Abstract
Context: Aquatic organisms (especially fish) require potent defense mechanisms to protect themselves against pathogen invasion and disease formation. The use of immunostimulants in fish culture can prevent the diseases through augmentation of both specific and non-specific immunity.
Objective: A study was conducted to investigate the efficacy of different dietary doses of Aegle marmelos (Linn.) Corr. Serr. (Rutaceae) leaf extract for the immune response and the disease resistance of the freshwater fish, Cyprinus carpio Linn. (Cyprinidae) infected by Aeromonas hydrophila Chester (Aeromonadaceae).
Materials and Methodology: Hematological, specific immune response, non-specific immune response and enzyme assay studies were performed on fish and were scrutinized after 50 days of feeding trial.
Results: Fish were challenged with Aeromonas hydrophila at a dose of 1.5 × 104 cells/mL through intraperitoneal injection, and the hematological changes, the immune response, the enzyme activity and the disease resistance of Cyprinus carpio against the pathogen were also studied for 20 days at 5-day intervals.
Discussion: The results obtained from the study demonstrated that the fish fed with leaf extract of Aegle marmelos incorporated into feed significantly enhanced the red blood cell count, white blood cell count, hemoglobin, phagocytic activity, nitroblue tetrazolium chloride assay, lysozyme, pathogen clearance and enzyme activity compared with the control group. The survivability was higher in the fish which consumed leaf extract-incorporated feed, and the fish group fed with 5 g diet showed highest percentage survival of the fish.
Conclusion: These results indicate that Aegle marmelos stimulates the immunity and makes the freshwater fish Cyprinus carpio more resistant to Aeromonas hydrophila.
Introduction
The intensity of fish farming is affected by many factors including the high sensitivity of fish to stress and the accelerated spread of disease in water. It is important for aquaculturists to focus their efforts to maintain their fish with sound health in order to obtain sustainable economic gains.
Intensive aquaculture practices provide much interest in understanding fish diseases. The fish, which are growing in immunosuppressed conditions, become highly susceptible to disease and the majority of these disease outbreaks are caused by bacterial infections. Bacterial diseases create many practical problems during the culture period of fish and these bacterial diseases can wipe out the entire population of fish in the culture system. Hence, aquaculture industries require further development to control disease outbreaks, which constitute one of the major setbacks for successful aquaculture.
The use of immunostimulants in fish culture for the prevention of diseases through augmentation of both specific and non-specific immunity is a promising new development in the field (CitationAnderson, 1992; CitationRao et al., 2006; CitationSakai, 1999), since the use of immunostimulants for the prevention of these diseases has not shown any side effects on fish. The use of antibiotics and live vaccines on the fish and on the environment are attractive alternative ways of controlling bacterial infections (CitationSiwicki et al., 1994; CitationMulero et al., 1998; CitationLogambal & Michael, 2000).
Aeromonads are fish pathogens noted for causing major problems for carp aquaculture. Aeromonas hydrophila Chester is more abundant in waters with a high organic load than in relatively unpolluted water (CitationGuojun et al., 2009).
Recent literature on the immunostimulant action of herbal drugs on fish shows that the Chinese herbs Astragalus radix Linn. (Fabaceae) and Ganoderma lucidum (Curtis) P. Kars (Ganodermataceae) on Cyprinus carpio Linn. (Cyprinidae) indicates that the ethanol or methanol triberbal solvent extracts positively influence the immune response and protect the health of goldfish against A. hydrophila infection (CitationHarikrishnan et al., 2009). The immunomodulation of Labeo rohita Hamilton (Cyprinidae) juveniles was evaluated in terms of immuno-hematological changes after dietary gelatinized and non-gelatinized challenge with A. hydrophila, which promoted the growth and protecting immunity in L. rohita (CitationVikas et al., 2007).
A study on the effect of dietary doses of Euglena viridis (O.F. Mueller) Ehrenberg (Euglenaceae) on the immune response and disease resistance of Labeo rohita fingerlings against infection with the bacterial pathogen Aeromonas hydrophila was performed and the study concluded that the Euglena stimulates the immunity and makes L. rohita more resistant to A. hydrophila infection (CitationDas et al., 2009). The plant extract derived from Cynodon dactylon (Linn.) Pers. (Poaceae) feed against white spot syndrome virus (WSSV) infection of shrimp Penaeus monodon Fabricius (Penaeidae) possessing potential prophylactic action was reported by CitationBalasubramanian et al., 2008. The regulatory roles of rutin extracted from Toona sinensis (A. Juss.) M. Roem (Meliaceae) in various functions such as physiological, innate non-specific immune responses to the pathogen Vibrio alginolyticus Miyamoto and Sakazaki (Vibrionaceae) in white shrimp Litopenaeus vannamei Boone (Penaeidae) were examined, and provided significant effect on immune response (CitationHsieh et al., 2008). The immunomodulatory effect of microbial levan on C. carpio juveniles against Aeromonas hydrophila infection shows that the effect is caused by the activation of non-specific phagocytes and the use of microbial polysaccharides, constituting an important parameter in reducing mortality in fish (CitationRairakhwada et al., 2007).
Cyprinus carpio (Common carp or European carp) is a widespread freshwater fish very closely related to the common goldfish Carassius auratus Linn. (Cyprinidae) and is the number one fish of aquaculture. The annual tonnage of common carp produced in China alone exceeds the weight of all other fish such as trout and salmon produced by aquaculture worldwide. The above literature study shows that this fish production is affected by diseases produced by pathogens, and research work is going on in this area to improve or stimulate the immune response of this fish to fight against pathogens (CitationWhyte, 2007).
The present study has been carried out with the aim of improving the immunity of infected fish (C. carpio) with herbal drug-containing feed. This feed provided better immunocompetence and disease resistance, in addition to satisfying the dietary nutrient requirements for maximum growth. The experiments were designed to improve the health of fish through the use of Aegle marmelos (Linn.) Corr. Serr. (Rutaceae) leaf extracts incorporated into feed as immunostimulant.
Methods
Fish collection and maintenance
Common carp (average weight 45.9 ± 1.5 g, 100 fish in both sex) were purchased from a private fish farm at Kallidaikurichi, Tamil Nadu, India. They were transported to the laboratory in an oxygenated bag and stocked in a tank (capacity 500 L) at a density of 1 g/L. The tank water was changed on alternate days and the fish were fed with a balanced fish diet (). The excess feed and fecal material were siphoned out once a day. The temperature of the water in the fish tank was kept between 28 and 29°C. Other physiochemical parameters of water were also analyzed systematically at 7-day intervals to maintain optimum levels of dissolved oxygen 6.8–7.2 mg/L, pH 7.7–8.5 and ammonia 0.08–0.12 mg/L throughout the experiment.
Table 1. Feed formulation used for the study.
Pathogen isolation and its sensitivity analysis
Aeromonas hydrophila was isolated from diseased fish C. carpio showing symptoms of hemorrhagic septicemia. After isolation of the strain, it was identified using a standard microbial identification test and the identified culture was maintained on tryptose soya agar slopes at 4°C for long-term preservation for infecting healthy fish. The isolated bacterial culture was tested for its sensitivity to crude leaf aqueous extract of Aegle marmelos by disc diffusion test (agar medium) using 100, 200, 300, 400, and 500 µg/mL concentrations of the crude extract. In consequence of this, the A. marmelos incorporated into artificial feed at different concentrations of 0, 5, 10, 20, 25 and 50 g plant leaf extract/kg feed was given as an immunostimulant to C. carpio.
