Abstract
Context: Pergularia daemia (Forsk) Chiov. (Asclepiadaceae) is a slender, hispid, fetid-smelling perennial climber and has been used for the treatment of inflammation, diabetes, malaria, asthma, and liver disorders. Ethnopharmacological surveys conducted among herbal practitioners of Western Ghats, Tamil Nadu, India, revealed that large numbers of laticiferous plant species are used as a source of herbal therapies, in which Pergularia daemia was commonly used to treat liver disease and jaundice.
Objective: The hepatoprotective effect of aqueous and ethanol extracts of Pergularia daemia roots by paracetamol and carbon tetrachloride (CCl4)-induced liver damage in rats was studied.
Materials and methods: The aqueous (PdAE) and ethanol (PdEE) extracts of Pergularia daemia were studied for their hepatoprotective effects on paracetamol and CCl4-induced liver damage on Wistar albino rats. The degree of protection was measured by physical changes (liver weight), biochemical (serum gultamic pyruvic transaminase, serum gultamic oxaloacetic transaminase, alkaline phosphatase, direct bilirubin, total bilirubin, cholesterol and decrease in protein), antioxidant enzymes (lipid peroxidation and glutathione levels), and histological changes.
Results: Pretreatment with PdAE and PdEE significantly prevented the physical, biochemical, antioxidant enzyme levels and histological changes induced by paracetamol and CCl4 in the liver. The effects of PdAE and PdEE were comparable to that of the standard drug silymarin. The ethanol extract was found to exhibit greater hepatoprotective activity than the aqueous extract.
Discussion and conclusion: These results indicate that Pergularia daemia could be useful in preventing chemically induced acute liver injury. From this study it can be concluded that the aqueous and ethanol extracts of P. daemia possess significant hepatoprotective activity.
Introduction
Liver, the key organ of metabolism and excretion, has an immense task of detoxification of xenobiotics, environmental pollutants, and chemotherapeutic agents. Hence, this organ is subjected to a variety of diseases and disorders. At present, in spite of an increasing need for agents to protect the liver from damage, modern medicine lacks a reliable liver protective drug. Therefore, a number of natural substances have been studied to evaluate the hepatoprotective activity.
Paracetamol and carbon tetrachloride are widely used toxicants to induce liver damage in experimental studies, and their toxicity has been studied extensively. The resulting hepatic injury is characterized by leakage of cellular enzymes into the bloodstream and by centrilobular necrosis. The hepatotoxicity of paracetamol has been attributed to the formation of toxic metabolites when a part of the paracetamol is activated by hepatic cytochrome P-450s to a highly reactive metabolite N-acetyl-p-benzoquinoneimine (NAPQI). NAPQI is initially detoxified by conjugation with reduced glutathione (GSH) to form mercapturic acid, which is excreted in urine (CitationGupta et al., 2004). After a toxic overdose of paracetamol, the quantity and rate of NAPQI formation may overwhelm the capacity of the liver to replenish its reduced glutathione stores. The CCl4 is converted into reactive metabolite, halogenated free radical by hepatic cytochrome P-450s (CitationPacker et al., 1978; CitationVan de Straat et al., 1987), which in turn covalently binds to cell membrane and organelles to elicit lipid peroxidation with subsequent tissue injury. High lipid peroxidation values indicate excessive free radical induced peroxidation and it is also a marker of hepatocellular damage (CitationBrattin et al., 1985; CitationPrabakaran et al., 2000).
