Abstract
Context: Holarrhena antidysenterica Wall. (Apocynaceae) is widely used in traditional medical system for treatment of constipation, colic, and diarrhea.
Aim: This study was carried out to provide pharmacological basis for medicinal use of Holarrhena antidysenterica in gastrointestinal disorders.
Materials and methods: Hydro-ethanolic crude extract of Holarrhena antidysenterica (HaCE) and its fractions were studied in various gastrointestinal isolated tissue preparations.
Results: In guinea pig ileum tissues, HaCE at 0.3-10 mg/mL caused pyrilamine-sensitive spasmogenic effect. When tested in spontaneously contracting rabbit jejunum preparations, HaCE (0.01-3.0 mg/mL) caused moderate stimulation, followed by a relaxant effect at next higher concentrations. In presence of pyrilamine, the contractile effect was blocked and the relaxation was observed at lower concentrations (0.01-0.3 mg/mL). HaCE inhibited the high K+ (80 mM)-induced contractions at concentration range of 0.01-1.0 mg/mL and shifted Ca++ concentration response curves to the right, like that caused by verapamil. Activity-directed fractionation revealed that the spasmogenic component was concentrated in the aqueous fraction, while the spasmolytic component was concentrated in the organic fraction.
Discussion and conclusion: These results indicate that the gut stimulant and relaxant activities of Holarrhena antidysenterica are mediated possibly through activation of histamine receptors and Ca++ channel blockade, respectively and this study provides sound mechanistic background for its usefulness in gut motility disorders such as constipation, colic, and possibly diarrhea.
Introduction
Holarrhena antidysenterica Wall. (Apocynaceae), commonly known as bitter oleander and locally as “inderjo tulkh” or “kurchi”, is a small deciduous tree found in Himalayan and sub-Himalayan tracts, ascending up to 1200 m (CitationBaquar, 1989). Its seeds are 1-2 cm long, linear-oblong, light brown, marked with linear lines and are bitter in taste (CitationBaquar, 1989; CitationNadkarni, 1976). The plant is used traditionally for a variety of health disorders, including colic, diarrhea, dysentery, constipation, flatulence, and urethrosis, as well as being considered useful as carminative, antispasmodic, astringent, anthelmintic, lithotriptic, diuretic, aphrodisiac, tonic, cardiosuppressant, and antihypertensive (CitationDuke et al., 2002; CitationUsmanghani et al., 1997). It is known to contain conessine, ergostenol, holarrhenine, kurchicine, resin, and tannin (CitationDuke, 1992; CitationKapoor, 1990).
Holarrhena antidysenterica has been reported to possess antimutagenic (CitationAqil et al., 2008), antibacterial (CitationAqil & Ahmad, 2007) and immunomodulatory (CitationAtal et al., 1986) properties. In this investigation we provide evidence that Holarrhena antidysenterica contains gut stimulatory and inhibitory constituents, mediating their effects via histaminergic and Ca++ antagonist pathways respectively, which may explains the folkloric use of the plant in gastrointestinal motility disorders such as constipation, colic, and diarrhea.
Materials and methods
Plant material, preparation of crude extract and fractions
Dried seeds of Holarrhena antidysenterica were from a local herbal store, identified by taxonomist Jhandar Shah, of the University of Malakand, Chakdara, NorthWest Frontier Province and a voucher specimen (HA-SE-01-08-71) was submitted to the herbarium of the Department of Biological and Biomedical Sciences, the Aga Khan University, Karachi. The plant material was cleaned and extracted with 70% aqueous ethanol by cold maceration and kept for three days with occasional shaking. It was filtered through a muslin cloth and then through a Whatman qualitative grade 1 filter paper. This procedure was repeated twice and the combined filtrate was evaporated on a rotary evaporator under reduced pressure (-760 mmHg) to a thick, semi-solid mass of brown color, i.e., the Holarrhena antidysenterica crude extract (HaCE), yielding approximately 18% w/w.