Diet formulation
The experimental feed was prepared by mixing the selected feed ingredients with known protein content accordingly to get 40.7% (). Fresh feed ingredients were procured in dry form and their quality was tested to confirm their protein content by Lowery method. The mixture of the feed ingredients was wetted with water and steam cooked in batches and cooled. To this feed, vitamin mix and cod liver oil were added and mixed thoroughly for even distribution. This feed mixture was then pelletized through hand pelletizer until between 1 and 4 mm and dried in shade to reduce the moisture content of the feed below 10%.
Medicated feed formulation
A. marmelos leaves were collected from Sri Paramakalyani temple Alwarkurichi, Tamil Nadu, India. All the required fresh leaves were taken from a single plant. A. marmelos medicated feeds at different doses of 0, 5, 10, 20, 25 and 50 g/kg feed were formulated by adding a prescribed amount of leaf extract to the pre-steam cooked and cooled feed mixture containing 40.7% protein.
Experimental design
The experimental fish C. carpio of uniform size (45.9 ± 1.5 g) were stocked in six troughs with ten fish each in triplicate (including control). The formulated feeds at various concentrations (0, 5, 10, 20, 25 and 50 g/kg) were given separately at 2% of body weight for a period of 50 days (the concentrations were fixed for the study from the minimum dose). After 50 days of feeding, all the experimental fish were given only control feed. At day 50 of immunomodulation the fish were infected with bacterial pathogen Aeromonas hydrophila through intraperitoneal injection at a dose of 1.5 × 104 cells/mL. After 5 days of infection, studies were carried out once every 5 days up to day 20 to observe the hematological and immunological changes and the biochemical responses. These responses were also studied prior to the challenge of pathogen, which served as control values.
Collection of blood and antiserum
The fish were bled serially using a tuberculin syringe with a 24-gauge needle from the caudal vein and the blood was collected in EDTA-rinsed small serological tubes. The blood (without anticoagulant) collected from the fish was kept overnight at 4°C for serum separation. The serum was separated by spinning down at 3000 rpm for 15–20 min in a centrifuge. The supernatant was collected and stored in sterile vials. The serum was kept at 57°C in a water bath for 30 min to inactivate the complement system and was stored at -20°C for further analysis.
Hematology
The red blood cell counts (RBC) (106 mm−3) were determined in a 1:200 dilution of blood sample in Hayem’s solution (Sigma-Aldrich, UK), and the white blood cell count (WBC) (104 mm−3) from a 1:20 dilution of the blood sample in Turk’s solution (Sigma-Aldrich, UK), with a Neubaeur hemocytometer (BiosciTech, Mumbai). Hemoglobin content (Hb:gm/dL) was determined by the cyanmethemoglobin method (CitationGowenlock, 1996); 20 µL of the anticoagulated blood was mixed with 4 mL of Drabkin’s reagent (Sigma-Aldrich, UK), and kept at room temperature for 4 min and read at 540 nm with a UV spectrophotometer.
Specific immune response
Antigen–antibody titration (bacterial agglutination assay)
Circulating antibody titers were performed in 96-well microtiter plates using two-fold dilutions. The titer was recorded as the highest dilution in which visible agglutination (mat-like observation) could be seen. Dot-like formation was considered a negative response (CitationVallinayagam, 1997).
Non-specific immune response
Assay of phagocytic activity
The phagocytic activity assay was performed by the following modified method of CitationSahoo and Mukherjee (2002). Blood sample (EDTA mixed) was mixed with an equal quantity of bacterial suspension (1:1) in Eppendorf tubes. The density of the bacterial culture was maintained throughout the experiment at 104 cells/mL in PBS. The mixture was incubated for 20 min at room temperature. After incubation a thin smear was prepared and fixed with absolute alcohol for 5 min. The smear was later stained with Giemsa stain for 5 min and the phagocytic cells engulfing the bacteria were counted (under microscope) as positive (CitationSeeley et al., 1990).
The percentage of bacteria ingested by phagocytes (phagocytic ratio) was calculated by Equation 1.
Nitroblue tetrazolium chloride assay
One drop of pooled heparinized blood (from 6 fish) was placed on a cover slip immediately after collection and was placed in a humid chamber (on 60 mm Petri dishes with a wet paper towel) and incubated for 30 min at room temperature for the neutrophils to stick on the glass. After incubation the cover slips containing the cells were transferred upside down to a clean glass slide containing a 50-µL drop of 0.2% filtered nitroblue tetrazolium chloride (NBT) solution and subsequently incubated for 30 min. The dark blue stained NBT-positive cells were counted under microscope (CitationSahoo & Mukherjee, 2002).
Serum lysozyme activity
Lysozyme activity was analyzed spectrophotometrically according to Santaram et al. (1997) and CitationSankaran et al. (1972). A standard suspension of Micrococcus lysodeikticus was prepared in 0.066 M phosphate buffer (pH 7). The serum (100 µL) was added to 2 mL of bacterial suspension and was incubated at 40°C for 20 min. After incubation the absorbance was read at 546 nm. The lysozyme content was determined on the basis of the calibration curve and the extinction measured. Standard solutions containing 2.5, 5, 7.5, 10 and 12.5 µL/mL of hen egg lysozyme in 0.066 M phosphate buffer were used to develop a standard curve.
Pathogen clearance in blood
Pathogen count in the blood sample was carried out by the following procedure of CitationVallinayagam (1997). The blood sample was serially diluted and the pathogens were counted by using pour plate technique with specific agar (thiosulfate citrate bile salts sucrose (TCBS) agar, Himedia, Mumbai, India) in the Petri dishes and then incubated at 37°C for 24 h. The values were expressed in cfu/mL.
Enzyme assay
Acid and alkaline phosphatase
Acid and alkaline phosphatase activity was estimated by the following method (CitationPattabiraman et al., 1998). A serum sample (10 μL) was mixed with 5 mL of substrate solution and the absorbance was read at 420 nm immediately. Then the substrate solution with serum sample was incubated at 37°C for 30 min and the absorbance was read at 420 nm. The optical density (OD) difference was noted and the activity was calculated by using Equation 2.
Serum peroxidase
Serum peroxidase was analyzed by the procedure of CitationMurugesan and Rajakumari (2005). To 1.4 mL of amino-antipyrine, 1.5 mL of H2O2 was added and to this mixture, 0.1 mL of serum sample was added and the extinction was measured at 510 nm for about 5 min. The solution without serum served as control and the standard solutions containing different concentrations of peroxidase in 0.066 M phosphate buffer were used to construct a standard curve. The results are expressed in IU/mL.
Disease resistance
The effects of plant leaf extract incorporated into feed for disease resistance on groups of fish (n = 20/group) were determined. The fish were artificially challenged with a dose of 1.5 × 104 cells/mL of live virulent pathogen A. hydrophila. The mortality was observed for 20 days and the average of a triplicate set was used for expression in terms of percentage of survival.
Statistical analysis
The data collected were statistically analyzed using two-way analysis of variance (ANOVA) to test the effects of experimental diets for all parameters. Student’s t-test was used to test differences among individual means and the control. The difference was recorded as significant when P <0.01 and P <0.05. This statistical work was performed manually with the help of Microsoft Excel program.
Results and discussions
The present study was carried out to evaluate the hematological and enzymatic changes in the freshwater fish C. carpio after being artificially infected with the pathogen A. hydrophila. The immunomodulatory effect of the leaf extract of A. marmelos was found by feeding various concentrations of the extract to the fish. The results obtained from the study are discussed hereunder.
The sensitivity of the A. marmelos leaf extract against the pathogen A. hydrophila was performed by agar plate diffusion method. The zone of inhibition obtained from the study shows that the extract at various concentrations (100, 200, 300, 400 and 500 µg/mL) has significant activity against the pathogen (A. hydrophila). Earlier literature has also shown that A. marmelos extracts possess antimicrobial activity against bacterial species (CitationMadasamy et al., 2003).
