Abstract
Context: In the absence of reliable liver-protective drugs in modern medicine, a large number of medicinal preparations are recommended for treatment of liver disorders.
Objective: The antioxidant, hepatoprotective and kidney protective activities of methanol extracts of Ficus carica Linn. (Moraceae) leaves and fruits and Morus alba Linn. root barks (Moraceae) are evaluated here.
Materials and methods: Liver and kidney damage were induced in rats by carbon tetrachloride in a subcutaneous dose of 1 mL (40% v/v in corn oil)/kg. The extract was given intraperitoneally at doses of 50 mg/kg (F. carica leaf and M. alba root bark) and 150 mg/kg (F. carica fruit). The activity of the extracts was comparable to that of silymarin, a known hepatoprotective agent. Antioxidant activity was evaluated by measuring blood glutathione (GSH) content, superoxide dismutase (SOD), catalase (CAT) activities, and malondialdehyde equivalent (MDA). Hepatoprotective activity was evaluated by measuring serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin, and total protein. These biochemical observations were supported by histopathological examination of liver sections. Kidney function was evaluated by measuring plasma urea and creatinine.
Results: Methanol extracts of Ficus carica and Morus alba showed potent antioxidant and hepatoprotective activities; in-depth chromatographic investigation of the most active extract (Ficus carica leaf extract) resulted in identification of umbelliferone, caffeic acid, quercetin-3-O-β-d-glucopyranoside, quercetin-3-O-α-l-rhamnopyranoside, and kaempferol-3-O-α-l-rhamnopyranoside.
Discussion and conclusion: These findings demonstrate that the phenolic constituents of Ficus carica leaf and Morus alba root bark are responsible at least in part for the observed protective effects.
Introduction
Liver intoxication has increased as a result of exposure to high levels of environmental toxins (CitationLee et al., 2004). Carbon tetrachloride (CCl4) is a potent hepatotoxin producing centrilobular hepatic necrosis (CitationRecknagel, 1983), developing liver and kidney damage, and finally can result in cancer (CitationRecknagel, 1967; CitationMasuda, 2006). Herbal preparations are recommended for treatment of liver disorders (CitationChatterjee, 2000). Evidence from literature is the hepatoprotective activity of plants related to family Moraceae such as Cudrania tricuspidata Carr. root bark (CitationTian et al., 2005), Ficus hispida Linn. leaf (CitationMandal et al., 2000), Ficus racemosa Linn. leaf (CitationMandal et al., 1998, Citation1999), Morus bombycis Koidzumi (CitationJin et al., 2005) and Morus alba Linn. leaf (CitationKalantari et al., 2009).
Ficus carica Linn. (Moraceae) is supposed to be native to Carica in Asia Minor (CitationBaily, 1953). In traditional medicine the roots are used in the treatment of leukoderma and ringworm, and its fruits have antipyretic, purgative and aphrodisiac properties, and have been shown to be useful in inflammations and paralysis (CitationNadkarni & Nadkarni, 1995; CitationKirthikar & Bzasu, 1996). Earlier chemical examination of this plant has shown the presence of psoralen, bergapten, umbelliferone (CitationSeong-Kuk et al., 1995; CitationLouis et al., 2000), β-sitosterol, campesterol, stigmasterol fucosterol, fatty acids (CitationJeong & Lachance, 2001), 6-(2-methoxy-Ζ-vinyl)-7-methyl-pyranocoumarin and 9,19-cycloarlane triterpenoid as an anticancer (CitationHongming et al., 1997; CitationWeiping et al., 1997) and antiproliferative agent; 6-O-acyl-β-d-glucosyl-β-sitosterol (CitationShai et al., 2001), calotropenyl acetate, and lupeol acetate (CitationSaeed & Sabir, 2002).
The root bark of the mulberry tree (Morus species) has been used by human beings for at least 4,000 years. Recent research has shown that this plant has free radical scavenging activities, hypolipidemic, antioxidant, antibacterial, antiviral, astringent, emollient, and anti-inflammatory properties (CitationChung et al., 2003; CitationDu et al., 2003; CitationSingab et al., 2006). A piperidine alkaloid (1-deoxynojirimycin) and glycoproteins (Morans A and 20K) were isolated as antidiabetic agents from Morus root bark and/or leaf (CitationYagi et al., 1976; CitationHikino et al., 1985; CitationKim et al., 1999). White mulberry leaf contains triterpenes (lupeol), sterols (β-sitosterol), bioflavonoids (rutin, moracetin, quercetin-3-triglucoside, and isoquercitrin), coumarins, volatile oil, alkaloids, amino acids, and organic acids (CitationDoi et al., 2001). On the other hand, the antioxidant potency of some phenolic compounds (flavonoids, stilbenes, and 2-arylbenzofurans) from Morus alba has been reported (CitationNomura et al., 1977, Citation1980; CitationFukai & Nomura, 1998; CitationSharma et al., 2001; CitationJin et al., 2002; CitationFukai et al., 2003). The present study was therefore undertaken to evaluate the hepatoprotective activity of both Ficus carica (fruit and leaf) and Morus alba (root bark) extracts.
Materials and methods
Chemicals used for biological study
All chemicals were of analytical grade and purchased from Sigma (St. Louis, MO). Carbon tetrachloride was obtained from Fisher Scientific (Fair Lawn, NJ). Silymarin (Legalon®) was obtained from Chemical Industries Development (CID) (Giza, Egypt). All the commercial kits used for the estimation of biochemical parameters were supplied by BioMed Diagnostics (Cairo, Egypt).
Apparatus and chemicals for phytochemical study
The UV spectra were measured using a Hitachi U–3200 spectrophotometer. The UV spectral data of the isolated compounds were recorded following standard procedures (CitationMabry et al., 1970). 1H-NMR and 13C-NMR spectra were recorded using a JEOL JNM-LA-500 spectrophotometer. Chemical shifts were given in δ values (ppm) with tetramethyl silane (TMS) as an internal standard. Fast atomic bombardment mass spectrometry (FAB-MS) data were registered in negative and positive ion mode using a JEOL JMS-DX 302 spectrophotometer. For column chromatographic fractionation and purification, ion exchange resin, diaion HP-20 (Nippon, Rensui, Japan), was used as a stationary phase. TLC was performed on silica gel 60 F254 sheets, 0.25 mm thickness, 10 × 20 cm (Fluka Chemika, Buchs, Switzerland). Sheets of Whatman paper No.1 MM was used for paper chromatography (PC) and No. 3 MM for preparative paper chromatography (PPC) (Whatman, Maidstone, Kent).