Activity-guided fractionation was carried out by using solvents of increasing polarity (CitationWilliamson et al., 1998). Approximately 40 g of HaCE was dissolved in 500 mL distilled water. The n-butanol was added to it and shaken vigorously. The mixture was allowed to separate into two layers. The n-butanol layer (lower) was removed. The same process was repeated twice more. All of the n-butanol layers were combined and evaporated to give the n-butanol fraction (HaB). The remaining layer was collected and evaporated to obtain the aqueous fraction (HaAq).
Chemicals
Acetylcholine perchlorate, atropine sulfate, pyrilamine sulfate, and verapamil hydrochloride were purchased from Sigma Chemicals, St. Louis, MO. All chemicals used were of the analytical grade available. Chemicals used for making Tyrode’s solution were: potassium chloride (Sigma), calcium chloride, glucose, magnesium chloride, sodium bicarbonate, sodium dihydrogen phosphate (Merck, Darmstadt, Germany) and sodium chloride (BDH Laboratory Supplies, Poole, UK).
Animals
Animals used in this study, such as guinea pigs (500–600 g) and rabbits (1–1.2 kg) of local breed and either sex, were housed at the Animal House of the Aga Khan University, maintained at 23–25°C and given a standard diet and tap water. Animals had free access to water but food was withdrawn 24 h prior to experiment. Guinea pigs were sacrificed by cervical dislocation and rabbits by blow on back of the head. Experiments performed complied with the rulings of the Institute of Laboratory Animal Resources, Commission on Life Sciences, CitationNational Research Council (1996) and approved by the Ethical Committee of the Aga Khan University.
Phytochemical analysis
Phytochemical screening of the plant extract was carried out for the presence of alkaloids, anthraquinones, coumarins, flavonoids, saponins, sterols, tannins, and terpenes in accordance to the reported procedures (CitationAkinyemi et al., 2005; CitationEdeoga et al., 2005). Briefly, alkaloids were detected by using Dragendorff’s reagent. Presence of saponins was detected based on the appearance of froth upon vigorous shaking of diluted samples. The observation of yellow fluorescence under ultraviolet light on examination of filter paper previously exposed to the vapors from boiling plant material indicated the presence of coumarins. For the detection of sterols and terpenes, plant material was treated with petroleum ether and subsequently extracted with CHCl3. The gradual appearance of green to pink (for sterols) and pink to purple (for terpenes) was then noted after treatment of CHCl3 layer with acetic anhydride and concentrated H2SO4 in succession. Plant material was detected as positive for flavonoids when it gave a yellow color with AlCl3 reagent and for tannins, when green or black was produced with aqueous FeCl3. Lastly, for detecting anthraquinones, the extract was dissolved in 1% HCl, then in benzene, and later if the extract showed pink, violet or red color with NH4OH, that indicated the presence of anthraquinones.
Experimentations
Guinea-pig ileum
The guinea pig was sacrificed by cervical dislocation; the ileum was dissected out and kept in Tyrode’s solution (CitationGilani et al., 1997). Segments about 2 cm length were mounted individually in a 10 mL tissue bath filled with Tyrode’s solution at 37°C and aerated with 95% O2 in CO2 (carbogen). The composition of the Tyrode’s solution in mM was: KCl 2.68, NaCl 136.9, MgCl2 1.05, NaHCO3 11.9, NaH2PO4 0.42, CaCl2 1.8 and glucose 5.55, with pH 7.4. A preload of 1 g was applied to each tissue and isotonic contractions were recorded using a Bioscience transducer coupled to a Harvard Oscillograph. After an equilibration period of 30 min, the tissues were repeatedly treated with sub-maximal concentration (0.3 μM) of ACh with 3 min intervals until constant responses were recorded. The contractile effect of the test material was assessed as a percentage of the maximum effect, produced by the control drug, histamine (1 μM).
Rabbit jejunum
The jejunum was dissected out after surgical opening of the rabbit abdomen, kept in Tyrode’s solution and cleaned of mesenteries (CitationGilani et al., 2005a). Segments about 2 cm in length were suspended in a 10 mL tissue bath containing Tyrode’s solution, maintained at 37°C and aerated with carbogen. Intestinal responses were recorded isotonically using Bioscience transducers coupled to a Harvard Oscillograph. Each tissue was allowed to equilibrate for at least 30 min before the addition of any drug and then stabilized with a sub-maximal concentration of ACh (0.3 μM). Under these conditions, rabbit jejunum exhibits spontaneous rhythmic contractions.
