) as standard drugs for antibacterial and antifungal activities, respectively. Stock solutions (1 mg/ mL) of all the extracts were prepared by dissolving in 1% DMSO while the stock solutions of the standard drugs were prepared by dissolving in sterile water for injection for the agar well assay. The micro-organisms were grown in double strength nutrient broth Indian Pharmacopoeia (IP) (bacteria), Sabouraud dextrose broth IP (Candida albicans) or potato dextrose broth IP (Aspergillus niger) and incubated at 37 ± 1°C for 24 h (for all bacterial strains), 28 ± 1°C for 2 days (Candida albicans) and 7 days (Aspergillus niger).
For the agar well assay, the concentration of microbes was adjusted to 50% transmittance under laminar flow hood using sterilized broth as blank. Of the microbial suspension, 1mL (about 106 CFU/ml) was plated with 20 mL melted and cooled, sterile agar medium on sterile Petri dishes. Three wells were drilled on the solidified medium seeded with the test organism using a sterile borer (6 mm diameter). An aliquot of 50 mL of either extract or standard was placed into the well in triplicates and further incubated at the required temperature. The solvents used for the extraction as well as 1% DMSO were used as negative controls. The antimicrobial efficacy was evaluated by measuring the zone of growth inhibition diameter at the end of the incubation period.
The MIC for each extract was determined for each micro-organism by the serial tube dilution method. The test extracts were dissolved in 1% DMSO to give a concentration of 100 µg/mL, while the standards were dissolved in sterile water for injection for obtaining the same concentration. Further dilutions were prepared in double strength nutrient broth (bacteria) and Sabouraud dextrose broth or potato dextrose broth (fungi) with the concentration of the extracts ranging from 50 to 1.56 µg/mL and concentration of the standards ranging from 5 to 0.15 µg/mL. The samples were incubated at 37 ± 1°C (bacteria) for 24 h, 28 ± 1°C for 2 days (Candida albicans) and 7 days (Aspergillus niger).
Statistical analysis
The antimicrobial zone of inhibition data are presented as mean ± standard error mean. The statistical analysis was performed using SigmaPlot 11 software from Systat, California. One-way analysis of variance (ANOVA) was done followed by Dunnett’s test and the value of p <0.05 was considered as significant.
Results
Antiplasmodial activity
Among all the fractions tested, the ethyl acetate extract showed the most significant schizonticidal effect with the IC50 values of 0.02 mg/mL against MRC-pf-2 and 0.07 mg/ mL against MRC-pf-56 strains of Plasmodium falciparum. The IC50 values for the standard drug chloroquine were found to be 0.51 and 1.3 µg/mL against MRC-pf-2 and MRC-pf-56 strains respectively. The remaining four extracts of Tagetes erecta were also profoundly able to arrest the ring stages of the parasites but exhibited schizonticidal activity at relatively higher concentrations in comparison to the ethyl acetate extract and standard (). The mean scores of schizonts per 200 asexual parasites for each individual treatment of tests, i.e. z values were found statistically significant (*p <0.001) in comparison to the general control. The new bithienyl constituent (compound 1) exhibited significant antiplasmodial efficacy against both strains of Plasmodium falciparum with IC50 values of 0.01 and 0.02 mg/mL against MRC-pf-2 and MRC-pf-56, respectively.
Table 1. Percentage (%) inhibition of schizonts by various successive extracts of the roots of Tagetes erecta and the new bithienyl compound 1 on chloroquine sensitive (MRC-pf-2) and chloroquine resistant (MRC-pf-56) strains of Plasmodium falciparum.
Antimicrobial activity
shows the diameter of zone of growth inhibition observed in the agar well assay for all extracts against each tested strain of micro-organism. The values were found statistically significant from standard at *p <0.001. The effect of petroleum ether extract on both B. subtilis and A. niger was half that of the standard. However, among all the extracts tested, the chloroform extract conferred the highest level of inhibition against S. aureus and C. albicans. The new thienyl compound (1) exhibited significant zones of inhibition against all the tested microbes in comparison to all the extracts, with maximum antibacterial effect against B. subtilis and antifungal effect against C. albicans.
Table 2. Antimicrobial activity of successive extracts of Tagetes erecta roots.
shows the MIC values of all extracts against all the tested strains of micro-organisms. The ethyl acetate and the petroleum ether extracts showed significant antibacterial activity against B. subtilis, each with a MIC value of 12.5 µg/mL. On the other hand, the chloroform extract exhibited significant antimicrobial activity against both S. aureus and C. albicans with a MIC value of 12.5 µg/mL. The MIC value for the standard drug, cephalexin, was 0.31 µg/mL for all tested Gram-positive and Gram-negative bacterial strains, while clotrimazole was specifically active on the fungal strains with a MIC value of 0.62 µg/mL.