Experimental animals
Adult female Swiss albino rats (100–120 g body weight) were obtained from the Egyptian Organization for Biological Product and Vaccines, Giza, Egypt. The animals were housed in cages with good ventilation and illumination, and had access to unlimited water and standard rodent chow. Animal care and handling was done according to the guidelines set by the World Health Organization, Geneva, Switzerland and approved by the Committee for Animal Care at the National Center for Radiation Research and Technology (NCRRT), Atomic Energy Authority (AEA).
Authentication of plants
The leaf and fruit of F. carica were collected from the Zoological garden in Giza, Egypt, and were air-dried. The root bark of M. alba was collected from the Delta region, Egypt, and was air-dried. Identification of plants was verified by Abd El Salam Mohamed Al-Nowiahi, Department of Taxonomy, Faculty of Science, Ain Shams University, Abbassia, Cairo, Egypt. A voucher specimen of authenticated F. carica leaf (FCL-2009), F. carica fruit (FCF-2009) and M. alba root bark (MRB-2009) were deposited at the Department of Pharmacognosy, Faculty of Pharmacy, Ain Shams University, Abbassia, Cairo, Egypt.
Preparation of the aqueous methanol extracts of leaf and fruit of F. carica and root bark of M. alba
Each fresh plant (2 kg) was air-dried in shade and reduced to fine powder, or pieces (in the case of Ficus carica fruits). After that, each was exhaustively extracted with water (15 L). The extracts were evaporated in vacuo at 40°C to dryness and residues were extracted with methanol until exhaustion. The combined methanol portions were evaporated in vacuo to dryness. The dried residues were made free from methanol and kept in tightly closed sample tubes for biological investigation.
Induction of liver damage in rats
Animals received a hepatotoxic compound (CCl4), administered subcutaneously (s.c.) twice a week as a 40% solution in corn oil in a dose of 1 mL/kg b.wt.
Toxicity studies
Acute toxicity study was performed for the methanol extracts in albino rats. The animals were fasted overnight and provided with only water, after which the extracts were administered intraperitoneally at a dose of 50 mg/kg and observed for 14 days. If mortality was observed in two out of three animals, the dose administered was assigned as the toxic dose. If the mortality was observed in one animal, the same dose was repeated again to confirm the toxic dose. If mortality was not observed, the procedure was repeated for a higher dose. One tenth of the maximum dose of the extract tested for acute toxicity was selected for evaluation of hepatoprotective activity (CitationSunilson et al., 2009), i.e. 50 mg/kg for both Ficus carica leaf and Morus alba root bark extracts and 150 mg/kg for Ficus carica fruit extract.
Experimental design
In the present study animals were divided into six main groups with six rats in each group:
Group 1: Control group left without any treatment.
Group 2: Animals received a hepatotoxic compound (CCl4), administered s.c. twice weekly as 40% solution in corn oil in a dose of 1 mL/kg bw, for eight weeks.
Group 3: Animals intraperitoneally (i.p.) administered silymarin three times weekly in a dose of 50 mg/kg bw (dissolved in DMSO), 1 week before and along with CCl4 (1 mL/kg bw, s.c., twice a week).
Group 4: Animals i.p. administered Ficus carica leaf (methanol extract) three times weekly in a dose of 50 mg/kg bw (dissolved in a mixture of saline and DMSO), 1 week before and along with CCl4 (1 mL/kg bw, s.c., twice a week).
Group 5: Animals i.p. administered Morus alba root bark (methanol extract) three times weekly in a dose of 50 mg/kg bw (dissolved in a mixture of saline and DMSO), along with CCl4 (1 mL/kg bw, s.c., twice a week).
Group 6: Animals i.p. administered Ficus carica fruit (methanol extract) three times weekly in a dose of 150 mg/kg bw (dissolved in saline), along with CCl4 (1 mL/kg bw, s.c., twice a week).
Groups 3-6 include rats subjected to different types of treatments a week before and along with administration of the hepatotoxic compound CCl4 (1 mL/kg bw, subcutaneously twice a week).
Blood sampling and plasma preparation
The animals were fasted overnight prior to sample collection. Animals were anethetized with diethyl ether then their abdomen were cut open and blood was collected by heart puncture and liver was dissected. The animals were sacrificed all at once after eight weeks from the beginning of hepatotoxicity induction. Whole blood was collected by heart puncture after light anesthesia with diethyl ether. The collected blood in heparinized tubes, was divided into two parts; one used as whole blood for the determination of reduced glutathione content (GSH) and superoxide dismutase (SOD), the other part of the sample was centrifuged at 3,500 rpm for 15 min and the separated plasma was collected in ependorff tubes and stored in a freezer at −20°C to be used for the determination of catalase activity levels, lipid peroxidation concentration measured as malondialdehyde (MDA) end product, aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP) activity levels, beside total protein, total bilirubin, plasma urea, and creatinine.
Tissue sampling (liver) and histopathological examination of hepatocytes
After animals were anesthetized, rat liver was dissected, washed with distilled water, dried on filter paper, weighed and kept in 10% formalin, then dehydrated with ethanol solution from 50 to 100%, embedded in paraffin and cut into 5 µm sections and stained using hematoxylin-eosin dye for photomicroscopic observation of hepatic cells (CitationGray, 1964; CitationMandal et al., 1999, Citation2000).
Estimation of biochemical parameters
Glutathione content was determined according to the method of CitationTietze (1969), SOD activity was easily measured according to the method of CitationMinami and Yoshikawa (1979), catalase was measured in plasma according to the colorimetric method of CitationSinha (1972) and lipid peroxidation was measured colorimetrically as described by CitationYoshioka et al. (1979), AST and ALT activity in plasma were determined by a colorimetric method as described by CitationReitman and Frankel (1957), ALP level was determined by a kinetic method according to the method of CitationHenry (1964), serum total protein was determined according to the biuret method described by CitationHenry et al. (1974), plasma total bilirubin was determined according to the method of CitationWalters and Gerarde (1970), urea in plasma was determined by an enzymatic colorimetric method as described by CitationPatton and Crouch (1977) and creatinine in plasma was determined kinetically without deproteinization by a colorimetric method as described by CitationHenry (1974).