Determination of calcium channel blocking effect
For the elucidation of Ca++ channel blocking (CCB) activity, high K+ (80 mM) was used to depolarize the preparations, as described by CitationFarre et al. (1991). K+ was added to the tissue bath, which produced a sustained contraction. Test materials were then added in a cumulative fashion to obtain concentration-dependent inhibitory responses (CitationVan Rossum, 1963). To confirm the Ca++ antagonist action of the test substance, the tissue was allowed to stabilize in normal Tyrode’s solution, which was then replaced with Ca++-free Tyrode’s solution containing EDTA (0.1 mM) for 30 min in order to remove Ca++ from the tissues. This solution was further replaced with K+-rich and Ca++-free Tyrode’s solution, having the following composition (mM): KCl 50, NaCl 91.04, MgCl2 1.05, NaHCO3 11.90, NaH2PO4 0.42, glucose 5.55 and EDTA 0.1. Following an incubation period of 30 min, control concentration-response curves (CRCs) of Ca++ were obtained. When the control Ca++ CRCs were found super-imposable (usually after two cycles), the tissue was pretreated with the plant extract for 60 min to test the possible CCB effect. The CRCs of Ca++ were reconstructed in the presence of different concentrations of the test material.
Statistical analysis
Data were expressed as mean ± standard error of mean (SEM, n = number of experiments) and the median effective concentrations (EC50 values) with 95% confidence intervals (CI). CRCs were analyzed by non-linear regression using GraphPad program (San Diego, CA).
Results
Phytochemical screening
HaCE was found to contain alkaloids, coumarins, flavonoids, saponins and tannins, while testing negative for other classes.
Effect of crude extract on ileum
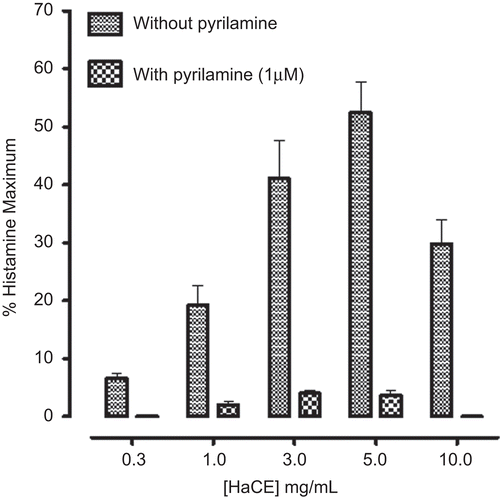
In guinea pig ileum, HaCE caused a concentration-dependent (0.3-5.0 mg/mL) contractile effect with decline at next higher concentration (10 mg/mL). The efficacy of the stimulant effect was 6.5 ± 2, 19.2 ± 8, 41 ± 16, 52.5 ± 12 and 25.8 ± 9.0% (mean ± SEM; n = 6) at 0.3, 1, 3, 5, and 10 mg/mL respectively, compared to histamine maximum contraction (), reflecting partial agonist effect. Pretreatment of the tissue with pyrilamine (1 μM) blocked the stimulatory effect of HaCE (), similar to that of histamine.