Pergularia daemia (Forsk.) Chiov. (Asclepiadaceae), known as “veliparuthi” in Tamil, “uttaravaruni” in Sanskrit and “utranajutuka” in Hindi (Hariknot plant; CitationKhare, 2007), has traditionally been used in the form of the whole plant, as an anthelmintic, laxative, antipyretic, expectorant, and to treat infantile diarrhea and malarial intermittent fever (CitationVarier, 1995; CitationKirtikar & Basu, 1999; CitationNadkarni, 2002). The aerial parts of this plant have been reported to exert various pharmacological activities, including antifertility (CitationGolam et al., 2001), antidiabetic (CitationWahi et al., 2002), hepatoprotection (CitationSureshkumar & Mishra, 2006, Citation2007, Citation2008a, Citation2008b), analgesic, antipyretic, and anti-inflammatory (CitationSathish et al., 1998). The roots of this plant have been used as an emetic (CitationMittal et al., 1962), and used to treat gonorrhea (CitationSamuelsson et al., 1991), asthma, and constipation (Reddy et al., 1988). From the literature, the roots of P. daemia have been proved to have central nervous system (CNS) depressant (CitationNikajoo, 2009), antioxidant, analgesic, anti-inflammatory and antipyretic activities (CitationBhaskar & Balakrishnan, 2009a, Citation2009b). Phytochemically the plant has been investigated for the presence of cardenolides, alkaloid, saponins (CitationSathish et al., 1998) and steroidal compounds (CitationAnjaneyulu et al., 1998).
Based on its diversified pharmacological properties and its use in liver disease in the traditional Indian system of medicine, an attempt has been made to validate the roots of Pergularia daemia for its hepatoprotective potential against paracetamol and carbon tetrachloride-induced hepatotoxicity in rats.
Materials and methods
Plant material
Roots of Pergularia daemia were collected from the Maruthamalai Hills, Coimbatore, India in November 2006 and identified by P. Jayaraman, Plant Anatomy Research Centre, Chennai, India. The voucher specimen of P. daemia (PARC/2007/52) has been preserved in our laboratory for further collection and reference. The roots were dried under shade, powdered with a mechanical grinder and passed through a 40-mesh sieve. The ethanol extract (PdEE) was obtained by macerating in 95% ethanol for 48 h using Soxhlet apparatus. The aqueous extract (PdAE) was prepared by maceration for about 72 h at room temperature. The solvents were removed from the extracts under reduced pressure by using a rotary vacuum evaporator (Buchi model, Jyoti Lab, Gwalior, India).
Phytochemical analysis
The PdAE and PdEE were subjected to tests to identify the presence of various phytoconstituents, i.e. alkaloids (Dragendorff’s test), steroids and terpenoids (Liebermann Burchard test), tannin and phenolic compounds (ferric chloride test), flavonoids (Shinoda test), amino acids (Ninhydrin test), etc., by the usual methods prescribed in standard texts (CitationKokate, 1994; CitationKhandelwal, 2004).
Experimental animals
Wistar albino rats (150-200 g) used in the present study were procured from listed suppliers Sri Venkateswara Enterprises, Bangalore, India. The animals were fed with standard pellet diet (Hindustan Lever, Bangalore) and water ad libitum. All the animals were acclimatized for a week before use. The experimental protocols were approved by the Institutional Animal Ethics Committee after scrutiny (IAEC No. Pcog/16/2008). The animals received the drug by oral gavage tube. All the animals were cared for under ethical consideration as per the CitationCPCSEA guidelines (2003) with regular inspections of rats.
Chemicals
All the chemicals and solvents were of analytical grade and were procured from Ranbaxy Fine Chemicals, Mumbai, India. The standard drug silymarin was obtained as a gift sample from Micro Labs, Bangalore, India. Standard kits for serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), alkaline phosphatase (ALP) and bilirubin were obtained from Span Diagnostics, Surat, India.
Acute toxicity studies
Healthy Wistar albino rats of either sex weighing 150-200 g maintained under standard laboratory conditions were used for acute oral toxicity test according to Organization for Economic Co-operation and Development guideline 423 (CitationOECD, 1996). A total of three animals were used, which received a single oral dose of (2000 mg/kg) of PdAE and PdEE. Animals were kept overnight fasting prior to administration of PdAE and PdEE. After administration of PdAE and PdEE food was withheld for a further 3–4 h. Animals were observed individually at least once during first 30 min after dosing, periodically during first 24 h (with special attention during the first 4 h) and daily thereafter for period of 3 days. Observations were done daily for changes in skin and fur, eyes, mucus membrane (nasal), respiratory rate, circulatory signs (heart rate), autonomic effect (salivation, lacrimation, perspiration, urinary incontinence and defecation) and central nervous system (drowsiness, gait, tremors and convulsion) changes.