Statistical analysis
Statistical analysis was done using SPSS software version 10. The inter-group variation was measured by one-way analysis of variance (ANOVA) followed by post hoc least significant difference (LSD) test. Results were expressed as mean ± SD (standard deviation). For all analysis, the level of statistical significance was set at P < 0.05.
Results
Biological study
Changes in blood glutathione content, superoxide dismutase (SOD) content, plasma catalase activity, and malondialdehyde (MDA) equivalent are presented in .
Table 1. Effect of methanol extracts of Ficus carica (F.c.) leaves, fruits and Morus alba (M.a.) root bark on blood glutathione (GSH) content, activity levels of superoxide dismutase (SOD), catalase (CAT) and malondialdehyde (MDA) content in rats that received CCl4.
After induction of liver damage with CCl4 in the experimental rats, blood glutathione content, SOD and catalase activities markedly decreased by 25.91, 27.67, and 27.62%, respectively, as compared to control, while MDA equivalent of CCl4-treated groups markedly increased by 27.17% as compared to control.
When hepatotoxic rats were treated intraperitoneally with F. carica leaf extract at a dose of 50 mg/kg, or fruit extract at a dose of 150 mg/kg, or M. alba root bark extract at a dose of 50 mg/kg for eight weeks, significant increase in glutathione, SOD, and catalase activity were observed, while MDA equivalent was markedly decreased. In comparison with rats with hepatotoxicity receiving silymarin, as a standard hepatoprotective, at a dose of 50 mg/kg, a marked increase in the levels of glutathione, SOD, and catalase and a significant decrease in MDA equivalent parallel to that of the tested extracts were observed when compared to rats with CCl4 alone.
Rats subjected to CCl4 regimen alone developed significant hepatocellular damage as evident from a significant elevation in plasma alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), total bilirubin, and significant decrease in total protein by 28, 30.51, 26.79, 64.61, and 23.66%, respectively, as compared to control. shows the effect of the different plant extracts on the above liver function parameters. Intraperitoneal administration of one of the tested extracts or silymarin reversed the hepatotoxicity significantly as evident from marked reduction in the CCl4-induced increase in ALT, AST, ALP and total bilirubin levels, and amelioration in the tremendously decreased level of plasma total protein as a result of CCl4 administration.
Table 2. Effect of methanol extracts of Ficus carica (F.c.) leaves, fruits and Morus alba (M.a.) root bark on plasma ALT, AST, ALP activity levels, total protein and total bilirubin in rats that received CCl4.
Kidney function tests are represented by measuring plasma urea and creatinine in rats as shown in and revealed that the CCl4 receiving group developed significant kidney damage as evident from a significant elevation in plasma urea and creatinine with a change of 28 and 35.77%, respectively, as compared to the control group. Intraperitoneal administration of F. carica leaf extract (50 mg/kg), or fruit extract (150 mg/kg), or M. alba root bark extract (50 mg/kg), reversed the kidney damage markedly and exhibited a significant reduction in the CCl4-induced elevation of plasma urea and creatinine. The same results were obtained in the silymarin group.
Table 3. Effect of methanol extracts of Ficus carica (F.c.) leaves, fruits and Morus alba (M.a.) root bark on plasma urea (UR) and creatinine (CR) contents in rats that received CCl4.
At the end of the eight weeks, mortality was observed in the CCl4-treated group and autopsy showed congested and enlarged liver. However, no mortality was observed in either the control or F. carica or M. alba extract-treated groups.
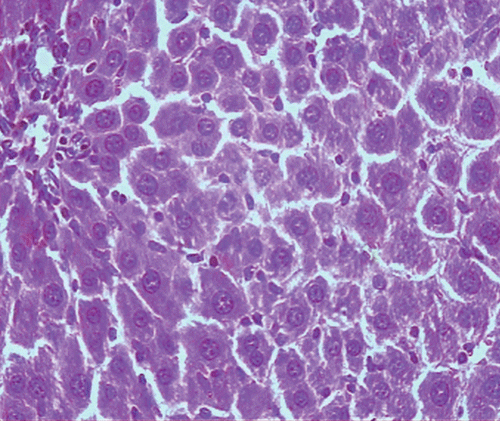
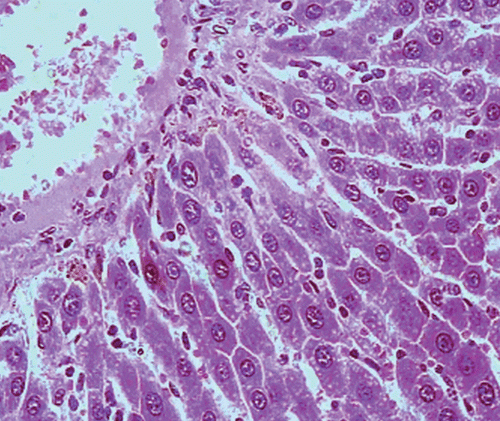
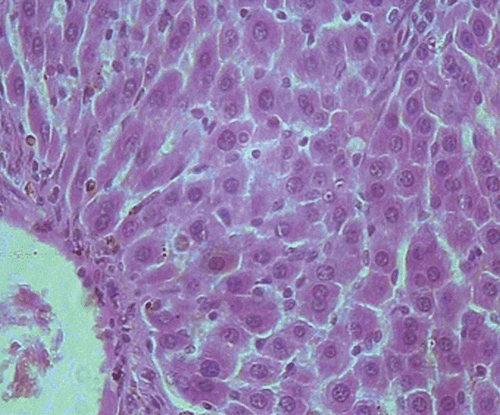
Histopathological examination of liver sections of the control group showed normal cellular architecture with distinct hepatic cell sinusoidal spaces and central vein (). Histopathological profiles of the liver from CCl4-treated rats revealed disarrangement of normal hepatic cells with intense centrilobular necrosis, vacuolization of cytoplasm with distinct fibrosis (). Liver sections of rats treated with silymarin at a dose of 50 mg/kg, i.p. showed significant signs of amelioration of CCl4-evoked liver injury which was evident from the presence of normal hepatic pods and the absence of necrosis and steatosis (). Administration of Ficus carica leaf extract to the experimental animals in a dose of 50 mg/kg, i.p. showed a significant improvement of the hepatocellular architecture over the CCl4-treated groups, as evident from a considerable reduction in necrosis and fibrosis, also the arrangement of cells in normal architecture (). Similar improvement occurred with M. alba root bark extract at a dose of 50 mg/kg i.p. () and F. carica fruit extract at a dose of 150 mg/kg i.p. ().