Effect of crude extract on jejunum
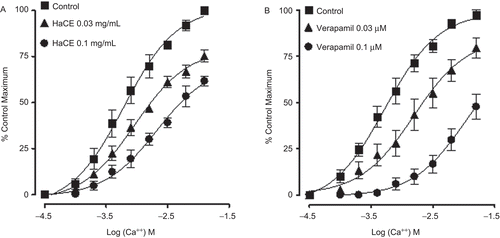
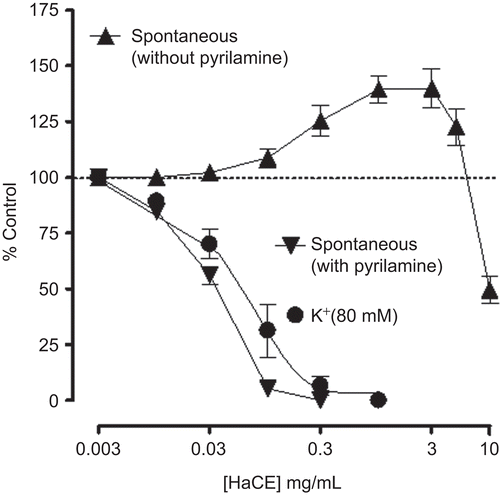
In isolated rabbit jejunum, HaCE (0.01-3.0 mg/mL) caused moderate stimulatory effect followed by weak spasmolytic effect at higher concentrations of 5 and 10 mg/mL (), because of the accompanied relaxant component. The spasmogenic effect was blocked in the presence of pyrilamine (0.1 μM) and the spasmolytic effect was observed at lower concentrations with EC50 value of 0.03 mg/mL (0.02-0.04, 95% CI, n = 4). When tested against high (80 mM) K+-induced contractions, HaCE caused relaxation with EC50 value of 0.07 mg/mL (0.04-0.1, n = 5) as shown in . HaCE concentration-dependently (0.03-0.1 mg/mL) shifted the Ca++ concentration-response curves to the right (), like that caused by verapamil ().
Figure 2. Concentration-response curves of the crude extract of Holarrhena antidysenterica (HaCE) on spontaneous contractions of isolated rabbit jejunum in the absence and presence of pyrilamine (0.1 μM) as well as on K+-induced contractions in jejunum preparations. Values shown are mean ± SEM, from four to seven determinations.
Effect of fractions on Ileum
In ileum, HaB was found devoid of any spasmogenic effect, while HaAq exhibited pyrilamine-sensitive marked stimulatory effect. The efficacy of the contractile effect was 12.5 ± 6.4, 21 ± 7.5, 47 ± 9.8 and 60 ± 9% at 0.3, 1, 3, and 5 mg/mL respectively, while at next higher concentration (10 mg/mL) did not produce further increase in response, rather a trend of decline in response was observed, as the response at this concentration was 50.7 ± 6.7%, of histamine maximum response ().
Effect of fractions on jejunum
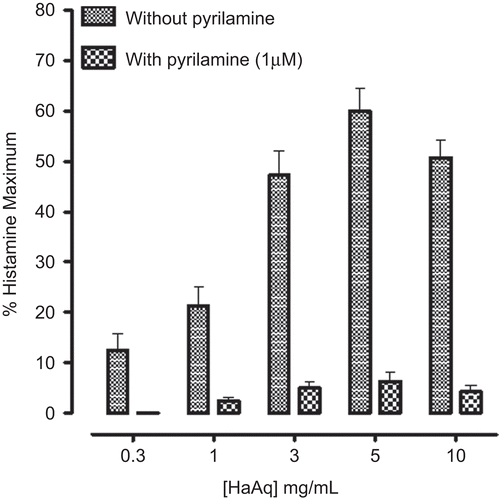
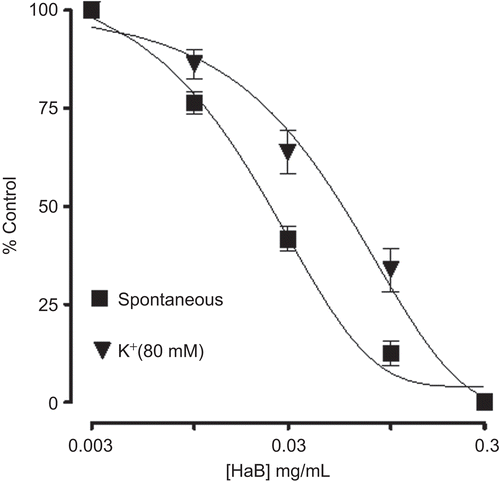
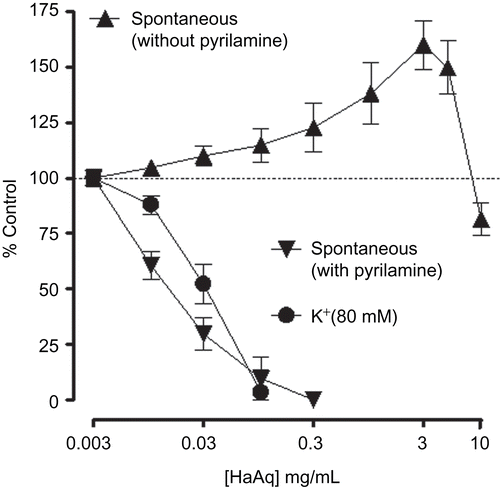
Like the parent crude extract, HaAq caused a concentration-dependent (0.01-3.0 mg/mL) spasmogenic effect in rabbit jejunum followed by relaxation at higher concentrations (5 and 10 mg/mL). In the presence of pyrilamine (0.1 μM), the spasmogenic effect was blocked and the spasmolytic effect was observed at lower concentrations () with EC50 value of 0.01 mg/mL (0.005-0.02, n = 3). In jejunum, relatively low concentration of pyrilamine was used, in comparison to that in ileum, so that it could not affect rhythmicity of jejunum spontaneous contraction. When tested against high K+-induced contraction, HaAq caused concentration-dependent relaxation with EC50 value of 0.05 mg/mL (0.02-0.06, n = 4) as shown in . HaB showed only inhibitory effect on spontaneous and (80 mM) K+-induced contraction with EC50 values of 0.02 mg/mL (0.02-0.04, n = 6) and 0.06 mg/mL (0.04-0.08, n = 4), respectively ().