Paracetamol-induced hepatotoxicity rats
The rats were divided into seven groups of 6 animals (n = 6) in each (CitationBhattacharya et al., 2003).
Group I: received water (5 mL/kg p.o.) for 9 days once daily, and served as normal control
Group II: received water (5 mL/kg p.o.) for 9 days once daily and paracetamol 1 g/kg, p.o. on day 7.
Group III: received standard drug silymarin (25 mg/kg p.o.) for 9 days once daily and paracetamol 1 g/kg, p.o. on day 7.
Groups IV and V: received PdAE (100 and 200 mg/kg respectively) for 9 days once daily and paracetamol 1 g/kg p.o. on day 7.
Groups VI and VII: received PdEE (100 and 200 mg/kg respectively) for 9 days once daily and paracetamol 1 g/kg p.o. on day 7.
CCl4-induced hepatotoxicity rat
The rats were divided into seven groups of six animals in each (n = 6) (CitationBhattacharya et al., 2003).
Group I: received water (5 mL/kg p.o.) for 9 days once daily, and served as normal control.
Group II: received water (5 mL/kg p.o.) for 9 days once daily and carbon tetrachloride 1 mL/kg in 50% v/v olive oil, s.c. on day 7.
Group III: received standard drug silymarin (25 mg/ kg p.o.) for 9 days once daily and carbon tetrachloride 1 mL/kg in 50% v/v olive oil, s.c. on day 7.
Groups IV and V: received PdAE (100 & 200 mg/kg) 9 days once daily and carbon tetrachloride 1 mL/kg in 50% v/v olive oil, s.c. on day 7.
Groups VI and VII: received PdEE (100 & 200 mg/kg) 9 days once daily and carbon tetrachloride1 mL/ kg in 50% v/v olive oil, s.c. on day 7.
Assessment of hepatotoxicity
After 48 h of paracetamol and carbon tetrachloride administration, the blood was obtained from animals by puncture of the retro-orbital plexus. The blood samples were allowed to clot for 45 min at room temperature. The serum was separated by centrifugation at 2,500 rpm at 30°C for 15 min and used for the estimation of various biochemical parameters including SGOT and SGPT (CitationReitman & Frankel, 1957), ALP (CitationKind & King, 1954), serum bilirubin (CitationD’Amour et al., 1965), serum protein (CitationLowry et al., 1951) and serum cholesterol (CitationSingh, 2001).
After collection of blood samples, the animals were sacrificed under deep ether anesthesia and their livers were excised immediately and washed with ice-cold saline and a 10% homogenate prepared in phosphate buffer (pH 7.0). The homogenate was centrifuged at 3,000 rpm for 15 min at 4°C and the supernatant was used for the estimation glutathione (CitationEllman, 1959) and lipid peroxidation (CitationOhkawa et al., 1979).
Morphological parameters such as weight of the animals and weight of the liver have also been used to evaluate the protective effect of the drug. Hepatotoxicity causes loss in liver weight/100 g body weight of rats (CitationAvadhoot & Rana, 1991; CitationBhanwra et al., 2000).
A portion of the liver tissue of all the animal groups was excised and was then washed with normal saline. The liver tissues were fixed in 10% buffered neutral formalin for 48 h and then with bovine solution for 6 h and were then processed for paraffin embedding. By using a microtome, sections of 5-mm thickness were taken and stained with hematoxylin and eosin. These sections were examined under light microscope using a magnification of 100× (CitationMankani et al., 2005).
Statistical significance
The results of the study were expressed as mean ± SEM, n = 6. ANOVA (CitationGennaro, 1995) was used to analyze and compare the data, followed by CitationDunnett’s (1964) test for multiple comparisons using statistical package Graphpad InStat 3.
Results
Preliminary phytochemical studies of PdAE and PdEE showed the presence of various phytochemicals including alkaloids, glycosides, steroids, flavonoids, saponin, tannin and phenolic compounds, terpenoids, carbohydrates, gums and mucilage (). The PdAE and PdEE were found to be non-toxic up to a dose of 2000 mg/kg.