Figure 1. Microphotograph of liver sections taken from rats of the control group. H and E staining (× 400).
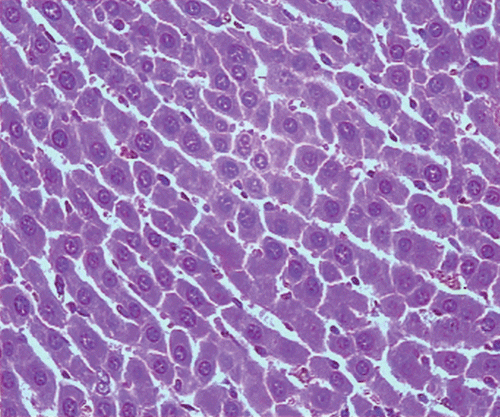
Figure 2. Microphotograph of liver sections taken from rats of CCl4 group, (1 mL/kg of 40% solution, s.c.). H and E staining (× 400).
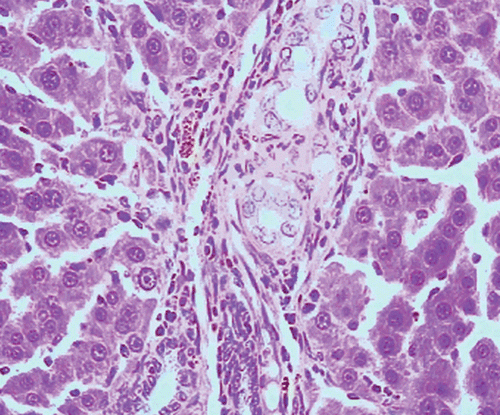
Figure 3. Microphotograph of liver sections taken from rats of silymarin treated group (50 mg/kg, i.p.). H and E staining (× 400).
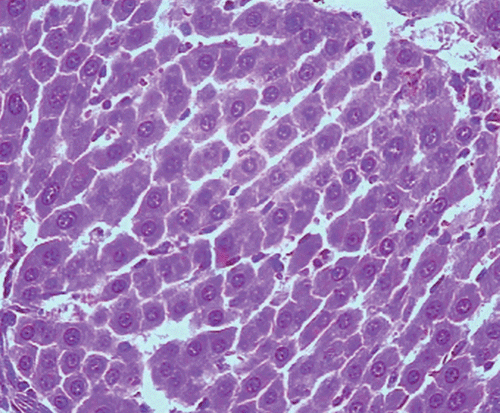
Figure 4. Microphotograph of liver sections taken from rats of F. carica leaves treated group (50 mg/kg, i.p.). H and E staining (× 400).
Phytochemical study
Ficus carica leaf extract (33.5 g) was fractionated over diaion HP-20 CC (100 L × 4.5 cm internal diameter (i.d.)), eluted with water (F-1), 50% MeOH (F-2), and finally with neat MeOH (F-3). Each fraction was concentrated under reduced pressure at a temperature not exceeding 50°C. F-2 (13.7 g) exhibited four spots on two-dimensional PC under short UV light (254 nm), so it was subjected to preparative paper chromatographic separation, (Whatman paper No.3 MM), using solvent composed of acetic acid:water (6:94). The chromatogram showed two blue bands and two dark purple bands. These bands were cut separately and immersed in methanol. The methanol solution of the separated bands was evaporated in vacuo at a temperature not exceeding 50°C to afford three pure compounds (umbelliferone, caffeic acid, and quercetin-3-O-β-d-glucopyranoside), the fourth dark purple band was then subjected to TLC (silica) chromatographic analysis, where it exhibited two dark purple spots which were isolated and purified using preparative silica TLC with solvent system CHCl3:MeOH (8:2); this afforded two pure compounds (quercetin-3-O-α-l-rhamnopyranoside and kaempferol-3-O-α-l-rhamnopyranoside). Identification of compounds was done by comparison with literature data.
Discussion
Carbon tetrachloride produces experimental liver damage that histologically resembles viral hepatitis (CitationLaflamme, 2000; CitationSreelatha et al., 2009). It is accumulated in hepatic parenchyma cells and metabolized to CCl3 by liver cytochrome P450-dependent monooxygenases (CitationRecknagel,1983; CitationRao et al., 2006). This free radical then combined with cellular lipid and proteins in the presence of oxygen to form a trichloromethyl peroxyl radical, which may attack lipids on the membrane of endoplasmic reticulum, faster than trichloromethyl free radical. Thus trichloromethyl peroxyl free radical leads to lipid peroxidation, the destruction of Ca+2 homeostasis, and finally results in cell death (CitationRao et al., 2006).
Hepatic fibrosis is a dynamic process caused by chronic liver injury due to various etiologies, eventually leading to cirrhosis. It is predominantly characterized by excessive accumulation of extracellular matrix caused by both an increased synthesis and decreased or unbalanced degradation of extracellular matrix, CCl4 is one of the most widely used hepatic toxins for experimental induction of hepatic fibrosis and cirrhosis in laboratory animals (CitationJiang et al., 2004; CitationSingab et al., 2005). CCl4-induced fibrosis or cirrhosis in experimental animals resembles human cirrhosis in some aspects of morphology and pathophysiology. In both cases regeneration of hepatocytes occurs after necrosis, and fibrotic infiltration is almost irreversible in the advanced stage of cirrhosis (CitationBeljaars et al., 2002). Thus, CCl4-induced hepatic fibrosis has also been used to assess the efficacy of anti-fibrotic reagents and to verify correlation between pathophysiological features of the liver and serum marker of fibrosis (CitationWU & Norton, 1996). The increase in the level of serum enzymes is an indication of cellular leakage and loss of functional integrity of the cell membrane in the liver (CitationMeyer & Twedt, 2000). The preventive action of liver damage induced by CCl4 has widely been used as a marker of the hepatoprotective activity of drugs in general (CitationWu & Norton, 1996; CitationNoaman, 2007).