Hematological responses
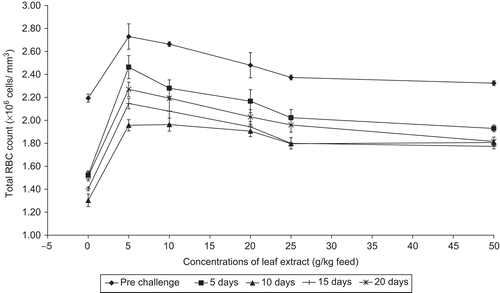
The effectiveness of the 50-day feeding regimes of A. marmelos medicated feed at different doses on C. carpio and those exposed to A. hydrophila for infection were recorded. The fish fed with feeds having leaf extract of A. marmelos were able to enhance their total RBC count significantly (P <0.05) and the results obtained show that the maximum RBC count of 2.73 ± 0.011 × 106 cells/mm3 was noticed in the feed having 5 g leaf extract of A. marmelos/kg. In control fish, the total RBC count was 2.19 ± 0.035 × 106 cells/mm3. After infection with the pathogen the RBC count was decreased in the control fish up to 10 days after challenge, followed by slight increase in RBC count. The fish fed with feeds incorporating leaf extract of Aegle marmelos also showed decreased RBC count up to 10 days after infection; later the RBC count increased marginally. Among all the concentrations of leaf extract incorporated feeds, the fish fed with feed having 5 g (P <0.01) leaf extract of A. marmelos showed higher RBC count compared with the other feeds (). The feed having 10 and 20 g (P <0.05) leaf extracts of A. marmelos showed significant difference in RBC count whereas the fish fed with 25 and 50 g plant leaf extract did not show any significant difference when compared with the control. The results obtained show that the leaf extract of A. marmelos has significant influence on the hematology of C. carpio.
WBC count
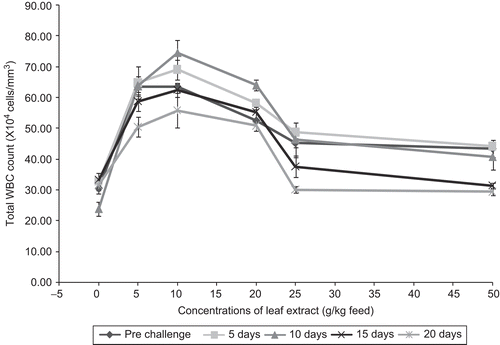
The WBC count was significantly increased in fish fed with feed having leaf extract of A. marmelos. After infection with the pathogen, the WBC count increased in fish fed with 10 g leaf extract for 10 days. After 10 days of infection, the WBC count was decreased. In the control, the WBC count increased on day 5 and then decreased on day 10, after which the WBC count increased slightly (). It is apparent from the figure that the feeds having leaf extract increased the total WBC count up to day 10 after infection with the maximum WBC count of 74.57 ± 4.004 × 104 cells/mm3 for 10 g leaf extract. Statistical analysis decisively indicates that all the concentrations of leaf extracts of A. marmelos enhanced the WBC count effectively at 1% level, when compared with the control.
Hemoglobin content
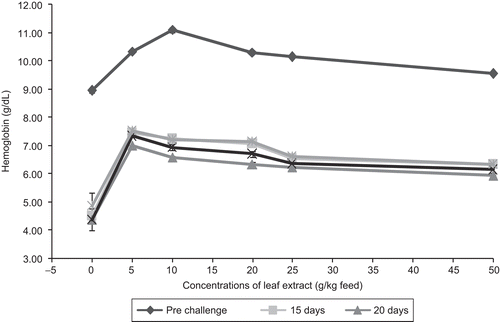
The hemoglobin content of the control fish was found to be 8.93 ± 0.056 g/dL. The hemoglobin content increased in the pre-challenged fish fed with feeds having A. marmelos leaf extract, and the maximum value of 11.09 ± 0.094 g/dL was noticed in the feed having 10 g leaf extract, followed by the feeds having 5, 20, 25 and 50 g leaf extracts of A. marmelos/kg feed. After infection with the pathogen, the hemoglobin content decreased in all fish, and after 10 days of infection the hemoglobin content increased with a maximum value of 7.53 ± 0.029 g/dL in fish fed with 5 g leaf extract of A. marmelos on day 20. reveals that all the values of mean hemoglobin content in challenged fish were significantly less than in pre-challenged fish. Among the challenged fish, the fish fed with feed having 50 g leaf extract/kg showed a lower concentration of hemoglobin throughout the period of experiment. Also it was found that there was no significant difference in the hemoglobin content between the concentrations when compared with the control, except fish fed with feed having 5 g (P <0.01). In general, all the concentrations showed significant variation (P <0.05) in the hemoglobin level from day 5 of post-challenge with pathogen.
Figure 3. Effect of different concentrations of leaf extract of Aegle marmelos on hemoglobin content (g/dL) in Cyprinus carpio infected with the bacterial pathogen Aeromonas hydrophila.
The results obtained from the hematological studies show that the fish fed with feeds having the leaf extract of A. marmelos significantly enhanced the RBC count, hemoglobin content and WBC count when compared to that of the control fish. CitationCooper et al. (1963) reported that mitochondria play a significant role in iron metabolism in developing erythrocytes. The results of the present study also suggested that the leaf extract of A. marmelos may induce erythropoiesis and lymphopoiesis resulting in the increase of RBC count, hemoglobin content and WBC count. The experiments conducted with feeds incorporating Acalypha indica Linn. fed to Oreochromis mossmbicus Peters (CitationAnita, 1998); A. marmelos fed to Catla catla Hamilton (CitationMadasamy, 2003) and Phyllanthus emblica Linn. fed to Cirrhinus mrigala Hamilton (CitationDiana, 2006) also showed that fish fed with lower concentrations of leaf extracts increased the RBC count, hemoglobin content and WBC count and the same values decreased at higher concentrations as observed in the case of A. marmelos in the present study (–). After challenge with A. hydrophila, the RBC count and hemoglobin content decreased for 10 days, indicating that the stress may be induced by the pathogens. In almost all infected fish the homostatic processes are extended beyond the normal limits due to stress (CitationPickering, 1981). CitationSuzumoto et al. (1977) also reported that the changes in blood profile of microbially infested fish, Oncorhynchus kisutch Walbaum especially documented a decline in erythrocytes and hemoglobin content. The decreased hemoglobin content may be brought about as a result of the swelling of RBC as well as poor mobilization of hemoglobin from the spleen and other hematopoietic organs in Ictalurus punctatus Rafinesque (CitationScott & Rogers, 1981). These facts support the present finding of significant decrease in erythrocyte and hemoglobin content in fish infected with bacterial pathogens, possibly due to hypochromic microcytic anemia caused by the bacteria. In the extract-treated group, the level of pathogenicity decreased when compared with the control fish. The decreased RBC counts and lower hemoglobin concentration in infected fish indicate that the RBCs are being destroyed by the leukocytosis activity in an erythrocytic anemia with subsequent erythroblastosis (CitationHaney et al., 1992). CitationKumar et al. (2006) in their experiments found that the RBC count and the hemoglobin content were significantly reduced due to bacterial challenge, but dietary starch (gelatinized and non-gelatinized) had no effect on it, whereas the dietary starch enhanced WBC count. CitationEllias (1976) suggested that lymphocytes are the executive cells in the specific immune mechanism of fish, and they become stimulated under stress conditions to fight against entry of foreign bodies into the blood stream. In the present investigation, the increase in WBC count may be the result of stimulation caused by the entry of pathogens. The increase in WBC count in fish fed with feeds having leaf extract in the present study may be also due to the induction of active compounds present in the leaf extract, consequent to infection. The hematological results of the present study reveal that the leaf extracts were able to reduce the immunosuppression caused by the pathogen.