Figure 5. Concentration-response curves of aqueous fraction of Holarrhena antidysenterica (HaAq) on spontaneous contractions of isolated rabbit jejunum in the absence and presence of pyrilamine (0.1 μM) as well as on K+-induced contractions in jejunum preparations. Values shown are mean ± SEM, from three to four determinations.
Discussion
In view of the well-known medicinal use of Holarrhena antidysenterica in constipation, its crude extract was tested for possible stimulatory effect on guinea pig ileum, a quiescent gut preparation considered useful for this purpose (CitationGhayur & Gilani, 2005b), where it produced excitatory effect, like that caused by histamine and ACh. Pretreatment of the tissues with pyrilamine, a histamine receptor (H1) antagonist (CitationHaley, 1983) blocked the contractile effect, while atropine, a muscarinic receptor antagonist (CitationArunlakshana & Schild, 1959), did not alter it, indicating that crude extract of Holarrhena antidysenterica causes gut stimulation possibly via activation of histaminergic receptors. There is enough evidence for histamine being an important cellular messenger of the gastrointestinal tract (CitationSchworer et al., 1994) and stimulates various smooth muscles including the gut through activation of H1 receptors (CitationHill, 1990). The observed stimulant effect of Holarrhena antidysenterica explains its medicinal use in hypomotility disorders of the gut. The maximum spasmogenic effect of the extract was around 52% of histamine maximum response and was followed by relaxation at next higher concentration, indicating the co-existence of spasmolytic constituents. The combination of gut inhibitory with stimulatory effect in Holarrhena antidysenterica is probably meant by nature not to allow the spasmogenic effect to go beyond the therapeutic limits beyond which it could have caused abdominal cramps, as is the case with chemical drugs used in constipation (CitationGilani et al., 2005a).
The presence of spasmolytic constituents may also explain the use of Holarrhena antidysenterica in hyperactive diseases of the gut such as abdominal spasm and diarrhea. Therefore, the spasmolytic effect was further investigated on rabbit jejunum, a spontaneously contracting gut preparation (CitationGilani et al., 2005c), thus allowing relaxant effect to be studied without induced contraction. When tested on rabbit jejunum, a pyrilamine-sensitive spasmogenic effect followed by spasmolytic effect was observed.