Hepatoprotective activity of the extracts was evaluated by their ability to lower the elevated levels of the serum enzymes resulting from CCl4 administration. The significant reduction in enzyme levels towards respective normal values mediated by the tested extracts is an indication of stabilization of the hepatocyte cell membrane as well as repairing of hepatic tissue damage caused by CCl4 (CitationNoaman et al., 2006).
Antioxidative action plays an important role in protection against carbon tetrachloride-induced liver injury (CitationXiong et al., 1998).There is increasing evidence for the hepatoprotective role of hydroxyl- and polyhydroxy organic compounds, particularly from vegetables, fruits and herbs (CitationBass, 1999).The consumption of fruits and vegetables (CitationPeschel et al., 2006) containing antioxidants has been found to offer protection against these diseases. In spite of the tremendous advances in modern medicine no effective drugs are available which stimulate liver functions and offer protection to the liver from damage or help to regenerate hepatic cells (CitationChattopadhyay, 2003).
Silymarin is one of the hepatoprotective natural compounds reported to possess many biological properties and perceived as natural remedies. Such compounds show protective effects against agent-induced hepatic injuries (CitationBerger & Kowdley, 2003; CitationLieber et al., 2003; CitationHedayat et al., 2004). Other studies state that silymarin exhibited antioxidant action (CitationSoto et al., 2003; CitationChlopcikova et al., 2004; CitationLieber, 2004) and showed promising antineoplastic and immunomodulatory effects (CitationAmirghofran et al., 2000; CitationHannay & Yu, 2003; CitationSchümann et al., 2003; CitationYoo et al., 2004). Silymarin has significant activation effects upon SOD and glutathione- related enzyme systems in rats (CitationSoto et al., 2003; CitationHedayat et al., 2004). Silymarin enhanced the activities of glutathione, glutathione reductase and glutathione S-transferase, and inhibited lipid peroxidation in the liver of normal rats (CitationSkottova et al., 2004).
Rats treated with M. alba root bark or F. carica fruit extracts showed similar antioxidant activity as those receiving silymarin, while rats receiving F. carica leaf extract, the was superior to silymarin and showed more potent antioxidant properties. It is clear that Ficus carica leaf extract increased blood glutathione level as compared to control. In this study, methanol extract of F. carica leaf exhibited great hepatoprotective activity. Therefore it was felt important to identify the constituents of this extract. Chromatographic investigation of the methanol extract of Ficus carica leaf resulted in isolation of different compounds namely; umbelliferone (a coumarin), caffeic acid, quercetin-3-O-β-d-glucopyranoside, quercetin-3-O-α-l-rhamnopyranoside, and kaempferol-3-O-α-l-rhamnopyranoside.
The hepatoprotective effect may be attributed to the presence of the isolated compounds, since coumarin compounds have hepatoprotective activity (CitationMatsuda et al., 1998; CitationLee et al., 2002; CitationPark et al., 2004), and caffeic acid has hepatoprotective activity (CitationJanbaz et al., 2004). Flavonoids (plant phenolic pigment products) such as quercetin (a flavonol compound) may delay oxidant injury and cell death by scavenging oxygen radicals; protecting against lipid peroxidation and thereby terminating the chain–radical reaction, chelating metal ions, to form inert complexes that cannot take part in the conversion of superoxide radicals and hydrogen peroxide into hydroxyl radicals (CitationDechameux et al., 1992; CitationIbrahim et al., 2006).
The expected mechanism of the hepatoprotective effect of F. carica leaf, against CCl4-induced liver damage, is the ability of its flavonol glycoside content to act as strong free radical scavengers intercepting those radicals involved in CCl4 metabolism by microsomial enzymes. Consequently, by trapping oxygen-related free radicals, the flavonol glycosides of F. carica leaf could hinder the free radical interaction with polyunsaturated fatty acids and would abolish the enhancement of the lipid peroxidative process leading to hepatic cell damage. The present results agree with previously reported data (CitationHewawasam et al., 2004; CitationJanbaz et al., 2004; CitationWang et al., 2004; CitationOrhan et al., 2007). In order to verify this finding the antioxidant activity of F. carica leaf was determined. The significant amelioration in the antioxidant parameters might result in the hepatoprotective action of flavonol glycosides against oxidative stress induced by CCl4. Consequently, the flavonol glycosides content of F. carica leaf are responsible for the abolishment of CCl4-induced hepatic damage through their strong antioxidant activity. Van Acker and his colleagues discussed the structural aspects of the antioxidant activity of flavonoids. Quercetin appears to be an extremely efficient radical scavenger. The strong inhibitory effect of quercetin was thought to be attributed to its additional phenolic group (3-OH). Also, kaempferol is a very good scavenger, even though it only has one hydroxyl group on the B ring (4′-OH), possibly due to the combination of other characteristics such as, a C2-C3 double bond, 3-OH group, and 4-keto group on ring C (CitationVan Acker et al., 1996, Citation1998). The mechanism of antiradical action of quercetin and its glycoside was evaluated by CitationAfanas’ev et al. (1989) and CitationAlbano et al. (1982) who referred the inhibitory effects of both quercetin and rutin to their ability to ameliorated the damage occurred by CCl4-dependent lipid peroxidation in rat liver microsomes. Therefore, the data reported herein reveal a protective potential of quercetin and kaempferol glycosides, the main constituents of F. carica leaf, against the acute hepatotoxicity induced by CCl4 in rats.
Worthy of mentioning, is that both F. carica (leaf and fruit) and M. alba (root bark) extracts were kidney protective against the damage induced by CCl4, and ameliorated the increase in plasma urea and creatinine levels caused by CCl4. The marked decrease in plasma urea content, (26.84% less than the control group), caused by Morus alba root bark extract may be attributed to the diuretic effect of M. alba root bark (CitationSingab et al., 2006), and since urea is excreted in urine (CitationRefsum & Stromme, 1974), subsequently a diuretic will decrease urea level in blood.