Specific immune response
Antigen antibody titration
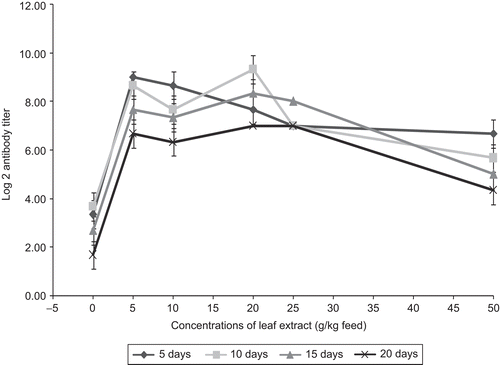
The antibody response to the pathogen A. hydrophila in A. marmelos medicated feed at different dose treatments in the experimental fish is presented in . The results indicate that the feeds with leaf extract strongly enhanced the primary immune response in comparison with control fish. The fish fed with leaf extract-incorporated feed developed immunological competence on day 5 after infection with the pathogen, whereas the control fish developed immune response on day 10 post-bacterial challenge. This clearly indicates that a single booster dose of leaf extract is sufficient to elicit a high antibody titer and the immunization schedule adopted gives a high degree of protection against the bacterial pathogens tested in common carp. Although all the concentrations of the leaf extract enhanced the antibody response and the lower concentrations (5 and 10 g) of A. marmelos were only able to stimulate higher antibody production, statistically no clear concentration dependency in the enhancement of antibody production was noticed. The previous studies indicated that leaf extracts of Ocimum sanctum Linn. (Lamiaceae) in Oreochromis mossambicus (CitationLogambal & Michael, 2000; CitationVenkatalakshmi & Michael, 2001) enhanced both primary and secondary immune responses to sheep erythrocytes, and they reported that the aqueous extract of O. sanctum leaves not only enhanced the magnitude of antibody response, but also significantly shortened the lag period of both primary and secondary immune responses. CitationDavis and Hayasaka (1984) showed that antibody titer rise on days 7 and 14 could be greatly enhanced by an intravenous injection of extract 2 days post-bacterial challenge. CitationVallinayagam (1997) reported that antibody production predominantly increased when the fish, O. mossambicus, were fed with vitamin C at 60 to 70 mg/kg body weight, and the peak response was seen on day 9 after administration. The present findings are in agreement with the reports on significant increase in hemagglutination antibody titers in O. mossambicus fed with Acalypa indica (CitationAnita, 1998), A. marmelos in Catla catla (Madaswamy, 2003), and Phyllanthus emblica in Cirrhinus mrigala (CitationRegina, 2006). The result obtained from the present study also reveals that the leaf extracts in the feed of carp were able to stimulate specific antibodies against the challenged bacteria and raise the humoral immunity.
Non-specific immune response
Nutritional modification has been used to prevent the outbreak or to reduce the severity of infections in fish (CitationChandra, 1996; CitationScrimshaw & San Giovanni, 1997). Studies regarding nutritional effects on fish immunity were focused on antibody-mediated immunity against non-specific events, on which fish seem to rely much more heavily than higher vertebrates (CitationLandolt, 1989). In this present investigation, several assays were carried out to test the efficacy of the leaf extract as immunostimulant.
Phagocytic activity
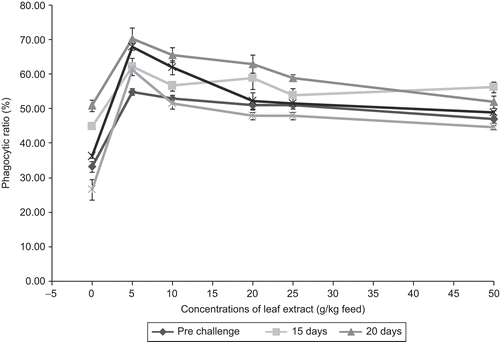
Phagocytosis is the most common cellular defense mechanism, and together with humoral components it constitutes the first line of defense mechanism against a parasite or an intruder. The phagocytic activity of a fish can be modulated by a wide range of endogenous and exogenous factors, which enhance or modulate the fish immune system (CitationSecombes, 1994). The present results clearly show that the leaf extract was able to stimulate the phagocytic activity and enhanced the phagocytic ratio significantly (). Phagocytic activity is mediated by cytokines such as macrophage activating factor secreted by peritoneal lymphocytes (CitationGraham & Secombes, 1988). From the results it is also clear that the chemotaxis process was stimulated by the lower concentrations (5 to 10 g) of the leaf extract of A. marmelos. The increase in phagocytic ratio indicates that the leaf extract stimulated the synthesis of chemotactic factors as observed in the study of CitationFujiki and Yano (1997). Phagocytic activity of blood leukocytes were increased in rainbow trout fed with 1% ginger (Zingiber officinale Roscoe) (CitationDugenci et al., 2003). CitationMisra et al. (2006a, Citation2006b) found that injection of different doses of tuftsin enhanced the phagocytic activity in Labeo rohita and maximum activity was noticed on day 42 after injection of tuftsin. In the present study, after infection with the pathogen, the phagocytic ratio was increased in all the fish which consumed feed with leaf extract, and the phagocytic ratio was higher up to 10 days after infection. The peak phagocytic ratio of 70.33 ± 3.21% was noticed in fish fed with feed incorporating 5 g leaf extract of A. marmelos/kg feed. In the control fish, the phagocytic ratio was found to be 33.33 ± 1.52%, and after infection with the pathogen A. hydrophila, the phagocytic ratio increased up to 51 ± 1.73% on day 10 of infection and the ratio further decreased up to day 20 of infection (). The fish fed with leaf extract of A. marmelos were significantly enhanced (P <0.01) the phagocytic ratio when compared with the control fish and the ratios were maximally significant (P <0.05) on day 10 of infection. The results also reveal that macrophage migration in the presence of exoantigen was enhanced with the incorporation of different levels of leaf extract of A. marmelos in fish feed.
NBT assay
NBT assay is a quick, inexpensive method focusing on the ability of phagocytes to reduce the dye by production of oxygen radicals. In vertebrate phagocytic cells, the oxygen-dependent defense mechanism consists of the generation of reactive oxygen intermediates (ROIS) with powerful microbicidal activity (CitationAllen et al., 1972; CitationBabior et al., 1973). These phagocytic cells can be elicited upon suitable stimulation by soluble components such as lectins, lipopolysaccharides (LPS), yeast, vitamin C, glucans, zymosan, leaf extracts, etc. The stimulation of the phagocytic cell membrane leads to increased consumption of oxygen, in which the reduction process is catalyzed by a membrane-bound enzyme, NAD(P)H-oxidase giving rise to superoxide (O2-). A number of oxides through various reactions lead to the production of hydrogen peroxide (H2O2), singlet oxygen (O21) hydroxyl radical (OH) and numerous other reactive products (CitationHigson & Jones, 1984; CitationMunoz et al., 2000). In the present study the NBT-positive cells were found to be significantly increased (P <0.01) with the increase in days after infection in fish fed with feeds of leaf extracts (). In control fish, the number of NBT positive cells was found to be 10 ± 1, and after infection with the pathogen A. hydrophila the NBT-positive cells increased and the peak of NBT-positive cells was found on day 10 after infection and the value was 17 ± 0.816. After day 10 of post-infection, the number of NBT-positive cells in the fish was decreased. The same trend of increase in the number of NBT-positive cells was noticed in fish fed with leaf extract of A. marmelos feed. Maximum number of 45 ± 0.816 NBT-positive cells was noticed on day 10 in fish fed with feed having 10 g of leaf extract of A. marmelos/kg feed. It is clear that a significant increase in the NBT-positive cells was also noticed in fish fed with feed having 5, 10 and 20 g of leaf extract of A. marmelos at the 1% level, whereas 25 and 50 g leaf extracts showed variations at the 5% level. Significant enhancement of NBT-positive cells in all concentrations was noticed during the days after infection with A. hydrophila. This is probably due to the increase in lysozyme activity. Lysozyme production is mainly based on the neutrophils and monocytes present in the blood. This is also reported by the fact that lysozyme activity in fish fed with feeds having leaf extract concentration was found to be higher than the control, suggesting the production of more NBT-positive cells as found in the present study ().