Table 1. Phytochemical screening of Pergularia daemia root extracts (PdAE and PdEE).
Paracetamol and CCl4 caused significant elevation of serum liver enzymes, bilirubin and cholesterol. Also, there was significant increase in lipid peroxidation products, malonaldehyde (MDA), and decrease in serum protein antioxidant enzymes glutathione (GSH) comparison with the control groups. Treatment with PdAE and PdEE (100 and 200 mg/kg) caused significant hepatoprotective effect almost comparable to that of silymarin, the known hepatoprotective agent (, , , and ). With the PdAE and PdEE at a dose of 100 mg/kg the effect was only marginal, whereas at higher dose (200 mg/kg) the drug effectively prevented the paracetamol and CCl4-induced liver damage. The ethanol extract (PdEE) was found to exhibit greater hepatoprotective activity than the aqueous extract (PdAE).
Table 2. Effect of PdAE and PdEE on serum enzyme and biochemical parameters in paracetamol-induced hepatotoxicity in rats.
Table 3. Effect of PdAE and PdEE on liver weight, glutathione (GSH) and lipid peroxidation (LPO) in paracetamol-induced hepatotoxicity in rats.
Table 4. Effect of PdAE and PdEE on serum enzyme and biochemical parameters in CCL4-induced hepatotoxicity in rats.
Table 5. Effect of PdAE and PdEE on liver weight, glutathione (GSH) and lipid peroxidation (LPO) in CCL4-induced hepatotoxicity in rats.
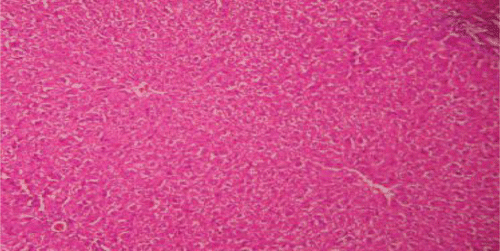
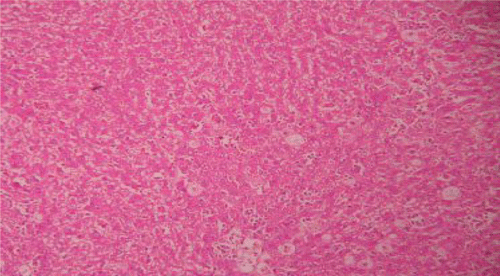
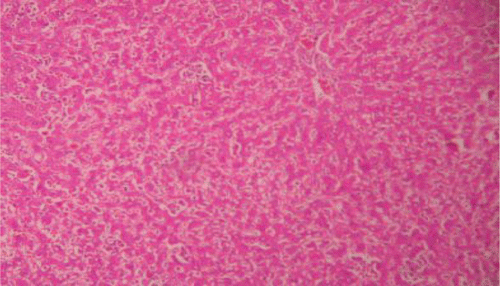
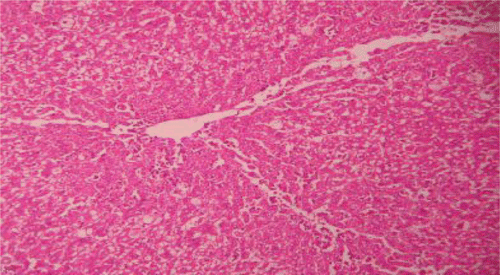
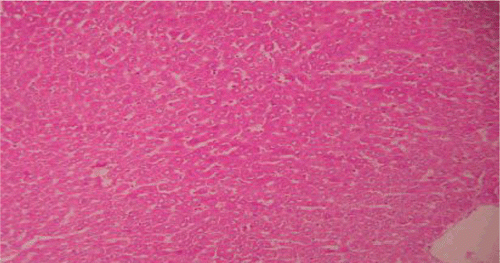
Histopathological examinations of livers damaged with paracetamol showed centrilobular necrosis, ballooning degeneration, inflammatory infiltration of lymphocytes and fatty changes (). The liver sections of control rats showing normal hepatic cells () and rats treated with the PdAE and PdEE showed well-preserved architecture, almost similar to silymarin treated groups (, and ). The liver of CCl4-intoxicated rats showed massive fatty changes, gross necrosis, and broad infiltration of lymphocytes and Kupffer cells around the central vein and loss of cellular boundaries (). The histological pattern of liver rats pre-treated with the PdAE and PdEE showed normal lobular pattern with fatty changes, necrosis and lymphocyte infiltration almost comparable to the control and silymarin-treated groups (, and ).