Conclusion
The results obtained revealed that methanol extract of Ficus carica leaf had the most potent antioxidant activity; these antioxidant properties are attributable to the ability of its phenolic constituents to quench reactive oxygen species. Also, the present study confirmed the kidney and liver protective action of both Ficus carica (leaf and fruit) and Morus alba (root bark) extracts against CCl4-induced damage in rats, which was comparable to that of a standard hepatoprotective drug, silymarin. The hepatoprotective effect is documented by the biochemical and histopathological data obtained. It may be speculated that the constituents of Ficus carica leaf (quercetin, kaempferol glycosides, caffeic acid, and coumarins such as umbelliferone) are to be responsible, at least in part, for the observed hepatoprotective effect. A possible mechanism of Morus alba extract as hepatoprotective may be due to its antioxidant effect which impairs the activation of carbon tetrachloride into the reactive form, it may be speculated that the high flavonoid content of Morus alba root bark, was responsible for the observed protective effects. Further studies need to be done on Ficus carica fruit extract to identify the active constituent(s) responsible for the hepatoprotective activity and elucidate the mechanism of action.
Acknowledgements
The authors are grateful to the National Center for Radiation Research and Technology (NCRRT), Atomic Energy Authority (AEA) for hosting the biological studies and providing the necessary facilities.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Afanas’ev IB, Dorozhko AI, Brodskii AV, Kostyuk VA, Potapovitch AI (1989): Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem Pharmacol 38: 1763–1769.
- Albano E, Lott KA, Slater TF, Stier A, Symons NSR, Tomasi P (1982): Spin-trapping studies on the free-radical products formed by metabolic activation of carbontetrachloride in rat liver microsomal fractions of isolated hepatocytes and in vivo in the rat. Biochem J 204: 593–603.
- Amirghofran Z, Azadbakht M, Karimi MH (2000): Evaluation of the immunomodulatory effects of five herbal plants. J Ethnopharmacol 72: 167–172.
- Baily LH (1953): The Standard Cyclopedia of Horticulture. Volume II. New York: Macmillan.
- Bass NM (1999): Is there any use for nontraditional or alternative therapies in patients with chronic liver disease? Curr Gastroenterol Rep 1: 50–56.
- Beljaars LM, Meijer DK, Poelstrak K (2002): Targeting hepatic stellate cells for cell-specific treatment of liver fibrosis. Front Biosci 7: 214–222.
- Berger J, Kowdley KV (2003): Is silymarin hepatoprotective in alcoholic liver disease? J Clin Gastroenterol 37: 278–279.
- Chatterjee TK (2000): Medicinal Plants with Hepatoprotective Properties in Herbal Opinions. Volume III. Calcutta: Books and Allied, p. 135.
- Chattopadhyay RR (2003): Possible mechanism of hepatoprotective activity of Azadirachta indica leaf extract. Part II. J Ethnopharmacol 89: 217–219.
- Chlopcikova S, Psotova J, Miketova P, Simanek V (2004): Chemoprotective effect of plant phenolics against anthracycline-induced toxicity on rat cardiomyocytes. Part I. Silymarin and its flavonolignans. Phytother Res 18: 107–110.
- Chung KO, Kim BY, Lee MH, Kim YR, Chung HY, Park JH, Moon JO (2003): In-vitro and in-vivo anti-inflammatory effect of oxyresveratrol from Morus alba L. J Pharm Pharmacol 55: 1695–1700.
- Dechameux T, Dubois F, Beauloye C, Walliaux-DeConinck S, Woniaux XR (1992): Effect of various flavonoids on lysosomes subjected to an oxidative stress. Biochem Pharmacol 44: 1243–1248
- Doi K, Kojima T, Makino M, Kimura Y, Fujimoto Y (2001): Studies on the constituents of the leaf of Morus alba L. Chem Pharm Bull 49: 151–153.
- Du J, He ZD, Jiang RW, Ye WC, Xu HX, But PP (2003): Antiviral flavonoids from the root bark of Morus alba L. Phytochemistry 62: 1235–1238.
- Fukai T, Nomura T (1998): Proof against 2-hydroxy-3-prenylflavone-oxygen complex by laser desorption/ionization time-of-flight mass spectrometry. Rapid Comm Mass Spectrom 12: 1945–1951.
- Fukai T, Satoh K, Nomura T, Sakagami H (2003): Antinephritis and radical scavenging activities of prenylflavonoids. Fitoterapia 74: 720–724.
- Gray P (1964): Handbook of Basic Microtechnique, third edition. New York: McGraw Hill, pp. 85–145.
- Hannay JA, Yu D (2003): Silibinin: A thorny therapeutic for EGF-R expressing tumors? Cancer Biol Ther 2: 532–533.
- Hedayat IS, Noaman E, Hassan N (2004): Evaluation of the role of Silybum marianum cultivated in Egypt against induced oxidative stress in γ-irradiated rats. Isot Radiat Res 36: 613–628.
- Henry RJ (1964): Clinical Chemistry: Principles and Technics. Determination of Alkaline Phosphatase by a Direct Colorimetric Method. New York: Harper & Row.
- Henry RJ (1974): Clinical Chemistry: Principles and Technics. Determination of Serum Creatinine by a Colorimetric Method, second edition. New York: Harper & Row, p. 525.
- Henry RJ, Cannon DC, Winkelman JW (1974): Clinical Chemistry: Principles and Technics. Determination of Total Protein by a Direct Colorimetric Method, second edition. New York: Harper & Row.
- Hewawasam RP, Jayatilaka KA, Pathirana C, Mudduwa LK (2004): Hepatoprotective effect of Epaltes divaricata extract on carbon tetrachloride-induced hepatotoxicity in mice. Indian J Med Res 120: 30–34.
- Hikino H, Mizuno T, Oshima Y, Konno C (1985): Validity of the oriental medicines. 80. Antidiabetes drugs. 4. Isolation and hypoglycemic activity of moran A, a glycoprotein of Morus alba root bark. Planta Med 2: 159–160.
- Hongming C, Weiping Y, Tianxin W, Mengshen C (1997): Research on the chemical structure and anticancer activity of 9, 19-cyclopropane-24, 25 ethyleneoxide-5-en-3β-spirostol. Zhongguo Yaowu Huaxue Zazhi 7: 46–47.