Figure 6. Effect of different concentrations of leaf extract of Aegle marmelos on number of glass adherent NBT assay positive cells in Cyprinus carpio infected with the bacterial pathogen, Aeromonas hydrophila.
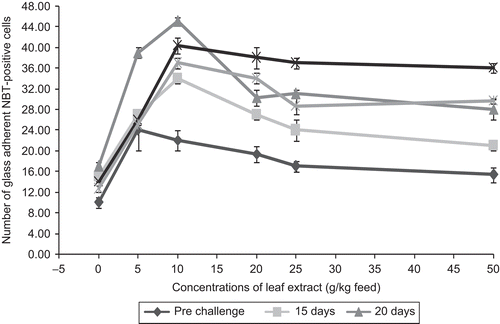
Figure 7. Effect of different concentrations of leaf extract of Aegle marmelos on serum lysozyme activity (µg/mL) in Cyprinus carpio infected with the bacterial pathogen Aeromonas hydrophila.
The leaf extract of A. marmelos administered through diet enhanced the non-specific defense mechanism in terms of increased number of activated neutrophils. This finding is in agreement with the earlier findings where number of NBT-positive cells in the blood of carp significantly increased at 20 and 30 days post-feeding with traditional Chinese medicine (TCM), a formulation from Astragalus root and Chinese Angelica root at 1% and 1.5% (CitationJian & Wu, 2004). Water soluble fractions of Crossandra infundibuliformis Linn. leaves also enhanced NBT positive cells in O. mossambicus (CitationVenkatesan et al., 2001). The fish Labeo rohita when fed with gelatinized and non-gelatinized starch with n-3 PUFA were enhanced in the NBT assay (Mirsa et al., 2006b). CitationKumari et al. (2007) reported that poly herbal formulation (Immunoplus) enhanced the NBT assay in L. rohita. The previous findings support the view that external stimulants such as plant extracts stimulate the activity of NBT-positive cells in the blood of fish, as evidenced by the results of the present investigation in common carp. This is supported by the fact that serum lysozyme and phagocytic ratio were also enhanced in fish fed with feeds having leaf extracts.
Serum lysozyme activity
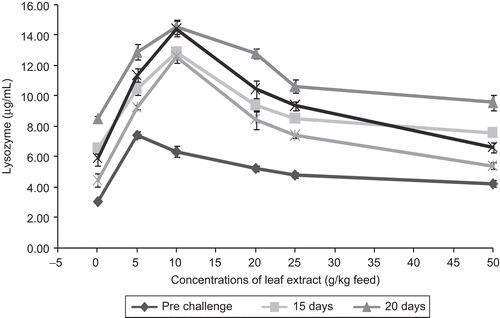
The lysozyme activity has been found to be modulated by a range of factors including stress, water temperature, injection of foreign materials, nutrients, etc. (CitationFletcher & White, 1973; CitationSakai, 1999). The major lysozyme secretory cells of higher vertebrates are the macrophage cells (CitationSchnydes & Baggioline, 1978). The results of the present investigation indicate that the leaf extract at various concentrations significantly enhanced the serum lysozyme activity in C. carpio (). The mean values of serum lysozyme activity obtained in C. carpio fed with feed incorporated with different concentrations of leaf extract of A. marmelos and infected with A. hydrophila are represented in . From the figure it is clear that in pre-challenged fish, the serum lysozyme activity of 7.44 ± 0.36 µg/mL was observed in fish fed with feed having 5 g leaf extract of A. marmelos/kg feed. It is apparent from the figure that the serum lysozyme activity was decreased after 10 days in all the fish fed with feeds having different concentrations of leaf extract, but the reduction was not prominent in the fish fed with feed having 10 g leaf extract of A. marmelos/kg feed, when compared to other experimental fish. These increased levels of lysozyme in fish fed with feeds with plant leaf extract could be the result of an increment in the number of phagocytes (macrophage) resulting in the secretion of more lysozyme by these cells. It can also be seen that the lower concentrations (5 to 10 g) of A. marmelos significantly enhanced the serum lysozyme activity.
The earlier reported literature also reveals that the medicinal plants are effective in enhancing serum lysozyme activity. CitationJian and Wu (2004) reported that serum lysozyme activity significantly increased when the fish, was fed with traditional Chinese medicine (TCM) formulation from Astragalus root (Radix astragali) and Chinese Angelica root (Angelica sinensis (Oliv.) Diels (Umbelliferae). Poly herbal formulation (Immunoplus) (CitationKumari et al., 2007) and Mangifera indica Linn. (CitationSahu et al., 2007) in the feed of L. rohita also enhanced the serum lysozyme activity. All the above studies show the effect of herbal plants on lysozyme activity and are in confirmation with the present study in which the leaf extracts also enhanced serum lysozyme activity.
Pathogen clearance
The average values of pathogen clearance in the blood of C. carpio fed with feeds having different concentrations of leaf extract of A. marmelos and infected with A. hydrophila are graphically shown in . From the figure it is evident that control fish showed 8.63 ± 0.15 × 104 cfu/mL of pathogen in the blood on day 5 after infection and on subsequent days the pathogen count in the blood was decreased, and on day 20 the count was found to be 5.5 ± 0.5 × 104 cfu/mL in control fish. It shows that the fish fed with feeds having A. marmelos leaf extract were also able to eliminate the pathogen from the blood significantly when compared with the control fish.
Figure 8. Effect of different concentrations of leaf extract of Aegle marmelos on pathogen clearance in the blood of the freshwater fish Cyprinus carpio infected with the bacterial pathogen Aeromonas hydrophila.
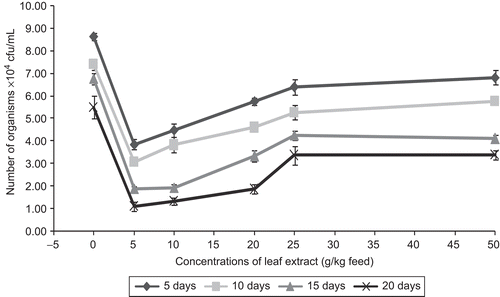
The data on the pathogen clearance in the blood of the freshwater fish C. carpio indicate that the feeds with 5 and 10 g leaf extracts of A. marmelos were able to eliminate the pathogen significantly at 1% level (P<0.01). All other feeds were not found to have a significant impact in respect of pathogen clearance.
The results of the pathogen clearance show that the fish consuming feeds with leaf extract were able to eliminate the pathogen from the blood significantly (p<0.01). The leaf extract enabled the fish to eliminate the pathogens on day 10 after bacterial challenge. This is also supported by the results of specific and non-specific immune parameters. The decrease in specific and non-specific immune response after day 10 may be due to the elimination of the pathogen from the fish body. CitationYoshida et al. (1993) noticed that bacterial counts in the blood and spleen decreased in the fish Clarias gariepinus Burchell when treated with glucan. Acalypha indica leaf extract in the feed of tilapia was also able to eliminate the pathogen from the kidney and blood (CitationSukumaran & Anita, 2001). CitationSukumaran and Vallinayagam (2002) also found that tilapia fed with vitamin C were able to eliminate the pathogen Pseudomonas fluorescens Flugge from the blood and kidney. Labeo rohita showed better bactericidal activity when they were fed with Achyranthes aspera Hook F. seeds (CitationRao et al., 2006). CitationSahu et al. (2007) and CitationMisra et al. (2006a) observed increased pathogen clearance in rohu when the fish were fed with Mangifera indica and tuftsin incorporated into feeds, respectively. On the basis of these reported results, it may be explained that the total immunostimulation property of the plant extracts may be the reason for better pathogen clearance, as indicated by total specific immune responses.