Figure 2. Liver section of paracetamol rats shows necrosis, ballooning degeneration and massive infiltration of lymphocytes.
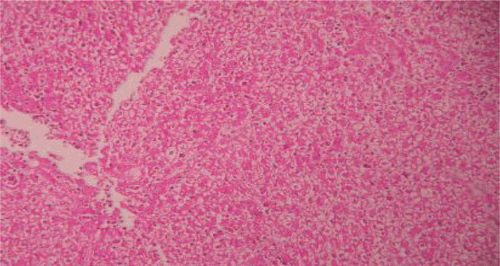
Figure 3. Liver section of silymarin and paracetamol rats shows moderate necrosis, moderate ballooning degeneration and little infiltration of lymphocytesries.
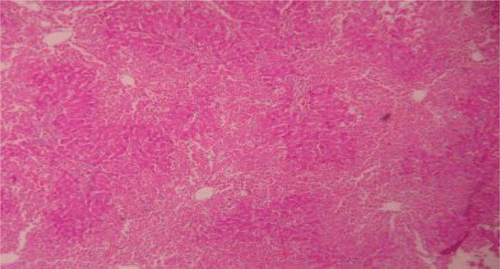
Figure 4. Liver section of PdAE (200 mg/kg) and paracetamol showing less fatty changes, mild necrosis, degeneration and little infiltration of lymphocytes.
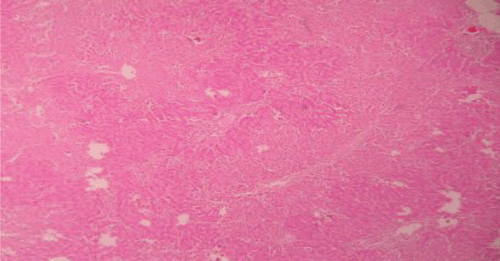
Figure 5. Liver section of PdEE (200 mg/kg) and paracetamol showing moderate fatty changes, necrosis, ballooning degeneration and no infiltration of lymphocytes.
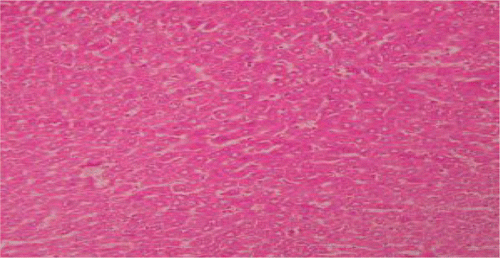
Figure 7. Liver section of silymarin and CCl4shows mild fatty changes, necrosis, mild ballooning degeneration and mild infiltration of lymphocytes.
Discussion
In the present study, PdAE and PdEE at a dose of 100 and 200 mg/kg caused significant inhibition in the levels of SGOT and SGPT towards the respective normal range and this is an indication of stabilization of plasma membrane as well as repair of hepatic tissue damage caused by paracetamol and CCl4. On the other hand, suppression of elevated ALP activities with concurrent depletion of raised bilirubin level and an increase in the total plasma protein content suggests the stability of biliary dysfunction in rat liver during hepatic injuries with toxicants (CitationMukherjee, 2002). These results indicate that PdAE and PdEE preserved the structural integrity of hepatocellular membrane and liver cell architecture damaged by CCl4 and paracetamol which was confirmed by histopathological examination.