Table 3. Minimum inhibitory concentration (MIC) of the successive extracts of Tagetes erecta roots and the newly reported compound 1.
Discussion
Schizont maturation inhibition assay has been used over the years as a classical in vitro technique for the preliminary antiplasmodial screening of plants and compounds from the traditional database, e.g., Calotropis procera (Ait). R.Br. (Asclepiadaceae), xanthones from Andrographis paniculata Nees (Acanthaceae), Hedyotis corymbosa Linn. (Rubiaceae), etc. (CitationSharma & Sharma, 2000; CitationDua et al., 2004; CitationMishra et al., 2009). The assay is primarily based on the inhibition of the mature ring stages of the parasites, i.e., schizonts. All the successive extracts of the roots of T. erecta exhibited dose-dependent inhibition of schizont maturation rate with IC50 values ranging from 0.01 to 0.31 mg/mL against chloroquine sensitive Plasmodium falciparum (MRC-pf-2) and from 0.07 to 0.49 mg/mL for the chloroquine resistant strain of Plasmodium falciparum. The ethyl acetate extract (0.5 mg/mL) showed the highest activity among the fractions, causing about 80% inhibition of schizont maturation.
Each extract showed discrete morphological changes in the mature ring parasitic stages, i.e., trophozoites and schizonts. In the presence of ethyl acetate extract, the trophozoites were smaller in size in comparison to the trophozoites in the negative control wells. The specific changes in morphology of the parasite affected by a particular extract may hint at different modes of action of the putative active principles in the extract. Compound 1 (0.5 mg/mL) caused a complete inhibition of the schizont maturation of the chloroquine sensitive strain of Plasmodium falciparum but produced about 84% inhibition against the resistant strain. The novel bithienyl compound showed significant schizonticidal activity at relatively lower concentrations than all the extracts for both the tested strains of Plasmodium, though these concentrations were higher in comparison to the standard drug chloroquine.
The different extract fractions showed varying degrees of antimicrobial efficacy in the agar well assay – an assay which serves as a useful preliminary tool in assessing the antimicrobial potency of compounds. The antimicrobial efficacy of the successive extracts was further quantified by the determination of MIC (the lowest concentration of the test substance which inhibited the growth of microorganisms). All successive extracts showed antimicrobial zones of inhibition against each organism that were statistically significant in comparison to the standards. Among all extracts, the chloroform fraction exhibited significant antibacterial activity against Staphylococcus aureus and antifungal effect against Candida albicans while the petroleum ether fraction showed significant inhibition against Bacillus subtilis and Aspergillus niger. Compound 1 conferred significant (*p<0.001) antimicrobial activity against all the tested strains of micro-organisms at significantly relatively lower concentrations than all the extracts with MIC values ranging between 3.12 and 6.25 µg/mL which was noticeably higher than the standards, cephalexin and clotrimazole. The chemical nature of the active principles responsible for the observed activities of all extracts remains to be elucidated. However, the preliminary phytochemical screening showed the presence of sterols, carbohydrates, glycosides, proteins, phenolic compounds and tannins (CitationGupta et al., 2009). The solvents used for successive extractions were individually tested for any antimicrobial activity and were found to be devoid of any effect. The observations also rule out any extraneous effect of the solvent, i.e., 1% DMSO that was used for the preparation of stock solutions of all extracts. The bithienyl constituent (compound 1) was found to possess significant (*P<0.001) efficacy against all tested strains of bacteria and fungi.
Conclusions
The successive extracts, namely, petroleum ether, chloroform, ethyl acetate, methanol and aqueous extract, of the roots of T. erecta exhibited noticeable schizonticidal activity against the chloroquine sensitive and resistant strains of Plasmodium falciparum. The extracts also showed noticeable antimicrobial activity against three Gram-positive bacteria (Staphylococcus aureus, Bacillus subtilis and Micrococcus luteus); two Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa) and two fungi (Candida albicans and Aspergillus niger). Comparatively, the newly isolated bithienyl constituent (compound 1) exhibited antiplasmodial and antimicrobial efficacy at much lower concentrations than the extracts. This is the first report on the antiplasmodial and antimicrobial potential of the roots of T. erecta and the results obtained strongly provide support for the ethnobotanical use of the plant. Detailed phytochemical examination of all the successive extracts to isolate the putative active principle(s) (other than compound 1) responsible for the observed activities is in progress in our laboratory.
Acknowledgements
The authors are grateful to C.R. Pillai and the late C. Usha Devi of the National Institute of Malaria Research, Indian Council of Medical Research, New Delhi, India, for providing Plasmodium falciparum cultures, and also to the staff there for imparting training on in vitro cultivation of malarial parasites, antimalarial testing and parasite counting.
Declaration of interest
There are no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- British Standards Institution (1968); Methods on Microbial Examination for Dairy Purposes, B.S. 4285. London, British Standards House.