- Ibrahim NK, Noaman E, Mansour SZ (2006): Role of quercetin and vitamin C in quenching oxidative damage induced by ionizing radiation and carbon tetrachloride in rats. Isot Radiat Res 38: 499–510.
- Janbaz KH, Saeed SA, Gilani AH (2004): Studies on the protective effects of caffeic acid and quercetin on chemical induced hepatotoxicity in rodents. Phytomedicine 11: 424–430.
- Jeong WS, Lachance PA (2001). Phytosterols and fatty acids in fig (Ficus carica, var. Mission) fruit and tree components. J Food Sci 66: 278–281.
- Jiang Y, Liu J, Waalkes M, Kang YJ (2004). Changes in the gene expression associated with carbon tetrachloride-induced liver fibrosis persist after cessation of dosing in mice. Toxicol Sci 79: 404–410.
- Jin WY, Na MK, An RB, Lee HY, Bae KH, Kang SS (2002): Antioxidant compounds from twig of Morus alba. Natural Prod Sci 8: 129–132.
- Jin YS, Sa JH, Shim TH, Rhee HI, Wang MH (2005): Hepatoprotective and antioxidant effects of Morus bombycis Koidzumi on CCl4-induced liver damage. Biochem Biophys Res Comm 329: 991–995.
- Kalantari H, Aghel N, Bayati M (2009): Hepatoprotective effect of Morus alba L. in carbon tetrachloride-induced hepatotoxicity in mice. Saudi Pharm J 17: 90–94.
- Kim ES, Park SJ, Lee EJ, Kim BK, Huh H, Lee BJ (1999): Purification and characterization of Moran 20K from Morus alba. Arch Pharm Res 22: 9–12.
- Kirthikar KR, Basu BD (1996): Indian Medicinal Plants. Vol. III, second edition. Allahabad, India: International Book Distributors, pp. 2329–2331.
- Laflamme DP (2000): Nutritional management of liver disease, in: Bonagura JD, ed., Kirk’s Current Veterinary Therapy XIII. Philadelphia: W.B. Saunders, pp. 693–697.
- Lee HS, Oh H, Kim T, Chai KY, Chung HT, Kwon TO, Jun JY, Jeong OS, Kmi YC, Yun YG (2002): Furocoumarins from Angelica dahurica with hepatoprotective activity on tacrine-induced cytotoxicity in Hep G2 cells. Planta Med 68: 463–464.
- Lee KJ, Woo ER, Choi CY, Shin DW, Lee DG, You HJ, Jeong HG (2004): Protective effect of acteoside on carbon tetrachloride-induced hepatotoxicity, Life Sci 74: 1051–1064.
- Lieber CS (2004): New concepts of the pathogenesis of alcoholic liver disease lead to novel treatments. Curr Gastroenterol Rep 6: 60–65.
- Lieber CS, Leo MA, Cao Q, Ren C, Decarli LM (2003): Silymarin retards the progression of alcohol-induced hepatic fibrosis in baboons. J Clin Gastroenterol 37: 336–339.
- Louis P, Patrick P, Andre M, Jean-Marie B, Andre F, Jean-Paul R (2000): Bergapten content in fig leaf. Annales des Falsifications de l’Expertise Chimiqui et Toxicologique 93: 427–435.
- Mabry TJ, Markham RK, Thomas MB (1970): The Systemic Identification of Flavonoids. New York: Springer Verlag.
- Mandal SC, Maity TK, Das J, Pal M, Saha BP (1998): Ficus racemosa affords antihepatotoxic activity against paracetamol-induced acute liver damage in rats. Natural Prod Sci 4: 174–179.
- Mandal SC, Maity TK, Das J, Pal M, Saha BP (1999): Hepatoprotective activity of Ficus racemosa leaf extract on liver damage caused by carbon tetrachloride in rats. Phytother Res 13: 430–432.
- Mandal SC, Saraswathi B, Ashok Kumar CK, Mohana Lakshmi S, Maiti BC (2000): Protective effect of leaf extract of Ficus hispida Linn. against paracetamol-induced hepatotoxicity in rats. Phytother Res 14: 457–459.
- Masuda Y (2006): Learning toxicology from carbon tetrachloride induced hepatotoxicity. Yakugaku Zasshi 126: 885–899.
- Matsuda H, Murakami T, Kageura T, Ninoniya K, Toguchida I, Nishida N, Yoshikawa M (1998): Hepatoprotective and nitric oxide production inhibitory activities of coumarin and polyacetylene constituents from the roots of Angelica furcijuga. Bioorg Med Chem Lett 8: 2191–2196.
- Meyer DJ, Twedt DC (2000): Effect of extrahepatic disease on the liver, in: Bonagura JD, ed., Kirk’s Current Veterinary Therapy XIII. Philadelphia: W.B. Saunders, pp. 668–671.
- Minami M, Yoshikawa H (1979): A simplified assay method of superoxide dismutase activity for clinical use. Clin Chim Acta 92: 337–342.
- Nadkarni KM, Nadkarni AK (1995): Indian Materia Medica. Volume 1, Bombay, India: Popular Prakashan, pp. 545–547.
- Noaman E (2007): Possible role of hesperidin for abrogating liver injury in rats. Isot Radiat Res 39: 599–617.
- Noaman E, Hedayat ISE, Mansour SZ (2006): Hepatoprotective effect of N-acetyl-l-cysteine on CCl4-induced liver damage in rats under oxidative stress of radiation exposure. Isot Radiat Res 37: 1616–1731.
- Nomura T, Fukai T, Katayanagi M (1977): Oxidative cyclization of morusin with manganese dioxide. Heterocycles 6: 1847–1854.
- Nomura T, Fukai T, Matsumoto J (1980): Studies on the constituents of the cultivated mulberry tree. 6. Oxidative cyclization of morusin. J Het Chem 17: 641–646.
- Orhan DD, Orhan N, Ergun E, Ergun F (2007): Hepatoprotective effect of Vitis vinifera L. leaf on carbon tetrachloride-induced acute liver damage in rats. J Ethnopharmacol 112: 145–151.
- Park EJ, Oh H, Kang TH, Sohn DH, Kim YC. (2004). An isocoumarin with hepatoprotective activity in Hep G2 and primary hepatocytes from Agrimonia pilosa. Arch Pharm Res 27: 944–946.