Thiobarbituric acid assay is the most popular method of estimation of malondialdehyde level, which is an indication of lipid peroxidation and free radical activity. The increase in lipid peroxidation, a degradative process of membranous polyunsaturated fatty acid has been suggested by the increase in MDA in CCl4- and paracetamol-induced toxicity in the liver. The increased lipid peroxidation results in changes in cellular metabolism of the hepatic and extra hepatic tissues, which ultimately leads to whole cell deformity and cell death (CitationSavides & Woehme, 1983; CitationWinrow et al., 1993). In the present study, an elevation of MDA levels of liver of animals treated with two toxicants was observed. The increase in MDA levels of liver suggest enhanced lipid peroxidation leading to tissue damage and failure of antioxidant defense mechanisms to prevent formation of excessive free radicals. Pre-treatment with PdAE significantly reduced the levels of lipid peroxidation.
Glutathione is one of the most abundant naturally occurring tripeptide, non-enzyme biological antioxidants present in liver (CitationGul et al., 2000). Its functions are concerned with the removal of free radicals such as H2O2 and superoxide radicals, maintenance of membrane protein, detoxification of foreign chemicals and biotransformation of drugs (CitationComporti et al., 1973, Citation1991). In the present study the decreased level of GSH has been associated with an enhanced level of lipid peroxidation in CCl4- and paracetamol-intoxicated groups of rats. Pre-treatment with PdAE significantly increased the level of glutathione in a dose-dependent manner. Thus PdAE may act by inducing the detoxifying enzymes and these enzymes may detoxify the reactive oxygen species (ROS) following administration of toxicants. This suggests that PdAE can reduce ROS that may lessen the oxidative damage to the hepatocytes and improve the activities of the liver antioxidant enzymes, thus protecting the liver from CCl4 and paracetamol.
Phytochemical reports revealed that PdAE and PdAE were found to contain higher concentrations of flavonoids and phenolic compounds, and preliminary phytochemical screening of these extracts showed various phytoconstituents including alkaloids, glycosides, steroids, flavonoids, saponin, tannin, phenolic compounds, terpenoids, carbohydrates, gums and mucilage (CitationBhaskar & Balakrishnan, 2009b). However, flavonoids (CitationPaya et al., 1993), terpenoids (CitationGao et al., 2004), saponins (CitationTran et al., 2001) and glycosides (CitationVijayan et al., 2003) are known to possess hepatoprotective activities in animals. Hence, the presence of secondary metabolites such as flavonoids, terpenoids, glycosides and saponins in these extracts may be responsible for hepatoprotective activity.
Conclusion
It can be concluded that the aqueous and ethanol extracts of roots of Pergularia daemia have significant hepatoprotection against paracetamol- and CCl4-induced hepatic damage in rats, as evidenced by the biochemical and histological parameters. Further investigation to determine the exact phytoconstituents responsible for its hepatoprotective effect is recommended.
Acknowledgement
The authors are grateful to B. Jayakar, Principal, Vinayaka Mission College of Pharmacy, Vinayaka Mission University, Salem, Tamil Nadu, India and A. Balasubaramaniam, Director, Technocrats Institute of Technology – Pharmacy, Bhopal, India for providing necessary facilities to conduct this study.
Declaration of interest
The authors report no conflict of interests. The authors alone are responsible for the content and writing of the paper.
References
- Anjaneyulu ASN, Raju DVSN, Srinivasa Rao S (1998): Chemical evaluation of Pergularia extensa. Indian J Chem 37B: 318–320.
- Avadhoot Y, Rana AC (1991): Hepatoprotective effect of Vitex negundo against carbon tetrachloride induced liver damage. Arch Pharm Res 14: 96–98.
- Bhanwra S, Singh J, Khosla P (2000): Effect of Azadirachta indica leaf aqueous extract on paracetamol induced liver damage in rats. Indian J Physiol Pharmacol 44: 64–68.
- Bhaskar VH, Balakrishnan N (2009a): In vitro antioxidant property of laticiferous plant species from Western Ghats Tamil Nadu, India. Int J Health Res 2: 163–170.
- Bhaskar VH, Balakrishnan N (2009b): Analgesic, anti-inflammatory and antipyretic activities of Pergularia daemia and Carissa carandas. Daru 17: 168–174.
- Bhattacharya D, Pandit S, Mukherjee R, Das N, Sur TK (2003): Hepatoprotective effect of Himoliv® polyherbal formulation in rats. Indian J Physiol Pharmacol 47: 435–440.