Enzyme assay
Phosphatase activity
Phosphatase enzyme is a liposome and acts as a non-specific mechanism. Acid phosphatase activity is widely considered to be a valuable parameter of macrophage activation (CitationSveinbjornsson & Seljelid, 1994).
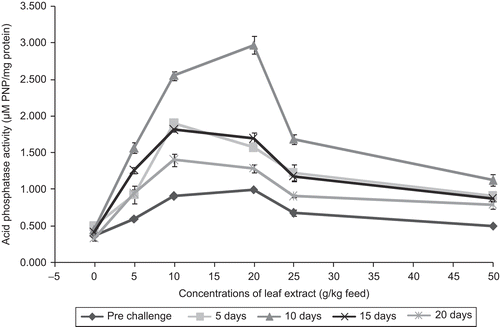
A. hydrophila-infected fish show an increasing trend of the acid phosphatase activity for 10 days and the maximum acid phosphatase activity of 1.898 ± 0.024 was observed in fish fed with 10 g leaf extract of A. marmelos feed. However, the peak value of serum acid phosphatase activity (2.97 µM PNP/mg protein) was observed in fish fed with 20 g leaf extract of A. marmelos feed on day 10 after infection. On day 20 after infection, the production was high in fish fed with 10 g extract of A. marmelos/kg feed, the value being 1.403 ± 0.085 µM PNP/mg protein (). Among the different concentrations tested, 10 g leaf extract performed better than other concentrations experimented. Feeding the fish with A. marmelos significantly enhanced the acid phosphatase activity (P < 0.01) when compared with the control, and significant enhancement was seen up to day 15 after infection.
Figure 9. Effect of different concentrations of leaf extract of Aegle marmelos on serum acid phosphatase (μg PNP/mg protein) in Cyprinus carpio infected with the bacterial pathogen Aeromonas hydrophila.
provides information about the alkaline phosphatase activity. It shows that the extracts were able to stimulate the alkaline phosphatase activity and the maximum alkaline phosphatase activity of 0.866 ± 0.034 µM PNP/mg protein was noticed in the fish fed with 10 g extract of A. marmelos/kg feed. It is also evident from that among the various concentrations of leaf extract experimented with in common carp, the 10 g extract feed showed the maximum serum alkaline phosphatase activity throughout the experiment. The fish fed with 10 g extract of A. marmelos were able to enhance the serum alkaline phosphatase up to day 15 after infection, the value being 2.237 ± 0.127 µM PNP/mg protein. All other concentrations were able to enhance the serum alkaline phosphatase activity only up to 10 days after infection.
Figure 10. Effect of different concentrations of leaf extract of Aegle marmelos on serum alkaline phosphatase (μg PNP/mg protein) in Cyprinus carpio infected with the bacterial pathogen Aeromonas hydrophila.
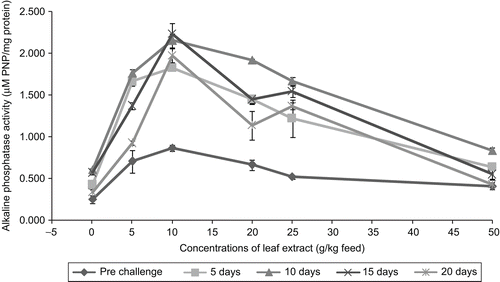
The results of the acid and alkaline phosphatase activity indicate that the fish C. carpio fed with feed having leaf extract of A. marmelos showed significant enhancement in the phosphatase activity when compared with control ( and ) suggesting that the enhancement of serum phosphatase activity in fish fed with leaf extract may possibly due to increase of macrophage cells, which in turn produced higher amount of phosphatase enzyme, resulting in enhanced enzyme activity as observed by CitationDalmo and Seljelid (1995), who reported that lipopolysaccharide (LPS) stimulated the macrophage cells for the higher enhancement of acid phosphatase when compared to the control macrophage cells. CitationPress et al. (1995) noticed the increased amount of acid phosphatase in tissues of Atlantic salmon administered with furunculosis vaccines. CitationRao et al. (2006) reported that Achyranthes aspera in feed enhanced the serum alkaline phosphatase activity in L. rohita, as observed in common carp in the present study.
Serum peroxidase
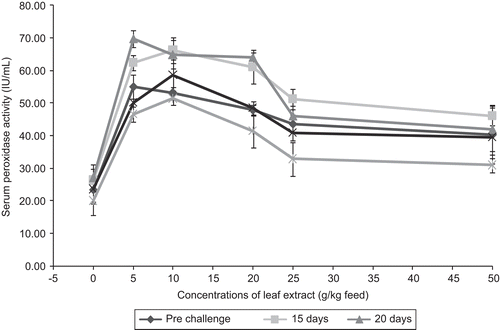
The release of myeloperoxide enzyme is mostly by azurophilic granules of neutrophils during oxidative respiratory burst activity, which is measured through the serum peroxidase activity. reveals that after infection with the pathogen on the control fish, the serum peroxidase activity was increased and the activity was found to be 27.10 ± 3.98 IU/mL up to 10 days after infection, and on subsequent days the serum peroxidase activity decreased. The fish which consumed feed having plant leaf extract showed higher serum peroxidase activity of 54.96 ± 3.67 IU/mL in fish fed with feed incorporating 5 g leaf extract of A. marmelos/kg feed, followed by the fish fed with feeds having 10, 20, 25 and 50 g leaf extracts of A. marmelos/kg feed. Of all the fish fed with leaf extracts of A. marmelos, the maximum serum peroxidase activity of 51.33 ± 1.93 IU/mL was noticed in fish fed with 10 g leaf extract of A. marmelos/kg feed. From the results obtained in the present study it is clear that the leaf extract at different concentrations was able to stimulate the serum peroxidase activity as evidenced from the increased enzyme activity in fish fed with feeds incorporating leaf extract of the plant, when compared with control fish. This is also supported by a study conducted by CitationKumari and Sahoo (2006), which showed that β-glucan enhanced the serum peroxidase activity in Clarias batrachus Linn. for about 21 days. In the present study, serum peroxidase activity, which is the indicator of humoral immune response that was found to be high up to 10 days after infection and subsequently the activity decreased, indicating that the leaf extract had stimulation effect on peroxidase activity up to 10 days after infection and subsequently the effect was found to be nullified resulting in the decrease in serum peroxidase activity. CitationCuesta et al. (2005) found that leukocyte peroxidase decreased in gilthead Sea bream (in both non-parasitized and parasitized) when compared with control. CitationEsteban et al. (2005) noticed that serum peroxidase made no significant difference when sea bream were fed with lactoferrin. Serum peroxidase levels reached a high during weeks 3 and 4 of feeding probiotics to sea bream (CitationDiaz-Rosales et al., 2006), whereas in the present study the enzyme activity was found to be at peak up to 10 days after infection, indicating the moderate effect of plant extract beyond 50 days after feeding with the medicated feed.