In earlier studies we observed that the relaxant effect of medicinal plants is usually mediated through blockade of calcium channels (CitationGilani et al., 2000, Citation2005b). To investigate whether the spasmolytic effect of Holarrhena antidysenterica is also mediated via a similar mechanism the extract was tested on high K+-induced contractions. High K+ (> 30 mM) is known to cause smooth muscle contractions through opening of voltage-dependent L-type Ca++ channels, thus allowing influx of extracellular Ca++ causing a contractile effect (CitationBolton, 1979) and the substance causing inhibition of high K+-induced contraction is considered an inhibitor of Ca++ influx (CitationGodfraind et al., 1986). HaCE relaxed the high K+-induced contractions, like that caused by verapamil, a standard Ca++ antagonist (CitationFleckenstein, 1977), indicating its CCB action. The Ca++ antagonist effect of Holarrhena antidysenterica was further confirmed when it shifted the Ca++ CRCs to the right, like that caused by verapamil. Ca++ antagonists have been shown to be beneficial in gut disorders resulting from hyperactivity such as abdominal cramps and diarrhea (CitationPasricha, 2006), hence the observed CCB effect justifies the medicinal use of Holarrhena antidysenterica in such conditions. Interestingly, such combinations of gut stimulatory and relaxant activities have been found commonly in plants used for two contrasting states of gut, i.e., constipation and diarrhea (CitationGhayur & Gilani, 2005b; CitationGilani et al., 2005a).
Activity-guided fractionation revealed that the spasmolytic component(s) is distributed among both the organic and aqueous fractions, but the spasmogenic component is separated in the aqueous fraction, which showed stimulant effect slightly stronger than the parent crude extract. The observed histaminergic and Ca++ antagonist effects of the plant might be due to the presence of tannins and flavonoids evident in the phytochemical screening, as the compounds of these classes have been reported to possess histamine- (CitationRead et al., 1970) and CCB-like (CitationDi Carlo et al., 1993; CitationRevuelta et al., 1997) actions, respectively. Saponins are also known as spasmogenic (CitationAwe et al., 1999; CitationGhayur & Gilani, 2005a); however, the contribution of other constituents accounting for reported effects cannot be ignored.
Conclusions
In conclusion, these data indicate that Holarrhena antidysenterica exhibits spasmogenic and spasmolytic effects, mediated through stimulation of histaminergic receptors and Ca++ antagonist mechanisms respectively, though additional mechanisms cannot be ruled out. Thus, this study provides sound pharmacological basis for the medicinal use of Holarrhena antidysenterica in gut disorders such as constipation, colic and diarrhea.
Declaration of interest
This study was supported by the Higher Education Commission of Pakistan, as indigenous M.Phil/PhD scholarship awarded to Aslam Khan.
References
- Akinyemi KO, Oladapo O, Okwara CE, Ibe CC, Fasure, KA (2005): Screening of crude extracts of six medicinal plants used in south-west Nigerian unorthodox medicine for anti-methicillin resistant Staphylococcus aureus activity. BMC Complement Altern Med 5: 6–11.
- Aqil F, Ahmad I (2007): Antibacterial properties of traditionally used Indian medicinal plants. Methods Find Exp Clin Pharmacol 29: 79–92.
- Aqil F, Zahin M, Ahmad I (2008): Antimutagenic activity of methanolic extracts of four ayurvedic medicinal plants. Indian J Exp Biol 46: 668–672.
- Arunlakshana O, Schild HO (1959): Some quantitative uses of drug antagonists. Br J Pharmacol 14: 48–58.
- Atal CK, Sharma ML, Kaul A, Khajuria A (1986): Immunomodulating agents of plant origin. I: Preliminary screening. J Ethnopharmacol 18: 133–141.
- Awe SO, Makinde JM, Olajide OA (1999): Cathartic effect of the leaf extract of Vernonia amygdalina. Fitoterapia 70: 161–165.
- Baquar SR (1989): Medicinal and Poisonous Plants of Pakistan. Karachi: Printas, p. 233.
- Bolton TB (1979): Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev 59: 606–718.
- Di Carlo G, Autore G, Izzo AA, Maiolino P, Mascolo N, Viola P, Diurno MV, Capasso F (1993). Inhibition of intestinal motility and secretion by flavonoids in mice and rats: Structure-activity relationships. J Pharm Pharmacol 45: 1054–1059.
- Duke JA (1992): Handbook of Phytochemical Constituents of GRAS Herbs and Other Economic Plants. Boca Raton, LA: CRC Press, pp. 292–293.
- Duke JA, Bogenschutz-Godwin MJ, Ducelliar J, Duke PAK (2002): Handbook of Medicinal Herbs (2nd Edn.). Boca Raton, USA: CRC Press, p. 219.
- Edeoga HO, Okwu DE, Mbaebie BO (2005): Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol 685–688.