- Brattin WJ, Glenda EA, Recknagel RO (1985): Pathological mechanisms in carbon tetrachloride hepatotoxicity. J Free Radical Biol Med 1: 27–38.
- Comporti M, Benedeti A, Chieli E (1973): Studies on in vitro peroxidation of lipids in ethanol treated rats. Lipids 8: 498–502.
- Comporti M, Maellaro E, Del Bello B, Casini AF (1991): Glutathione depletion, its effect on other antioxidant systems and hepatocellular damage. Xenobiotica 21: 1067–1076.
- CPCSEA (2003): Committee for the Purpose of Control and Supervision on Experimental Animals guidelines for laboratory animal facility. Indian J Pharmacol 35: 257–274.
- D’Amour FF, Blood FR, Belden DA (1965): The Manual for Laboratory Work in Mammalian Physiology. Chicago: University of Chicago Press, pp. 126–128.
- Dunnett CW (1964): New tables for multiple comparisons with a control. Biometrics 20: 482–491.
- Ellman GL (1959): Tissue sulfhydryl groups. Arch Biochem Biophys 17: 214–226.
- Gao J, Tang X, Dou H, Fan Y, Zhao X, XU Q (2004): Hepatoprotective activity of Terminalia catappa L leaves and its two terpenoids. J Pharm Pharmacol 56: 1449–1555.
- Gennaro AR. (1995): Remington’s The Science and Practice of Pharmacy, Vol. I, nineteenth edition. Easton, PA: Mack Publishing, pp. 111.
- Golam S, Gafur MA, Shah Alam Bhuiyan M, Khurshid Alam AHM, Helal U, Biswas M, Hassan P, Mannan A, Faruk O, Khan M, Chowdhury AKA (2001): Antifertility activity of Pergularia daemia. The Sciences 1: 22–24.
- Gul M, Kutay FZ, Temocin S, Hanninen O (2000): Cellular and clinical implications of glutathione. Indian J Exp Biol 38: 625–634.
- Gupta M, Mazumder UK, Kumar TS, Gomathi P, Kumar RS (2004): Antioxidant and hepatoprotective effects of Bauhinia racemosa against paracetamol and carbon tetrachloride induced liver damage in rats. Iranian J Pharmacol Therapeutics 3: 12–20.
- Khandelwal KR (2004): Practical Pharmacognosy, eleventh edition. Pune, India: Nirali Prakashan, pp. 149–153 and 157–159.
- Khare CP (2007): Indian Medicinal Plants: An Illustrated Dictionary. New York: Springer Science, pp. 472.
- Kirtikar KR, Basu BD (1999): Indian Medicinal Plants, Vol II. Dehradun: International Book Distributors, pp. 1546–1547.
- Kind PRN, King EJ (1954): Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J Clin Pathol 7: 322–326.
- Kokate CK (1994): Practical Pharmacognosy, fourth edition. Delhi: Vallaph Prakashan, pp. 107–111.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951): Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275.
- Mankani KL, Krishna V, Manjunatha BK, Vidya SM, Singh SJ, Manohara YN, Raheman A, Avinash KR (2005): Evaluation of hepatoprotective activity of stem bark of Pterocarpus marsupium Roxb. Indian J Pharmacol 37: 165–168.
- Mittal OP, Tamm C, Reichstein T (1962): The glycosides of Pergularia extensa. Helv Chim Acta 45: 907–924.
- Mukherjee PK (2002): Quality Control of Herbal Drugs. New Delhi: Business Horizons Pharmaceutical Publications, pp. 531.
- Nadkarni AK (2002): Indian Materia Medica, Volume I. Mumbai: Popular Prakashan, pp. 266.
- Nikajoo LT. (2009): Central nervous system depressant activity of alcohol and aqueous root extracts of Pergularia daemia (Forsk.) Chiov. Pharmacologyonline 1: 119–124.
- OECD (1996): Test no. 423; Acute oral toxicity – Acute toxic class method. Guidelines for the Testing of Chemicals. Paris: Organisation for Economic Cooperation and Development.