- Cappucino JG, Sherman N (1999): Microbiology – A Laboratory Manual, fourth edition. Harlow, Addison Wesley Longman.
- Chattopadhyay R, Kumar S (2009): Malaria vaccine: Latest update and challenges ahead. Indian J Exp Biol, 47, 527–536.
- CSIR (1976): The Wealth of India – Raw Material, volume X. New Delhi, India: Council for Scientific and Industrial Research.
- Das KC, Tripathi AK (1997): 6-Hydroxy kaempferol-7-O-β-d-alloside from Tagetes erecta. Fitoterapia, 68, 477.
- Dash AP, Valecha N, Anvikar AR, Kumar S (2008): Malaria in India: Challenges and opportunities. J Biosci, 33, 583–592.
- Dua VK, Ohja VP, Roy R, Joshi BC, Valecha N, Usha Devi C, Bhatnagar MC, Sharma VP, Subbarao SK (2004): Anti-malarial activity of some xanthones isolated from the roots of Andrographis paniculata. J Ethnopharmacol, 95, 247–251.
- El-Emary NA, Ali AA (1983): Revised phytochemical study of Tagetes erecta. Fitoterapia, 54, 9–12.
- Garg SC, Dengre SL (1986): Antibacterial activity of essential oil of Tagetes erecta Linn. Hind Antibiot Bull, 28, 27–29.
- Grover GS, Rao JT (1978): In vitro antimicrobial studies of the essential oil of Tagetes erecta. Perfum Flav Int, 3, 28.
- Gupta P, Vasudeva N, Sharma SK (2009): Pharmacognostical study and preliminary phytochemical screening of the roots of Tagetes erecta Linn. Hamdard Med, 52 153–160.
- Hadden WL, Watkins RH, Levy LW, Ragalado E, Rivadeneira DM, Van Breemen RB, Schwartz SJ (1999): Carotenoid composition of marigold (Tagetes erecta) flower extract used as nutritional supplement. J Agric Food Chem, 47, 4189–4194.
- Janakiram T, Rao TM (1996): Marigold: Tagetes spp. Indian Horticult, 41, 1, inside cover.
- Khan MT, Evans FJ (1996): Clinical evaluation of Tagetes erecta in the treatment of parakeratosis. Phytotherapy Res, 10, 5186–5188.
- Khan MT, Potter M, Birch I (1996): Podiatric treatment of hyperkaratotic plantar lesions with marigold Tagetes erecta. Phytotherapy Res, 10, 211–214.
- Kirtikar KR, Basu BD (1975): Indian Medicinal Plants, Volume II. Allahabad, India, Lalit Mohan Basu.
- Lambros C, Vanderburg JP (1979): Sychronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol, 65, 418–420.
- Mishra K, Dash AP, Swain BK, Dey N (2009): Antimalarial activities of Andrographis paniculata and Hedyotis corymbosa and their combination with curcumin. Malaria J, 8, 26–34.
- Nanir SP, Kadu BB (1987): Effect of some medicinal plants extract on some fungi. Acta Bot Ind, 15, 170–175.
- Penna CA, Radice M, Gutkind GO, VanBaren C, Broussalis A, Muschietti L, Martino V, Ferraro G (1994): Antibacterial and antifungal activities of some Argentinian plants. Fitoterapia, 65, 172–174.
- Rasoanaivo P, Petitjean AM, Ratsimamanga-Urverg S, Rakoto- Ratsimamanga A (1992): Medicinal plants used to treat malaria in Madagascar. J Ethnopharmacol, 37, 117–127.
- Roy S, Saha KD (2005): Malaria – old disease with threat of epidemic. Your Health, 54, 3–5.
- Sharma P, Sharma JD (2000): In-vitro schizonticidal screening of Calotropis procera. Fitoterapia, 71, 77–79.
- Trager W, Jensen JB (1976): Human malaria parasites in continuous culture. Sci, 193, 673–675.
- Tripathy AK, Gupta KR (1991): Plant phenolics of Tagetes erecta. Fitoterapia, 62, 91–92.
- Tripathy AK, Khan SA, Vaishnava MM, Gupta KR (1992): Compounds of taxonomic significance in Tagetes. Fitoterapia, 63, 85.
- Valecha N, Biswas S, Badoni V, Bhandari KS, Sati OP (1994): Antimalarial activity of Artemisia japonica, Artemisia maritima and Artemisia nilegarica. Indian J Pharmacol, 26, 144–146.
- Vasudeva N, Gupta P, Sharma SK (2007): A new bithienyl constituent from Tagetes erecta Linn. roots. Indian J Heterocy Ch, 16, 303–304.
- Vasudevan P, Kashyap S, Sharma S (1997): Tagetes: A multipurpose plant. Biosci Technol, 62, 29–35.
- WHO (2002): Roll Back Malaria Infosheet 1 of 11. Geneva: World Health Organization.