- Farre AJ, Columbo M, Fort M, Gutierrez B (1991): Differential effects of various Ca++ antagonists. Gen Pharmacol 22: 177–181.
- Fleckenstein A (1977): Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Ann Rev Pharmacol Toxicol 17: 149–166.
- Ghayur MN, Gilani AH (2005a): Gastrointestinal stimulatory and uterotonic activities of dietary radish leaves extract are mediated through multiple pathways. Phytother Res 19: 750–755.
- Ghayur MN, Gilani AH (2005b): Pharmacological basis for the medicinal use of ginger in gastrointestinal disorders. Dig Dis Sci 50: 1889–1897.
- Gilani AH, Aziz N, Khan MA, Shaheen F, Jabeen Q, Siddiqui BS, Herzig JW (2000): Ethnopharmacological evaluation of the anticonvulsant, sedative and antispasmodic activities of Lavandula stoechas L. J Ethnopharmacol 71: 161–167.
- Gilani AH, Bashir S, Janbaz KH, Khan A (2005a): Pharmacological basis for the use of Fumaria indica in constipation and diarrhea. J Ethnopharmacol 96: 585–589.
- Gilani AH, Bashir S, Janbaz KH, Shah AJ (2005b): Presence of cholinergic and calcium channel blocking activities explains the traditional use of Hibiscus rosasinensis in constipation and diarrhoea. J Ethnopharmacol 102: 289–294.
- Gilani AH, Shah AJ, Ghayur MN, Majeed K (2005c): Pharmacological basis for the use of turmeric in gastrointestinal and respiratory disorders. Life Sci 76: 3089–3105.
- Gilani AH, Shaheen F, Christopoulos A, Mitchelson F (1997): Interaction of ebeinone, an alkaloid from Fritillaria imperialis, at two muscarinic acetylcholine receptor subtypes. Life Sci 60: 535–544.
- Godfraind T, Miller R, Wibo M (1986): Calcium antagonism and calcium entry blockade. Pharmacol Rev 38: 321–416.
- Haley TJ (1983): Physical and biological properties of pyrilamine. J Pharm Sci 72: 3–12.
- Hill SJ (1990): Distribution, properties, and functional characteristics of three classes of histamine receptor. Pharmacol Rev 42: 45–83.
- Kapoor LD (1990): Hand Book of Ayurvedic Medicinal Plants. Boca Raton, LA: CRC Press, pp. 205–206.
- Nadkarni KM (1976): Indian Materia Medica, third edition. Bombay: Popular Prakashan, pp. 634–651.
- National Research Council (1996): Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press, pp. 1–7.
- Pasricha PJ (2006): Treatment of disorders of bowel motility and water flux; antimemetics; agents used in biliary and pancreatic disease, in: Brunton LL, Lazo JS, Parker KL, Gilman AG, eds, Goodman and Gilman’s The Pharmacological Basis of Therapeutics, eleventh edition. New York: McGraw-Hill, pp. 983–1008.
- Read GW, Naguwa GS, Wigington JJ, Lenney JF (1970): A histamine liberator in the tannin fraction of the Eucalypts. Lloydia 33: 461–471.
- Revuelta MP, Cantabrana B, Hidalgo A (1997): Depolarization-dependent effect of flavonoids in rat uterine smooth muscle contraction elicited by CaCl2. Gen Pharmacol 29: 847–857.
- Schworer H, Reimann A, Ramadori G, Racke K (1994): Characterization of histamine H3 receptors inhibiting 5-HT release from porcine enterochromaffin cells: Further evidence for H3 receptor heterogeneity. Naunyn Schmiedebergs Arch Pharmacol 350: 375–379.
- Usmanghani K, Saeed A, Alam MT (1997): Indusyunic Medicine. Karachi: University of Karachi Press, pp. 255–256.
- Van Rossum JM (1963): Commutative concentration-response curves. II: Techniques for the making of concentration-response curves in isolated organs and the evaluation of drug parameters. Arch Int Pharmacodyn Ther 143: 299–330.
- Williamson EM, Okpako DT, Evans FJ (1998): Selection, Preparation and Pharmacological Evaluation of Plant Material. Chichester: John Wiley, pp. 15–23.