- Ohkawa H, Ohishi N, Nagi K (1979): Assay of lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal Biochem 95: 351–358.
- Packer JE, Slater TF, Wilson RL (1978): Reactions of the carbon tetrachloride related peroxy free radical (CC 130.2) with amino acids: Pulse radiolysis evidence. Life Sci 23: 2617–2620.
- Paya M, Ferrandiz ML, Sanz MJ, Alcaraz MJ (1993): Effects of phenolic compounds on bromobenzene mediated hepatotoxicity in mice. Xenobiotica 23: 327–333.
- Prabakaran M, Rangasamy A, Devaki T (2000): Protective effect of Hemidesmus indicus against rifampicin and isoniazid-induced hepatotoxicity in rats. Fitoterapia 71: 55–59.
- Reddy MB, Reddy KR, Reddy MN (1998): A survey of medicinal plants of Chenchu tribes of Andhra Pradesh, India. Int J Crude Drug Res 26: 189–196.
- Reitman S, Frankel S (1957): Colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transminases. Am J Clin Pathol 28, 56–63.
- Samuelsson G, Farah MH, Claeson P, Hagos M, Thulin M, Hedberg O, Warfa AM, Hassan AO, Elmi AH, Abdurahman AD, Elmi AS, Abdi YA, Alin MH (1991): Inventory of plants used in traditional medicine in Somalia-Plants of the families Acanthaceae-Chenopodiaceae. J Ethnopharmacol 35: 25–63.
- Sathish CJ, Sharma RA, Jain R, Mascolo N, Capasso F, Vijayvergia R, Mittal C (1998): Ethnopharmacological evaluation of Pergularia daemia (Forsk.) Chiov. Phytother Res 12:378–380.
- Savides MC, Woehme F (1983): Acetaminophen and its toxicity. J App Toxicol 3: 96–111.
- Singh SP (2001): Practical Manual of Biochemistry, fourth edition. New Delhi: CBS Publishers and Distributors, pp. 140–141.
- Suresh kumar SV, Mishra SH (2006): Hepatoprotective effect of extracts of Pergularia daemia Forsk. J Ethnopharmacol 107: 164–168.
- Sureshkumar SV, Mishra SH (2007): Hepatoprotective activity of extracts from P. daemia Forsk. against carbon tetrachloride-induced toxicity in rats. Pharma Mag 13: 187–191.
- Sureshkumar SV, Mishra SH (2008a): Hepatoprotective effect of Pergularia daemia (Forsk.) ethanol extract and its fraction. Indian J Exp Biol 46: 447–452.
- Sureshkumar SV, Mishra SH (2008b): In vitro evaluation of hepatoprotective activity of P. daemia Forsk. Pharma Mag 4(16)8: 298–302.
- Tran QI, Adnyana IK, Tezuka Y, Nagaoka T, Tran QK, Kadota S (2001): Triterpene saponins from Vietnamese gingeng (Panax vietnamensis) and their hepatocyte protective activity. J Nat Prod 64: 456–461.
- Van de Straat R, De Vries J, Debets AJ, Vermueulein NP (1987): The mechanism of prevention of paracetamol-induced hepatotoxicity by 3, 5-dialkyl substitution. The role of glutathione depletion and oxidative stress. Biochem Pharmacol 36: 2065–2070.
- Varier PS (1995): Indian Medicinal Plants: A Compendium of 500 Species, Volume IV. Hyderabad: Orient Longman, pp. 236–238.
- Vijayan P, Prashanth HC, Vijayaraj P, Dhanaraj SA, Badami S, Suresh B (2003): Hepatoprotective effect of the total alkaloid fraction of Solanum pseudocasicum leaves. Pharm Biol 41: 443–448.
- Wahi AK, Ravi J, Hemalatha S, Singh PN (2002): Anti-diabetic activity of Daemia extensa, J Nat Remedies 2: 80–83.
- Winrow VR, Winyard PG, Morris CJ, Black DR (1993): Free radical in inflammation: Secondary messengers and mediators of tissue destruction. Br Med Bull 49: 506–517.