- Patton CJ, Crouch SR (1977): A colorimetric method for the determination of serum urea. Anal Chem 49: 464–469.
- Peschel W, Snchez-Rabaneda F, Dickmann W, Plesehen A, Gartiza I, Jimnez D, Lamuela-Raventos R, Buxaderas S, Codina C (2006): An industrial approach in the search of natural antioxidants from vegetables and fruit wastes. Food Chemistry 97: 137–150.
- Rao GM, Rao CV, Pushpangadan P, Shirwaikar A (2006): Hepatoprotective effects of rubiadin, a major constituent of Rubia cordifolia Linn. J Ethnopharmacol 103: 484–490.
- Recknagel RO (1983): A new direction in the study of carbon tetrachloride hepatotoxicity. Life Sci 33: 401–408.
- Recknagel RO (1967): Carbon tetrachloride hepatotoxicity. Pharmacol Rev 19: 209–250.
- Refsum HE, Stromme SB (1974): Urea and creatinine production and excretion in urine during and after prolonged heavy exercise. Scand J Clin Lab Investig 33: 247–254.
- Reitman S, Frankel S (1957): A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Path 28: 53–56.
- Saeed MA, Sabir AW (2002): Irritant potential of triterpenoids from Ficus carica leaf. Fitoterapia 73: 417–420.
- Schümann J, Prockl J, Kiemer AK, Vollmar AM, Bang R, Tieg G (2003): Silibinin protects mice from T cell-dependent liver injury. J Hepatol 39: 333–340.
- Seong-Kuk K, Dong-Ok C, Hee-Jong C (1995): Purification and identification of antimicrobial substances in phenolic fraction of fig leaf. Han’guk Nonghwa Hakhoechi 38: 293–296.
- Shai R, Yoel K, Ruth R, Michael S, Raphael M (2001): Suppressors of cancer cell proliferation from fig (Ficus carica) resin: Isolation and structure elucidation. J Nat Prod 64: 993–996.
- Sharma R, Sharma A, Shono T, Takasugi M, Shirata A, Fujimura T, Machii H (2001): Mulberry moracins: Scavengers of UV stress generated free radicals. Biosci Biotechnol Biochem 65: 1402–1405.
- Singab AN, El-Beshbishy HA, Sinkkonen J, Pihlaja K (2006): Hypolipidemic and antioxidant effects of Morus alba L. (Egyptian mulberry) root bark fractions supplementation in cholesterol-fed rats. Life Sci 78: 2724–2733.
- Singab ANB, Youssef DTA, Noaman E, Kotb S (2005): Hepatoprotective effect of flavonol glycosides rich fraction from Egyptian Vicia calcarata Desf. against CCl4-induced liver damage in rats. Arch Pharm Res 28: 791–798.
- Sinha AK (1972): Colorimetric assay of catalase. Anal Biochem 47: 389–394.
- Skottova N, Kazdova L, Oliyarnykm O, Veceram R, Sobolova L, Ulrichova J (2004): Phenolics-rich extracts from Silybum marianum and Prunella vulgaris reduce a high sucrose diet induced oxidative stress in hereditary hypertriglyceridemic rats. Pharmacol Res 50:123–130.
- Soto C, Recobe R, Barron H, Alvarez C, Favaril L (2003): Silymarin increases antioxidant enzymes in alloxan-induced diabetes in rat pancreas. Comp Biochem Physiolc Toxicol Pharmacol 136: 205–212.
- Sreelatha S, Padma PR, Umadevi M (2009): Protective effects of Coriandrum sativum extracts on carbon tetrachloride-induced hepatotoxicity in rats. Food Chem Toxicol 47: 702–708.
- Sunilson JAJ, Muthappan M, Das A, Suraj R, Varatharajan R, Promwichit P (2009): Hepatoprotective activity of Coccinia grandis leaf against carbon tetrachloride induced hepatic injury in rats. Int J Pharmacol 5: 222–227.
- Tian YH, Kim HC, Cui1 JM, Kim YC (2005): Hepatoprotective constituents of Cudrania tricuspidata. Arch Pharm Res 28: 44–48.
- Tietze F (1969): Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal Biochem 27: 502–522.
- Van Acker SA, Best A, Vander VW (1998): Structural aspects of antioxidant activity of flavonoids, in: Rice-Evans CA, Packer L, eds., Flavonoids in Health and Disease. New York: Marcel Dekker, pp. 221–251.
- Van Acker SA, Van den Berg DJ, Tromp MN (1996): Structural aspects of antioxidant activity of flavonoids. Free Rad Biol Med 20: 331–342.
- Walters M, Gerarde H (1970): Ultramicromethod for the determination of conjugated and total bilirubin in serum or plasma. Microchem J 15: 231–236.
- Wang BJ, Liu CT, Tseng CY, Wu CP, Yu ZR (2004): Hepatoprotective and antioxidant effects of Bupleurum kaoi Liu (Chao et Chuang) extract and its fractions fractionated using supercritical CO2 on CCl4-induced liver damage. Food Chem Toxicol 42: 609–617.
- Weiping Y, Hongming C, Tianxin W, Mengshen C (1997): A new coumarin compound with anticancer activity. Zhongcaoyao 28: 3–4.
- Wu J, Norton PA (1996): Animal models of liver fibrosis. Scand J Gastroenterol 31: 1137–1143.
- Xiong Q, Hase K, Tezuke Y, Tani T, Namba T, Kadota S (1998): Hepatoprotective activity of phenylethanoids from Cistanche deserticola. Planta Med 64: 120–125.
- Yagi M, Kouno T, Aoyagi Y, Murai H (1976): The structure of moranoline, a piperidine alkaloid from Morus species. Nippon Nogei Kagaku Kaishi 50: 571–572.
- Yoshioka T, Kawada K, Shimada T, Mari U (1979): Lipid peroxidation in maternal and cord blood and protective mechanism against activated oxygen toxicity in the blood. Am J Obstet Gynecol 135: 372–376.
- Yoo HG, Jung SN, Hwang YS, Kim MH (2004): Involvement of NF-kappaB and caspases in silibinin-induced apoptosis of endothelial cells. Int J Mol Med 13: 81–86.