Survival percentage
shows the survival percentage of C. carpio fed with extract of A. marmelos and infected with A. hydrophila. From the results obtained, it is clear that the fish fed with feed having 5 g A. marmelos showed higher survival rates (96.67 ± 2.98%) when compared with those in other concentrations. It is clear that the fish which consumed 0 g (control) A. marmelos showed the survival percentage of 60%. It explained that the extracts of A. marmelos enhanced the survival percentage significantly at 1% level.
Table 2. Survival percentage of the freshwater fish Cyprinus carpio infected with the bacterial pathogen Aeromonas hydrophila.
Conclusion
Results of the present investigation on disease resistance concluded that the incorporation of leaf extract of A. marmelos feed protects C. carpio fish against the pathogen A. hydrophila. The extract when supplementing the feed enhanced protection when challenged with live pathogen A. hydrophila. The mechanism by which survival was augmented appears to be positively correlated with increased phagocytosis, neutrophil activity, lysozyme activity, etc. Among the different concentrations tested the groups fed continuously with feed of lower concentrations (5 and 10 g) of A. marmelos resulted in maximum protection (). Earlier studies have revealed that dietary supplementation enhanced disease resistance as observed in common carp in the present study.
In general, immuno-stimulants were found to stimulate antibody response, lysozyme and phagocytosis and other immunological function in fish (CitationSakai, 1999). This study also reveals the scope of using extracts of A. marmelos as an immuno-prophylactic for health management in the culture of carp. From the study it is concluded that the leaf extract of A. marmelos had significant immunostimulant activity against A. hydrophila infecting freshwater C. carpio fish.
Acknowledgements
The authors are grateful to the Vice Chancellor, MS University, Tirunelveli, India for providing the necessary facilities for this study. V. P. is grateful to BRNS-DAE, India for support for the work.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Allen RC, Sternholm RL, Steele RH (1972): Evidence for the generation of an electronic excitation state(s) in human polymorpho nuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Comm 47: 679–684.
- Anderson DP (1992): Immunostimulants, adjuvants and vaccine carriers in fish: Application to aquaculture, in: Faisal M, Hetrick IM, eds. Annual Review of Fish Diseases. USA: Pergamon, Fairviewpark, New york, USA, 281–307.
- Anita A (1998): Influence of Acalypha indica extract on humoral immune responses and growth in tilapia, Oreochromis mossambicus infected with Pseudomonas fluorescens. M.Sc. thesis, SPKCES, M.S. University, Tirunelveli, India.
- Babior BM, Kipnes RS, Curnutte JT (1973): The production by leukocytes of super oxide, a potential bactericidal agent. J Clin Invest 52: 741–744.
- Balasubramanian G, Sarathi M, Venkatesan C, Thomas J, Sahul Hameed AS (2008): Studies on the immunomodulatory effect of extract of Cyanodon dactylon in shrimp, Penaeus monodon, and its efficacy to protect the shrimp from white spot syndrome virus (WSSV). Fish Shellfish Immunol 25: 820–828.
- Chandra RK (1996): Nutrition, immunity and infection from basic knowledge of dietary manipulation of immune responses to practical application of ameliorating suffering and improving survival. Proc Natl Acad Sci USA 93: 14304–14307.
- Cooper RG, Welster LI, Harris JW (1963): Role of mitochondria in iron metabolism of developing erythrocytes. J Clin Invest 42: 926–930.
- Cuesta A, Rodriguez A, Esteban MA, Meseguer J (2005): In vivo effects of propolis, a honey bee product, gilthead seabream innate immune responses. Fish Shellfish Immunol 18: 71–80.
- Dalmo RA, Seljelid R (1995): The immunomodulatory effect of LPS, laminaran and sulphated laminaran [(1,3)-d-glucan] on Atlantic salmon, Salmo salar L. macrophages in vitro. J Fish Dis 18: 175–185.
- Das BK, Pradhan J, Sahu S (2009): The effect of Euglena viridis on immune response of rohu, Labeo rohita (Ham.). Fish Shellfish Immunol 26: 871–876.
- Davis JL, Hayasaka SS (1984): The enhancement of resistance of the American eel, Anguilla rostrata, Lesuer, to a pathogenic bacterium, Aeromonas hydrophila by an extract of the tunicate, Ecteinascidia turbinata. J Fish Dis 7, 311–316.
- Diana J (2006): Influence of leaf extract Phyllanthus emblica on the growth and immune responses in freshwater fish, Cirrhinus mrigala. M.Sc. thesis, SPKCES M.S.University, Tirunelveli, India.
- Diaz-Rosales P, Salinas I, Rodriguez A, Cuesta A, Chabrillon M, Balebona MC, Morinigo MA, Esteban MA, Meseguer J (2006): Gilthead seabream (Sparus aurata L.) innate immune response after dietary administration of heat-inactivated potential probiotics. Fish Shellfish Immunol 20: 482–492.
- Dugenci SK, Arda N, Candan A (2003): Some medicinal plants as immunostimulant for fish. J Ethnopharmacol 88: 99–106.
- Ellias AE (1976): Leucocytes and related cells in the plaice, Pleuronectes platessa. J Fish Biol 8: 143–156.
- Esteban MA, Rodriguez A, Cuesta A, Meseguer J (2005): Effects of lactoferrin on non-specific immune responses of gilthead seabream (Sparus auratus L.). Fish Shellfish Immunol 18: 109–124.
- Fletcher TC, White A (1973): Lysozyme activity in plaice (Pleuronectes platessa L.) Experimenta 29: 1283–1285.
- Fujiki K, Tomoki Y (1997): Effects of sodium alginate on the non-specific defence system of the common carp (Cyprinus carpio L.). Fish Shellfish Immunol 7: 417–427.
- Gowenlock AH (1996): Varley’s Practical Clinical Biochemistry, sixth edition. New Delhi: CBS Publishers, 664–665.
- Graham S, Secombes CJ (1988): The production of a macrophage activating factor from rainbow trout, Salmo gairdneri leucocytes. Immunol 65: 293–297.
- Guojun Y, Ardo L, Thompson KD, Adams A, Jeney Z, Jeney G (2009): Chinese herbs (Astragalus radix and Ganoderma lucidum) enhance immune response of carp, Cyprinus carpio and protection against Aeromonas hydrophila. Fish & Shellfish Immunol 26: 140–145.
- Haney DC, Hursh DA, Mix MC, Winton JR (1992): Physiological and haematological changes in chum salmon artificially infected with erythrocytic necrosis virus. J Aquat Animal Health 4: 48–57.
- Harikrishnan R, Balasundaram C, Kim M-C Kim, J-S, Han Y-J Heo, M-S (2009): Innate immune response and disease resistance in Carassius auratus by triherbal solvent extracts. Fish & Shellfish Immunol 27: 508–515.
- Higson FK, Jones OTJ (1984): The generation of active oxygen species by stimulated rainbow trout leucocytes in whole blood. Comparative Biochem Physiol 77: 783–587.
- Hsieh T-J, Wang J-C, Hu C-J, Li C-T, Kuo CM, Hsieh S-L (2008): Effects of rutin from Toona sinensis on the immune and physiological responses of white shrimp (Litopenaeus vannamei) under Vibrio alginolyticus challenge. Fish Shellfish Immunol 25: 581–588.
- Jian J, Wu Z (2004): Influences of traditional Chinese medicine on non-specific immunity of Jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol 16: 185–191.
- Kumar V, Sahu NP, Pal AK, Kumar S (2006): Immunomodulation of Labeo rohita juveniles due to dietary gelatinized and non-gelatinized starch. Fish Shellfish Immunol 23: 341–353.
- Kumari J, Sahoo PK (2006): Dietary β-1,3 glucan potentiates innate immunity and disease resistance of Asian catfish, Clarias batrachus (L.). J Fish Dis 29: 95–101.
- Kumari J, Sahoo PK, Giri SS (2007): Effects of polyherbal formulation “Immuplus” on immunity and disease resistance of Indian major carp, Labeo rohita at different stages of growth. Indian J Exp Biol 45: 291–298.
- Landolt ML (1989): The relationship between diet and the immune response of fish. Aquaculture 79: 193–206.
- Logambal SM, Michael RD (2000): Immunostimulatory effect of Azadirachtin, Oreochromis mossambicus (Peters). Indian J Exp Biol 38: 1092–1096.
- Madasamy D (2003): Influence of plant extract Aegle marmelos on Growth and immune responses in freshwater fish, Catla catla infected with bacterial pathogen, Pseudomonas aeruginosa. M.Sc. thesis, SPKCES, M.S. University, Tirunelveli, India.
- Misra CK, Das BK, Mukherjee SC, Meher PK (2006a): The immunomodulatory effects of tuftsin on the non-specific immune system of Indian major carp, Labeo rohita. Fish Shellfish Immunol 20: 728–738.
- Misra S, Sahu NP, Paul AK, Xavier B, Kumar S, Mukherjee SC (2006b): Pre and post challenge immuno haematological changes in Labeo rohita juveniles fed gelatinized or non-gelatinized carbohydrate with n-3 PUFA. Fish Shellfish Immunol 21: 346–356.
- Mulero V, Esteban MS, Munoz J, Mesegucr J (1998): Dietary intake of levamisole enhances the immune response and disease resistance of the marine teleost gilt head seabream (Sparus aurata L.). Fish Shellfish Immunol 8: 49–62.
- Munoz M, Cedeno R, Rodriguez J, Van der Knaap WPM, Mialhe E, Bachere E (2000): Measurement of reactive oxygen intermediate production in haemocytes of the penaeid shrimp, Penacus vannamei. Aquaculture 191: 89–107.
- Murugesan AG, Rajakumari C (2005): Environmental Science and Biotechnology – Theory and Techniques. Chennai: MJP Publishers, pp. 371–396.
- Pattabiraman TN (1998): Laboratory Manual in Biochemistry, third edition. New Delhi: All India Publishers, pp. 154–155.
- Pickering AD (1981): Introduction in the concept of biological stress, in: Pickering AD, ed., Stress and Fish. New York: Academic Press, pp. 1–10.
- Press McL C, Reitan LJ, Landsverk T (1995): Antigen retention and enzyme reactivity in the spleen of Atlantic salmon, Salmo salar L. following administration of injectable furunculosis vaccines. J Fish Dis 18:199–210.
- Rairakhwada D, Pal AK, Bhathena ZP, Sahu NP, Jha A, Mukherjee SC (2007): Dietary microbial levan enhances cellular non-specific immunity and survival of common carp (Cyprinus carpio) juveniles. Fish & Shellfish Immunol 22: 477–486.
- Rao VY, Das BK, Jyotyrmavee P, Chakrabarthi R (2006): Effect of Achyranthes aspera on the immunity and survival of Labeo rohita infected with Aeromonas hydrophila. Fish Shellfish Immunol 20: 263–273.
- Regina M (2006): Influence of leaf extract of Phyllanthus emblica (Linn) on immunological, haematological and biochemical responses in freshwater fish, Cirrhinus mrigala artificially infected with bacterial pathogen, Pseudomonas fluorescens. M.Sc. Thesis, SPKCES, M.S. University, Tirunelveli, India.
- Sahoo PK, Mukherjee SC (2002): Influence of high dietary α-tocopherol intakes on specific immune responses, non specific resistance factors and disease resistance of healthy and aflatoxin B1-induced immunocompromised Indian major carp. Labeo rohita (Hamilton). Aquaculture Nutrition 8: 159–167.
- Sahu S, Das BK, Pradhan J, Mohn Patra BC, Mishra BK, Sarangi N (2007): Effect of Mangifera indica kernel as a feed additive on immunity and resistance to Aeromonas hydrophila in Labeo rohita fingerlings. Fish Shellfish Immunol 23: 109 -118.
- Sakai M (1999): Current research status of fish immunostimulant. Aquaculture 172: 63–92.
- Sankaran K, Gurnani S (1972): On the variation in the catalytic activity of lysozyme in fishes. Indian J Biochem Biophys 9: 162–165.
- Schnydes J, Baggioline M (1978): Secretion of lysosomal hydrolases by stimulated and non-stimulated macrophages. J Exp Med 148: 453–450.
- Scott AL, Rogers WA (1981): Haematological effects of prolonged sublethal hypoxia on Channel catfish. Ictalurus punctatus (Rafinesque). J Fish Biol 18: 591–601.
- Scrimshaw NS, San Giovanni JP (1997): Synergism of nutrition, infection and immunity: An overview. Am J Clin Nutrition 66: 4645–4779.
- Secombes CJ (1994): Enhancement of fish phagocytic activity. Fish Shellfish Immunol 4: 421–436.
- Seeley KR, Gillespie PD, Weeks BA (1990): A simple technique for the rapid spectrophotometric determination of phagocytosis by fish macrophages. Mar Environ Res 30: 123–128.
- Siwicki AK, Anderson DP, Rumsey GL (1994): Dietary intake of immunostimulants by rainbow trout affects non-specific immunity and protection against furunculosis. Vet Immunol Immunophathol 41: 125–139.
- Sukumaran N, Vallinayagam S (2002): Effect of vitamin C on humoral immune responses in tilapia, Oreochromis mossambicus (Peters), in: Book of Abstracts – World Aquaculture Society, April 23-27 Held at Beijing, China, p. 734.
- Sukumaran N, Anita A (2001): Immunomodulatory effects of Azadirachta indica and Acalypha indica extract in tilapia, Oreochromis mossambicus infected with Pseudomonas fluorescence. Proceedings of the International Technical and Trade Conference of Tilapia held at Kaulampur, Malaysia, Infofish, May, pp. 28–30.
- Suzumoto BK, Schreek CB, McIntyre JD (1977): Relative resistances of three transferrin genotypes of Coho salmon (Oncorhynchus kisutch) and their haematological responses to bacterial kidney disease. J Fisheries Res Board of Canada 34: 1–8.
- Sveinbjornsson B, Seljelid R (1994): Aminated β 1,3-d polyglucose activates salmon pronephros macrophages in vitro. Veterinary Immunol Immunopathol 41: 113–123.
- Vallinayagam S (1997): Influence of Vitamin C on humoral immune responses in Tilapia, Oreochromis mossambicus (Peters) infected with Pseudomonas fluorescens. M.Sc. thesis, SPKCES, M.S. University, Tirunelveli, India.
- Venkatalakshmi S, Michael RD (2001): Immunostimulation by leaf extract of Ocimum sanctum Linn. in Oreochromis mossambicus (Peters). J Aquaculture Tropics 16: 1–10.
- Venkatesan P, Binu Ramesh C, Dinakaran M (2001): Immunostimulation by leaf extract of Crossandra infundbuliformis in Oreochromis mossambicus (Peters). National Workshop on Aquaculture Medicine. Kochi, Kerala, India, Jan 18-20.
- Vikas K, Sahu NP, Pal AK, Shivendra K (2007): Immunomodulation of Labeo rohita juveniles due to dietary gelatinized and non-gelatinized starch. Fish Shellfish Immunol 23: 341–353.
- Whyte SK (2007): The innate immune response of finfish: A review of current knowledge. Fish Shellfish Immunol 23: 1127–1151.
- Yoshida T, Sakai M, Kitao T, Khlil SM, Araki S, Saitoh R, Ieno T Inglis, V (1993): Immunodulatory effects of the fermented products of chicken egg, EF203, on rainbow trout, Oncorhynchus mykiss. Aquaculture 109: 207–214.